Abstract
Neural circuits in the spinal cord transform instructive signals from the brain into well-coordinated locomotor movements by virtue of rhythm-generating components. Although evidence suggests that excitatory interneurons are the essence of locomotor rhythm generation, their molecular identity and the assessment of their necessity have remained unclear. Here we show, using larval zebrafish, that V2a interneurons represent an intrinsic source of excitation necessary for the normal expression of the locomotor rhythm. Acute and selective ablation of these interneurons increases the threshold of induction of swimming activity, decreases the burst frequency, and alters the coordination of the rostro–caudal propagation of activity. Thus, our results argue that V2a interneurons represent a source of excitation that endows the spinal circuit with the capacity to generate locomotion.
Keywords: central pattern generator, premotor interneurons, motor behavior, synaptic transmission
The generation of motor behavior involves decision making, selection, initiation, and execution (1–9). The spinal cord acts as an interface to process descending commands from the brain and sensory inputs from the periphery (5, 8, 10–16). Many insights into the organization and function of circuits underlying motor behavior have been gained from studies of spinal networks controlling locomotor movements (6, 13, 17–24). The basic locomotor activity requires the interplay of ipsilateral excitatory drive and crossed inhibition, which ensures the alternating pattern between the two sides of the spinal cord. Whereas inhibitory interneurons ensure the coordination of motoneurons controlling antagonistic muscles, excitatory interneurons are believed to represent the core components for the generation of the locomotor rhythm.
Determining the identity of the interneurons at the origin of excitation necessary for the normal generation of locomotor activity is central for understanding the principles of organization and function of locomotor circuits. However, the molecular identity of these excitatory interneurons has remained unclear. A prominent class of excitatory interneurons is the V2a interneurons, defined by Chx10 expression and derived from the p2 progenitor domain with homologous counterparts across vertebrate species (21, 25–27). In larval zebrafish and newborn mice, V2a interneurons have been shown to project to motoneurons and to be recruited in a frequency-dependent manner (28–32). These properties are consistent with the possibility that this class of interneurons represents a source of excitation within the spinal locomotor circuit.
In newborn mice, however, genetic elimination of V2a interneurons has been reported to have little effect on the rhythmic output of the locomotor circuits induced by pharmacological means (33, 34). The absence of tangible effects on the excitability of the locomotor circuit in the absence of V2a interneurons led to the conclusion that these interneurons are not essential for rhythm generation, but they help to stabilize the rhythm and activate commissural pathways that maintain left–right coordination (33–35). Therefore, the source of ipsilateral excitation contributing to the generation of the locomotor rhythm is still unclear.
Here we investigate the contribution of V2a interneurons to rhythm generation in the spinal locomotor network using acute and specific ablation in zebrafish. We show that elimination of V2a interneurons increases the induction threshold of swimming activity by electrical and pharmacological stimulation. When swimming was elicited, the duration of the swimming episodes and the burst frequency were significantly reduced by ablation of V2a interneurons. In addition, the propagation of the activity wave along the rostro–caudal body axis was significantly slowed down. Our findings define V2a interneurons in zebrafish as one source of excitation in the spinal locomotor network: they contribute to the normal generation of swimming activity, set its baseline frequency range, and define the coordination of the rostro–caudal propagation of activity. Thus, these interneurons endow the spinal network with important features that are compatible with a role in locomotor rhythm generation.
Results
V2a Interneurons Represent a Source of Excitation in the Spinal Circuit.
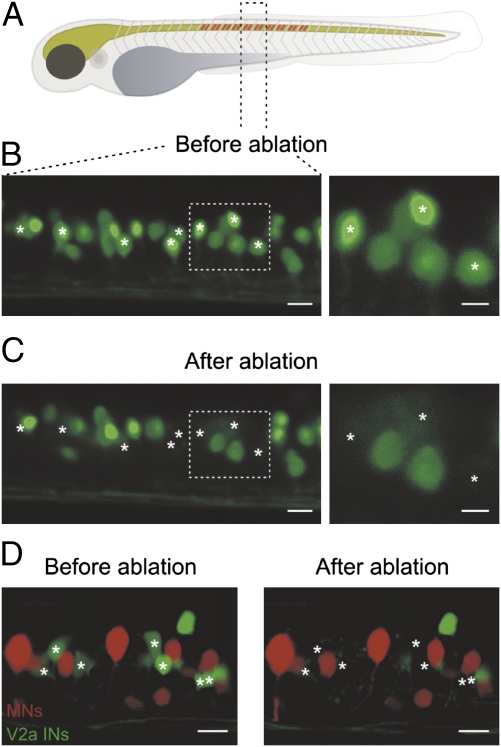
V2a interneurons provide monosynaptic excitatory input to motoneurons and are rhythmically active during swimming in embryonic and larval zebrafish (28, 31). To test directly whether these interneurons are important for producing swimming activity, we used a photoablation technique. For these experiments, we used transgenic zebrafish in which GFP expression is driven by the Chx10 promotor, which selectively labels V2a interneurons (28). V2a interneurons were ablated across 10 segments in the midbody region using a two-photon laser microscope (Fig. 1A). In most experiments, ∼30% of V2a interneurons (15 of 50 GFP-labeled interneurons) in each of the 10 segments were specifically ablated (Fig. 1 B and C) and the success of the ablation was confirmed by confocal scanning of the spinal cord before and after ablation (Fig. 1 B and C). We further tested the possible unspecific damage of neighboring neurons following ablation of V2a interneurons. In these experiments, motoneurons were backfilled by injection of rhodamine dextran in muscles of larval zebrafish expressing GFP in V2a interneurons. Motoneurons remained unaffected by ablation of neighboring V2a interneurons (Fig. 1D), indicating that the ablation does not induce secondary damage in adjacent neurons.
Fig. 1.
Ablation of V2a interneurons in the larval zebrafish. (A) V2a interneurons were ablated over 10 segments in the midbody region of larval zebrafish. (B) Reconstruction of 1.5 segments of the spinal cord in zebrafish using confocal microscopy before the ablation of V2a interneurons. (C) The spinal cord was scanned and reconstructed after photoablation of eight V2a interneurons (asterisks). (Right) Expanded image of the area indicated by the dashed boxes. (Left scale bar, 10 μm and Right scale bar, 5 μm.) (D) Motoneurons were prelabeled with rhodamine dextran in larval zebrafish expressing GFP in V2a interneurons. Ablation of V2a interneurons did not produce any secondary damage in adjacent motoneurons. (Scale bar, 10 μm.)
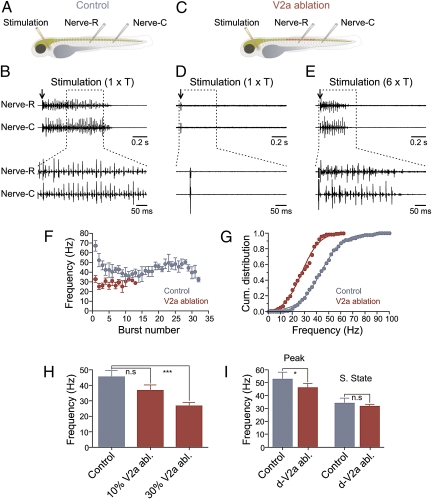
Swimming activity was compared between controls and animals in which V2a interneurons were ablated. To induce swimming, a glass stimulation electrode was placed at the level of the otic vesicle and the motor activity was monitored by recording peripheral nerves in the ablated region and in a more caudal segment (Fig. 2A). In control animals, threshold stimulation (T; 5–20 μA; mean = 15 ± 2 μA) induced a bout of swimming activity characterized by a propagating wave of activity from rostral to caudal segments (Fig. 2B). In animals in which ∼30% of V2a interneurons were ablated, threshold stimulation did not elicit any rhythmic activity in the peripheral nerves (Fig. 2 C and D). To induce swimming activity, higher stimulation intensities (∼6 × control threshold; 50–150 μA; mean = 86 ± 2 μA) were required (Fig. 2E). Furthermore, the duration of the swimming bout was significantly shorter (P < 0.001) in zebrafish with V2a ablation and amounted to 0.35 ± 0.04 s (n = 12) compared with 0.65 ± 0.07 s (n = 12) in control (Fig. 2F).
Fig. 2.
Ablation of V2a interneurons affects the swimming activity. (A) Experimental setup showing the position of the stimulation and recording electrodes in control animals. (B) A bout of swimming activity is induced by electrical stimulation (arrow) at the level of the otic vesicle at threshold intensity (1 × T). (C) Experimental setup showing the region of the animal where V2a interneurons are ablated together with the position of the stimulation and recording electrodes. (D) Stimulation with threshold intensity (1 × T; arrow) fails to induce swimming activity. (E) Locomotor activity is induced only by a higher stimulation intensity (6 × T; arrow). (F) Burst frequency and bout duration are decreased in V2a interneuron-ablated animals. (G) Cumulative distribution of the burst frequency in control and animals with V2a interneuron ablation. (H) Graph showing the effect of ablation of different proportions of V2a interneurons on the burst frequency. (I) Ablation of dorsally located V2a interneurons (d-V2a abl.) decreased the peak frequency (peak) without affecting the steady-state (s. state) frequency during the swimming episode induced by stimulation of the rostral part of the animals.
To determine the effect of V2a ablation on the excitability of the spinal locomotor circuit, we analyzed the cumulative distribution of the swimming frequencies, which was shifted toward lower values in animals with V2a interneuron ablation (Fig. 2G). In control, the burst frequency ranged between 16 and 94 Hz with the half-maximum frequency of 43.5 Hz (n = 12). After ablation of V2a interneurons, the frequency ranged between 7 and 61 Hz and the half-maximum frequency significantly decreased to 27.9 Hz (n = 12) (P < 0.005; two-sample Kolmogorov–Smirnov test; Fig. 2G). We also examined the effect of V2a ablation on the left–right alternation pattern during swimming. In the five animals tested, ablation of V2a interneurons did not affect the phase relationship between the activity of the motor nerves on the left and right sides.
In some experiments, 10% of V2a interneurons (5 of 50 GFP-labeled interneurons) per segment were ablated across 10 segments. In these animals, threshold electrical stimulation induced a significantly shorter bout of swimming activity (0.43 ± 0.04 s; P < 0.05; n = 5) with a mean burst frequency of 36.7 ± 3.5 Hz (n = 5), which was not significantly different (P > 0.05) from control, which had a mean frequency of 45.4 ± 4.2 Hz (n = 12; Fig. 2H). The mean frequency in animals with 30% of V2a interneurons ablated was 26.8 ± 2.3 Hz, which was significantly different from that of control animals (P < 0.001; Fig. 2H). We then tested whether ablation of dorsally located V2a interneurons, known to be recruited only at high frequency swimming or escape (28–31), preferentially affects fast swimming frequencies. Ablation of dorsal V2a interneurons (∼15% per segment) decreased the peak frequency from 58.5 ± 5.2 Hz to 46.1 ± 3.2 Hz (P < 0.05; n = 8; Fig. 2I) without affecting the steady-state frequency (control = 34.1 ± 3.8 Hz; V2a ablation = 31.7 ± 1.6 Hz; P > 0.05; n = 8; Fig. 2I). Ablation of dorsal V2a interneurons did not significantly affect the duration of the swimming episode (0.64 ± 0.11 s; n = 8). These results together indicate that V2a interneurons contribute to excitatory drive in the spinal locomotor circuit.
V2a Interneurons Generate Swimming Activity.
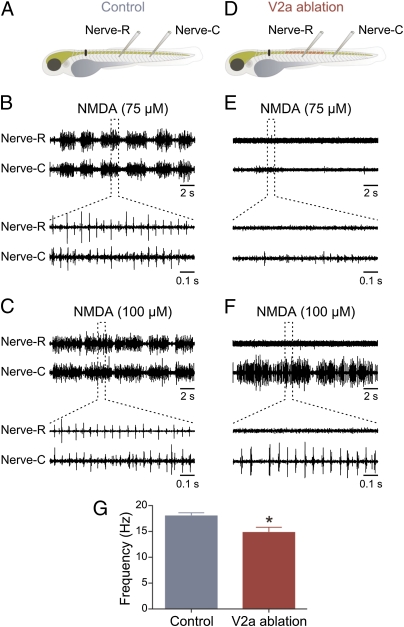
The above results show that V2a interneurons are important for normal activity of the swimming circuit. Although V2a act as premotor interneurons and are likely to activate other interneurons (28), they could also serve as a relay of descending inputs to the swimming circuit without any direct involvement in rhythm generation (33). To test this possibility, the descending input was substituted with pharmacological activation by NMDA to provide a tonic excitatory drive sufficient to activate the swimming circuit in the spinal cord. In these experiments the animals were spinalized and two concentrations of NMDA (75 and 100 μM) were tested in control and in fish with ∼30% of V2a interneurons ablated. In control, application of NMDA (75 μM; n = 7) induced swimming activity, which was monitored by recording motor nerves in two different segments along the rostro–caudal axis (Fig. 3A). The locomotor activity consisted of repetitive swimming bouts, which are characteristic of larval zebrafish (Fig. 3B) (36–38). Application of a higher NMDA concentration (100 μM; n = 7) increased the duration of the swimming bouts (Fig. 3C). In animals with V2a interneuron ablation (Fig. 3D), application of the lower NMDA concentration (75 μM; n = 7) failed to induce any rhythmic activity in the motor nerve recorded in the region of the ablation and only elicited feeble activity in the more caudal “intact” region (Fig. 3E). In these animals, swimming activity was induced only by the higher NMDA concentration and in most experiments only in the segments caudal to the site of ablation of V2a interneurons (100 μM; n = 7; Fig. 3F). In three of seven preparations, swimming activity was also recorded in the rostral motor nerve located in segments where V2a interneurons were ablated. The burst frequency induced by the higher NMDA concentration (100 μM) was 18.0 ± 0.6 Hz (n = 7) in control and was significantly lowered (P < 0.05) to 14.8 ± 1.0 Hz (n = 7) in animals with V2a ablation (Fig. 3G). These results show that the ability to induce swimming activity by NMDA was decreased by ablation of V2a interneurons and even when swimming was elicited, the burst frequency was reduced, suggesting that they are not only relaying descending inputs.
Fig. 3.
Ablation of V2a interneurons affects swimming activity induced by NMDA. (A) Experimental setup showing the site of the spinalization (black bar) and the position of recording electrodes in control animals. (B) Application of NMDA (75 μM) induces locomotor activity consisting of episodic swimming bouts. (C) Application of a higher concentration of NMDA (100 μM) prolongs the swimming bout duration. (D) Experimental setup showing the region of the animal where V2a interneurons are ablated as well as the position of the spinalization (black bar) and recording electrodes. (E) Application of a lower NMDA concentration (75 μM) fails to induce swimming activity. (F) Application of a higher concentration of NMDA (100 μM) induces swimming activity in the segments caudal to the region where V2a interneurons are ablated. (G) Graph showing the mean locomotor burst frequency in control and in animals with V2a interneuron ablation.
Change in Swimming Is Not the Result of Unspecific Ablation of Neurons.
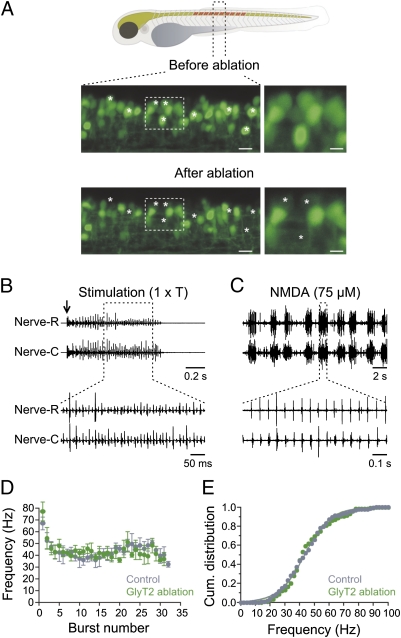
The two-photon laser ablation allows for targeting specific neurons without affecting neighboring ones. However, the decrease in excitability of the spinal circuit could still be the result of the ablation of neurons per se and not the consequence of the specific elimination of V2a interneurons. To rule out this possibility, we ablated glycinergic interneurons by using transgenic zebrafish with GFP expression driven by the promoter of the glycine transporter 2 (GlyT2) (31). The same number of glycinergic interneurons (i.e., 15 neurons) was ablated over the same number of segments (10 segments) as for the V2a interneurons (Fig. 4A). Threshold stimulation at the level of the otic vesicle in animals with GlyT2 interneuron ablation elicited a bout of swimming activity (n = 8; Fig. 4B). The duration of the swimming bout induced by electrical stimulation in animals with GlyT2 interneuron ablation was similar to that in control (GlyT2 bout duration: 0.75 ± 0.1 s; n = 8; Fig. 4D). The cumulative distribution of the burst frequency was also similar to that of control animals with a half-maximum frequency of 43.6 Hz (range: 16–93 Hz; n = 8; Fig. 4E).
Fig. 4.
Ablation of glycinergic (GlyT2) interneurons does not alter swimming activity. (A, Upper) Drawing of a zebrafish showing the region where GlyT2 interneurons are ablated. (Lower) Confocal reconstruction of 1.5 segment of the spinal cord before and after photoablation of GlyT2 interneurons. Asterisks indicate the position of the ablated interneurons. (Right) High magnification of the regions indicated by the dashed boxes. (Left scale bar, 10 μm and Right scale bar, 5 μm.) (B) Electrical stimulation at threshold intensity (1 × T; arrow) induces a bout of swimming activity in animals with GlyT2 interneurons ablated. (C) Application of a lower NMDA concentration (75 μM) induces swimming activity both in the region of the ablation and in more caudal segments. (D) Burst frequency and duration of the swimming bout recorded in animals with GlyT2 interneurons ablated is similar to control animals. (E) Cumulative distribution of the burst frequency in control and in animals with GlyT2 interneurons ablated.
Similar to control (Fig. 3B), application of the lower concentration of NMDA (75 μM; n = 6) was able to elicit repetitive swimming bouts in animals with GlyT2 ablation (Fig. 4C). Ablation of the GlyT2 interneurons had an influence on the onset and pattern of swimming. In these animals, the time to the onset of swimming activity was shortened and the activity usually switched to continuous swimming with prolonged NMDA application (75 μM; n = 6). These results indicate that an ablation of glycinergic interneurons has little impact on the excitability of the spinal locomotor circuit. Thus, the increase of the threshold to trigger swimming activity after ablation of V2a cannot be attributed to the ablation per se but rather to the specific elimination of this class of excitatory interneurons.
V2a Interneuron Ablation Changes the Intersegmental Coordination.
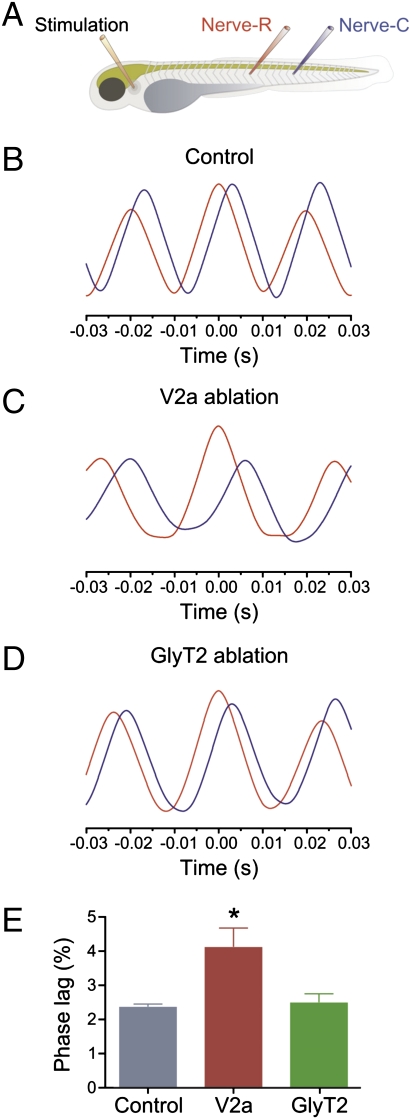
The propagation of the wave of activity from rostral to caudal segments with a constant phase lag is an important characteristic of swimming. This characteristic is mediated by intersegmental coordination mechanisms that ensure the precise propagation of activity along the different segments of the spinal cord. V2a interneurons are likely to contribute to the intersegmental coordination, because they have descending axons projecting over several segments, allowing them to relay excitatory drive along the rostro–caudal axis (28, 39). To test this possibility, we examined the delay between swimming bursts in the rostral and caudal parts of the animal using auto- and cross-correlation analysis (Fig. 5A).
Fig. 5.
Ablation of V2a interneurons changes the intersegmental coordination during swimming. (A) Experimental setup showing the position of the stimulation and recording electrodes. Rostral and caudal recording electrodes are placed five segments apart from each other in all these experiments. (B) Graph showing auto- (red) and cross-correlation (blue) of the activity recorded in a rostral and caudal segment. Swimming activity propagates in a rostro–caudal direction. (C) Ablation of V2a interneurons increases the delay of propagation of the swimming activity along the rostral–caudal axis of the animals. (D) Ablation of GlyT2 interneurons does not change the delay of propagation of swimming activity. (E) Graph showing mean phase lag per segment in control (blue), in animals with V2a interneuron ablation (red), and in animals with GlyT2 interneuron ablation (green).
In control animals, there was a constant delay between the activity of the rostral (auto-correlation, red curve) and the caudal (cross-correlation, blue curve) motor nerve with a phase lag per segment of 2.3 ± 0.1% of the cycle duration (n = 7; Fig. 5 B and E). Ablation of V2a interneurons increased the delay of activity between the rostral (red curve) and caudal (blue curve) motor nerves, resulting in a significant increase of the phase lag per segment to 4.1 ± 0.5% (P < 0.05; n = 7; Fig. 5 C and E). Ablation of GlyT2 interneurons, on the other hand, did not affect the rostro–caudal delay during swimming (phase lag per segment = 2.5 ± 0.3%; P > 0.05; n = 6; Fig. 5 D and E). These results show that the specific ablation of V2a interneurons not only altered the threshold activation of the spinal swimming circuit, but it also affected the intersegmental coordination.
Discussion
V2a Interneurons as a Source of Excitatory Drive Within the Locomotor Circuit.
Neural circuits in the spinal cord transform command signals from the brainstem into well-coordinated locomotor patterns that drive the sequence of activation of muscles to produce motion. The ability of spinal circuits to generate the locomotor rhythm emanates from the existence of an intrinsic source of excitability that sets the activity tone of the constituent neurons of these circuits. The molecular identity of the excitatory interneurons underlying the locomotor rhythm has been unclear (6, 18, 22). Our analysis now suggests that V2a interneurons represent a possible source of excitation that contributes to generating swimming activity. Even a partial ablation of the V2a interneuron population produces major changes. It affects the threshold for initiation of locomotion, decreases the swimming frequency, and modifies the rostro–caudal delay.
A similar decrease in the excitability was also seen when swimming activity was induced by activation of NMDA receptors, indicating that it is due to a change in the level of excitability of the spinal circuit rather than a perturbation of integration of descending inputs. The propagation of activity along the rostro–caudal axis of the spinal cord was also altered by ablation of V2a interneurons. These interneurons do indeed project their axons caudally (28, 39), which allows them to participate in the coordination of activity across the different segments to mediate the undulatory swimming movement. Thus, our results identify V2a interneurons in zebrafish as an intrinsic source of glutamatergic excitation within the spinal circuit, which contributes to the generation of locomotion and the coordination of its propagation across different segments.
Linking Connectivity and Function of V2a Interneurons in the Locomotor Circuit.
In embryonic and larval zebrafish, V2a interneurons have been divided into two classes dependent on whether they are activated during strong (escape) or weak (swimming) locomotor activity (28, 40). The former make monosynaptic connections with both primary and secondary motoneurons (28, 41), whereas the latter are presumed to project only to secondary motoneurons (28). V2a interneurons seem to be organized into different circuits to produce locomotor movements at different speeds (21, 23, 24). They thus appear to play an important role for the generation of locomotor activity in zebrafish; however, a direct assessment of their involvement in the generation of rhythm in the spinal circuit has been missing.
The connectivity of V2a interneurons in zebrafish is reminiscent of that previously described in the swimming circuits of lamprey and tadpoles (7, 17, 23, 42–44). Recent evidence in rodents showed the existence of an anatomical substrate for connections between V2a interneurons and motoneurons (45–48). On the basis of their connectivity scheme and activity during locomotion (28, 31, 32, 49, 50), V2a interneurons have been suggested to serve as the main source of on-cycle excitation and represent the prime candidates for excitatory interneurons at the core of the locomotor generating circuit (6, 19, 22). However, elimination of V2a interneurons in mice did not induce any obvious defects that could link these interneurons to the rhythm-generating circuits (33, 34, 51). These interneurons seem to be preferentially activated at faster locomotor frequencies and excite commissural interneurons to ensure the alternating pattern of activity on both sides of the body (32–34). In newborn mice, the little impact of the genetic elimination of V2a interneurons on drug-induced rhythm generation capacity of the spinal cord was considered to imply that this class of interneurons is not essential for normal rhythm generation. It is, however, possible that the organization of the locomotor network is different between zebrafish and mice. V2a interneurons may have acquired a different role in the mouse compared with zebrafish and seem to be mostly involved in ensuring left–right alternation at high locomotor frequency (33, 34, 51). In addition, the generation of the rhythm underlying locomotion in mice could involve overlapping classes of interneurons and elimination of one of these classes (i.e., V2a interneurons) is not sufficient to prevent the normal expression of the rhythmic activity in the spinal circuits (6, 18, 22).
V2a Interneurons as a Part of the Rhythm Generation Circuit in Zebrafish.
Several lines of evidence support the possibility that V2a interneurons are a source of excitation that contributes to the generation of the locomotor rhythm. First, their ablation increases the threshold to trigger swimming activity by electrical stimulation and pharmacologically. Second, even when the spinal circuit generated swimming activity, the range of burst frequencies was always shifted toward lower values compared with control. Third, in many preparations, swimming activity could be expressed only by the segments caudal to the ablation sites, suggesting that in these cases the elimination of V2a interneurons impaired the ability of rhythm generation.
V2a are premotor interneurons that could also relay descending inputs to the rhythm generation circuit in the spinal cord. If this were the case, their ablation should indeed disrupt the generation of swimming activity induced by electrical stimulation, but it should not affect NMDA-induced locomotion. The latter should provide sufficient excitatory drive to compensate for the absence of descending inputs and trigger the locomotor rhythm and thus bypass any upstream relay interneurons. The generation of the locomotor rhythm was not only disrupted in the region of the spinal cord where V2a interneurons were ablated, but the threshold for its expression by NMDA was increased in the intact part with a significant decrease in the burst frequency. Alternatively, V2a interneurons could only serve as last-order interneurons conveying a rhythmic pattern generated by an upstream circuit. However, this cannot account for the change in the burst frequency during swimming induced by either electrical stimulation or NMDA. Our results are incompatible with a role of V2a interneurons as merely conveying descending activity to the rhythm generation circuit or as last-order premotor interneurons. Overall, the impact of V2a ablation on the excitability of the spinal swimming circuit expressed as a decrease in burst frequency combined with an increase in its activation threshold argue that this interneuron class contributes to the excitability of the locomotor circuit in zebrafish.
Experimental Procedures
Zebrafish Lines and Care.
All experimental protocols were approved by the animal research ethical committee, Stockholm. Zebrafish were raised and kept according to established procedures in a core facility at the Karolinska Institute. Two transgenic zebrafish lines were used (Chx10:GFP and GlyT2:GFP) and were described previously. All experiments were carried out in 4- to 5-d-old transgenic larval zebrafish at room temperature (∼22 °C).
Backfilling of Motoneurons.
Motoneurons were backfilled in anesthetized larval zebrafish with 0.03% tricaine methanesulfonate (MS222; Sigma-Aldrich) and tetramethylrhodamine dextran (3000 MW, Invitrogen) was injected into the muscles. The animals were left to recover for 1–2 h and to allow the retrograde transport of the tracer to motoneuron somata.
Laser Ablation of GFP-Labeled Interneurons.
Larval zebrafish were first anesthetized and then transferred to the ablation chamber, embedded in 1.5% low-melt agarose and covered with zebrafish extracellular solution, containing 0.03% MS-222. The chamber was then placed under the confocal microscope (LMS 510; Carl Zeiss). A total of 10, 15, or 30% of GFP-labeled V2a interneurons per segment was photoablated individually using a two-photon laser (wavelength 800 nm) over 10 segments in the midbody region of the animal.
Zebrafish Preparations.
The fish were anesthetized in 0.03% MS-222 in extracellular solution and then pinned down using tungsten pins placed through the notochord in a Sylgard-lined recording chamber. The skin was removed from both sides of the body with fine forceps before the fish were paralyzed with 6.25 μM α-bungarotoxin (Sigma-Aldrich) for 10 min. The larvae were placed dorsal side up to monitor left–right alternating activity during swimming or the rostro–caudal propagation of activity. The preparation was then perfused with extracellular solution for 15 min before the start of the experiment.
Electrophysiology.
Extracellular recordings were performed from the motor nerves running through the intermyotomal clefts from two segments either opposite to each other or along the rostro–caudal axis of the fish. Swimming activity was induced by electrical stimulation or by application of NMDA. For electrical stimulation, a glass electrode was placed at the level of the otic vesicle and current stimulation. The stimulation intensity was gradually increased until a bout of swimming activity could be reliably elicited.
Data Acquisition and Analysis.
Data were digitized and recorded on a personal computer. Data analysis was performed using correlation analysis in Spike2 (Cambridge Electronic Design). All values are given as mean ± SEM. Unless otherwise stated, the significance of differences of means between experimental groups and conditions was analyzed using Student's t test. Means were considered statistically significant at P values of <0.05.
Supplementary Material
Acknowledgments
We thank Drs. K. Dougherty, R. Hill, O. Kiehn, and S. Grillner for comments and critical discussion of this manuscript. This work was funded by a grant from the Swedish Research Council, European Commission [Seventh Framework Programme (FP7), Spinal Cord Repair] and Karolinska Institutet. J.A. received postdoctoral fellowships from the German Research Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115377109/-/DCSupplemental.
References
- 1.Deliagina TG, Zelenin PV, Orlovsky GN. Encoding and decoding of reticulospinal commands. Brain Res Brain Res Rev. 2002;40:166–177. doi: 10.1016/s0165-0173(02)00199-6. [DOI] [PubMed] [Google Scholar]
- 2.Grillner S. Biological pattern generation: The cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Hultborn H, et al. How do we approach the locomotor network in the mammalian spinal cord? Ann N Y Acad Sci. 1998;860:70–82. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- 4.Jankowska E. Spinal interneuronal networks in the cat: Elementary components. Brain Res Brain Res Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 7.Roberts A, Li WC, Soffe SR. How neurons generate behavior in a hatchling amphibian tadpole: an outline. Front Behav Neurosci. 2010;4:16. doi: 10.3389/fnbeh.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- 9.Büschges A, Scholz H, El Manira A. New moves in motor control. Curr Biol. 2011;21:R513–R524. doi: 10.1016/j.cub.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Arrenberg AB, Del Bene F, Baier H. Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci USA. 2009;106:17968–17973. doi: 10.1073/pnas.0906252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubuc R, et al. Initiation of locomotion in lampreys. Brain Res Brain Res Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 12.El Manira A, Pombal MA, Grillner S. Diencephalic projection to reticulospinal neurons involved in the initiation of locomotion in adult lampreys Lampetra fluviatilis. J Comp Neurol. 1997;389:603–616. [PubMed] [Google Scholar]
- 13.Grillner S. The motor infrastructure: From ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 14.Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci. 2010;13:246–252. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- 15.Kyriakatos A, et al. Initiation of locomotion in adult zebrafish. J Neurosci. 2011;31:8422–8431. doi: 10.1523/JNEUROSCI.1012-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 17.Berkowitz A, Roberts A, Soffe SR. Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Front Behav Neurosci. 2010;4:36. doi: 10.3389/fnbeh.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brownstone RM, Bui TV. Spinal interneurons providing input to the final common path during locomotion. Prog Brain Res. 2010;187:81–95. doi: 10.1016/B978-0-444-53613-6.00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fetcho JR, Higashijima S, McLean DL. Zebrafish and motor control over the last decade. Brain Res Brain Res Rev. 2008;57:86–93. doi: 10.1016/j.brainresrev.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriel JP, et al. Principles governing recruitment of motoneurons during swimming in zebrafish. Nat Neurosci. 2011;14:93–99. doi: 10.1038/nn.2704. [DOI] [PubMed] [Google Scholar]
- 21.Goulding M. Circuits controlling vertebrate locomotion: Moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grillner S, Jessell TM. Measured motion: Searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts A, Li WC, Soffe SR, Wolf E. Origin of excitatory drive to a spinal locomotor network. Brain Res Brain Res Rev. 2008;57:22–28. doi: 10.1016/j.brainresrev.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Wyart C, et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461:407–410. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 26.Kimura Y, Satou C, Higashijima S. V2a and V2b neurons are generated by the final divisions of pair-producing progenitors in the zebrafish spinal cord. Development. 2008;135:3001–3005. doi: 10.1242/dev.024802. [DOI] [PubMed] [Google Scholar]
- 27.Ladle DR, Pecho-Vrieseling E, Arber S. Assembly of motor circuits in the spinal cord: Driven to function by genetic and experience-dependent mechanisms. Neuron. 2007;56:270–283. doi: 10.1016/j.neuron.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean DL, Fan J, Higashijima S, Hale ME, Fetcho JR. A topographic map of recruitment in spinal cord. Nature. 2007;446:71–75. doi: 10.1038/nature05588. [DOI] [PubMed] [Google Scholar]
- 30.McLean DL, Fetcho JR. Spinal interneurons differentiate sequentially from those driving the fastest swimming movements in larval zebrafish to those driving the slowest ones. J Neurosci. 2009;29:13566–13577. doi: 10.1523/JNEUROSCI.3277-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci. 2008;11:1419–1429. doi: 10.1038/nn.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong G, Sharma K, Harris-Warrick RM. Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord. Nat Commun. 2011;2:274. doi: 10.1038/ncomms1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crone SA, et al. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60:70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiehn O, et al. Probing spinal circuits controlling walking in mammals. Biochem Biophys Res Commun. 2010;396:11–18. doi: 10.1016/j.bbrc.2010.02.107. [DOI] [PubMed] [Google Scholar]
- 36.Drapeau P, et al. Development of the locomotor network in zebrafish. Prog Neurobiol. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 37.Masino MA, Fetcho JR. Fictive swimming motor patterns in wild type and mutant larval zebrafish. J Neurophysiol. 2005;93:3177–3188. doi: 10.1152/jn.01248.2004. [DOI] [PubMed] [Google Scholar]
- 38.McDearmid JR, Drapeau P. Rhythmic motor activity evoked by NMDA in the spinal zebrafish larva. J Neurophysiol. 2006;95:401–417. doi: 10.1152/jn.00844.2005. [DOI] [PubMed] [Google Scholar]
- 39.Higashijima S, Schaefer M, Fetcho JR. Neurotransmitter properties of spinal interneurons in embryonic and larval zebrafish. J Comp Neurol. 2004;480:19–37. doi: 10.1002/cne.20279. [DOI] [PubMed] [Google Scholar]
- 40.Ritter DA, Bhatt DH, Fetcho JR. In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. J Neurosci. 2001;21:8956–8965. doi: 10.1523/JNEUROSCI.21-22-08956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fetcho JR. Excitation of motoneurons by the Mauthner axon in goldfish: Complexities in a “simple” reticulospinal pathway. J Neurophysiol. 1992;67:1574–1586. doi: 10.1152/jn.1992.67.6.1574. [DOI] [PubMed] [Google Scholar]
- 42.Buchanan JT, Grillner S. Newly identified ‘glutamate interneurons’ and their role in locomotion in the lamprey spinal cord. Science. 1987;236:312–314. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- 43.Buchanan JT, Grillner S, Cullheim S, Risling M. Identification of excitatory interneurons contributing to generation of locomotion in lamprey: Structure, pharmacology, and function. J Neurophysiol. 1989;62:59–69. doi: 10.1152/jn.1989.62.1.59. [DOI] [PubMed] [Google Scholar]
- 44.Dale N, Roberts A. Dual-component amino-acid-mediated synaptic potentials: Excitatory drive for swimming in Xenopus embryos. J Physiol. 1985;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur J Neurosci. 2007;26:3003–3015. doi: 10.1111/j.1460-9568.2007.05907.x. [DOI] [PubMed] [Google Scholar]
- 46.Lundfald L, et al. Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur J Neurosci. 2007;26:2989–3002. doi: 10.1111/j.1460-9568.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 47.Stepien AE, Arber S. Probing the locomotor conundrum: Descending the ‘V’ interneuron ladder. Neuron. 2008;60:1–4. doi: 10.1016/j.neuron.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 48.Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron. 2010;68:456–472. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 49.Dougherty KJ, Kiehn O. Firing and cellular properties of V2a interneurons in the rodent spinal cord. J Neurosci. 2010;30:24–37. doi: 10.1523/JNEUROSCI.4821-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong G, et al. Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. J Neurosci. 2010;30:170–182. doi: 10.1523/JNEUROSCI.4849-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dougherty KJ, Kiehn O. Functional organization of V2a-related locomotor circuits in the rodent spinal cord. Ann N Y Acad Sci. 2010;1198:85–93. doi: 10.1111/j.1749-6632.2010.05502.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.