Males have been short-changed in the X chromosome department: they only get one copy of the chromosome and the genes that it carries, whereas females get two. Differences in gene dose often translate into differences in protein levels, potentially putting males at a disadvantage compared with their sisters. Female placental and marsupial mammals inactivate one of their two X chromosomes, effectively silencing gene expression from the inactive X and seemingly putting themselves at the same disadvantage as their brothers. In PNAS, Pessia et al. (1) show that female X chromosome inactivation (XCI) is not in fact an act of solidarity by females but rather a result of the evolutionary tug of war over gene expression and sex chromosome dosage compensation that occurs between the sexes on the X chromosome.
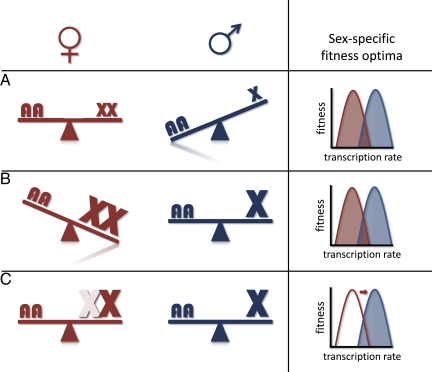
Not all genes are dosage-sensitive (2). However, the potential for problems related to reduced gene dose amplifies as the sex chromosomes diverge and the gene content of the Y chromosome deteriorates (3), leaving progressively more and more of the X chromosome present in only one copy in males. Ohno was the first to propose that selection would act in males to increase expression of X-linked genes to compensate for reduced gene dosage, restoring expression to levels observed before the origin of male monosomy (4). Ohno also suggested that XCI in females is a consequence of selection in males for dosage compensation. This is because gene expression levels are highly correlated between males and females, and selection for hyperexpression of the single male X would also cause overexpression of the two X chromosomes in females. For dosage-sensitive genes, overexpression in one sex is likely to be as harmful as underexpression in the other, setting up a battle between the sexes over optimal transcription levels. XCI resolves this conflict by effectively reducing gene dose in females as well, thereby aligning selection in both sexes to hypertranscribe X-linked genes (Fig. 1).
Fig. 1.
Sexual conflict over X chromosome transcription and the evolution of XCI. The single copy of the X chromosome in males results in selection to hypertranscribe dosage-sensitive X-linked genes (A), which balances out the X and autosomes in males but results in overtranscription of X-linked genes in females (B). In both A and B, males (blue) and females (red) have distinctly different transcriptional optima. This sexual conflict is resolved by XCI, which equalizes effective gene dose and realigns optimal transcription levels for both sexes (C).
Ohno's hypothesis (4) predicts that X-linked genes will be expressed in both males and females at a level normally observed for genes present in two copies (i.e., autosomal genes), as this represents the ancestral state of affairs for these loci before sex chromosome divergence caused gene dose imbalance. If Ohno was wrong and XCI is a mechanism of solidarity simply to balance gene dose of the X between the sexes regardless of the ancestral expression levels, then X-linked genes will be expressed on average less than genes on autosomes.
A recent surge of papers has revealed that dosage compensation varies a great deal and that many animals compensate only a minority of genes on the sex chromosomes (5–8). There is an unresolved controversy at the moment regarding the efficacy of X chromosome dosage compensation in therian mammals (9–11), but regardless of the debate, genes on the therian X chromosome show a range of dosage compensation as well. This range suggests that selection for dosage compensation varies greatly across loci on the sex chromosomes. By choosing a subset of mammalian genes known to be dosage-sensitive (2, 12), Pessia et al. (1) exploit this variance to test the requirements of Ohno's hypothesis. They find that dosage-sensitive X-linked genes are indeed expressed in both sexes at similar levels to loci on the autosomes. In contrast, the remainder of genes on the X chromosome seem not to be hypertranscribed and therefore are expressed less than the autosomal average, presumably because reduced gene dose in males and XCI in females has not been accompanied with selection for hypertranscription at these sites.
Pessia et al. (1), therefore, have not only refined the predictions regarding the evolution of sex chromosome dosage compensation in therian mammals but (arguably more importantly) revealed the extensive sexual conflict over dosage compensation on the X chromosome (13). Sex chromosome divergence and gene loss on the Y results in a battle between males and females over optimal transcription levels. XCI represents the armistice ending this battle, because it realigns effective gene dose between the sexes, thereby resolving their conflict over optimal transcription rates. The strength of sexual conflict over gene dose and transcription could go a long way to explaining the variation in dosage compensation observed, where different genes and even species are placed along a continuum with large differences in gene dose effects at one end and complete dosage compensation at the other end.
Selection for sex chromosome dosage compensation is therefore a delicate balancing act between male and female optimal expression levels, which are expected to vary among different types of
For dosage-sensitive genes, overexpression in one sex is likely to be as harmful as underexpression in the other.
genes (2, 14) and different developmental stages (15) and across tissues (16, 17). Variation in the relative strength of male- versus female-specific selection plays an essential part in determining the outcome of this conflict as does the intensity of sexual selection. Additionally, this conflict has the potential to interact with the degeneration of the Y chromosome, theoretically halting the loss of certain genes where dosage is important and potentially arresting sex chromosome divergence.
Despite acknowledging the role of this conflict, the early stages of sex chromosome divergence and dosage compensation evolution are still difficult to envisage. X chromosome monosomy in males can have severely deleterious consequences if just a few dosage-sensitive genes are affected. How these fitness costs are managed without hypertranscription simultaneously occurring with the loss of dosage-sensitive Y chromosome genes remains a mystery. Stay tuned for additional developments.
Acknowledgments
A.E.W. is supported by a Natural Environment Research Council studentship, and work in the laboratory of J.E.M. is supported by both the Biotechnology and Biological Sciences Research Council and European Research Council Grant 260233.
Footnotes
The authors declare no conflict of interest.
See companion article on page 5346.
References
- 1.Pessia E, Makino T, Bailly-Bechet M, McLysaght A, Marais GAB. Mammalian X chromosome inactivation evolved as a dosage-compensation mechanism for dosage-sensitive genes on the X chromosome. Proc Natl Acad Sci USA. 2012;109:5346–5351. doi: 10.1073/pnas.1116763109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papp B, Pál C, Hurst LD. Dosage sensitivity and the evolution of gene families in yeast. Nature. 2003;424:194–197. doi: 10.1038/nature01771. [DOI] [PubMed] [Google Scholar]
- 3.Hughes JF, et al. Strict evolutionary conservation followed rapid gene loss on human and rhesus Y chromosomes. Nature. 2012;483:82–86. doi: 10.1038/nature10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno S. Sex Chromosomes and Sex Linked Genes. Berlin: Springer; 1967. [Google Scholar]
- 5.Deakin JE, Hore TA, Koina E, Marshall Graves JA. The status of dosage compensation in the multiple X chromosomes of the platypus. PLoS Genet. 2008;4:e1000140. doi: 10.1371/journal.pgen.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh Y, et al. Dosage compensation is less effective in birds than in mammals. J Biol. 2007;6:2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mank JE. The W, X, Y and Z of sex-chromosome dosage compensation. Trends Genet. 2009;25:226–233. doi: 10.1016/j.tig.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicoso B, Bachtrog D. Lack of global dosage compensation in Schistosoma mansoni, a female-heterogametic parasite. Genome Biol Evol. 2011;3:230–235. doi: 10.1093/gbe/evr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng XX, et al. Evidence for compensatory upregulation of expressed X-linked genes in mammals, Caenorhabditis elegans and Drosophila melanogaster. Nat Genet. 2011;43:1179–1185. doi: 10.1038/ng.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharchenko PV, Xi RB, Park PJ. Evidence for dosage compensation between the X chromosome and autosomes in mammals. Nat Genet. 2011;43:1167–1169. doi: 10.1038/ng.991. [DOI] [PubMed] [Google Scholar]
- 11.Xiong YY, et al. RNA sequencing shows no dosage compensation of the active X-chromosome. Nat Genet. 2010;42:1043–1047. doi: 10.1038/ng.711. [DOI] [PubMed] [Google Scholar]
- 12.Makino T, McLysaght A. Ohnologs in the human genome are dosage balanced and frequently associated with disease. Proc Natl Acad Sci USA. 2010;107:9270–9274. doi: 10.1073/pnas.0914697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mank JE, Hosken DJ, Wedell N. Some inconvenient truths about sex chromosome dosage compensation and the potential role of sexual conflict. Evolution. 2011;65:2133–2144. doi: 10.1111/j.1558-5646.2011.01316.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang LQ, Li WH. Mammalian housekeeping genes evolve more slowly than tissue-specific genes. Mol Biol Evol. 2004;21:236–239. doi: 10.1093/molbev/msh010. [DOI] [PubMed] [Google Scholar]
- 15.Domazet-Lošo T, Tautz D. A phylogenetically based transcriptome age index mirrors ontogenetic divergence patterns. Nature. 2010;468:815–818. doi: 10.1038/nature09632. [DOI] [PubMed] [Google Scholar]
- 16.Duret L, Mouchiroud D. Determinants of substitution rates in mammalian genes: Expression pattern affects selection intensity but not mutation rate. Mol Biol Evol. 2000;17:68–74. doi: 10.1093/oxfordjournals.molbev.a026239. [DOI] [PubMed] [Google Scholar]
- 17.Khaitovich P, et al. Parallel patterns of evolution in the genomes and transcriptomes of humans and chimpanzees. Science. 2005;309:1850–1854. doi: 10.1126/science.1108296. [DOI] [PubMed] [Google Scholar]