Abstract
Stroke causes brain dysfunction and neuron death, and the lack of effective therapies heightens the need for new therapeutic targets. Here we identify prokineticin 2 (PK2) as a mediator for cerebral ischemic injury. PK2 is a bioactive peptide initially discovered as a regulator of gastrointestinal motility. Multiple biological roles for PK2 have been discovered, including circadian rhythms, angiogenesis, and neurogenesis. However, the role of PK2 in neuropathology is unknown. Using primary cortical cultures, we found that PK2 mRNA is up-regulated by several pathological stressors, including hypoxia, reactive oxygen species, and excitotoxic glutamate. Glutamate-induced PK2 expression is dependent on NMDA receptor activation and extracellular calcium. Enriched neuronal culture studies revealed that neurons are the principal source of glutamate-induced PK2. Using in vivo models of stroke, we found that PK2 mRNA is induced in the ischemic cortex and striatum. Central delivery of PK2 worsens infarct volume, whereas PK2 receptor antagonist decreases infarct volume and central inflammation while improving functional outcome. Direct central inhibition of PK2 using RNAi also reduces infarct volume. These findings indicate that PK2 can be activated by pathological stimuli such as hypoxia-ischemia and excitotoxic glutamate and identify PK2 as a deleterious mediator for cerebral ischemia.
Keywords: excitotoxicity; RNA interference; Akt; Erk1,2; SAP/JNK
Ischemic injuries such as stroke are devastating neurological insults that affect about 750,000 people in the United States each year (1). During such injury, rapid release of glutamate triggers an increase in free cytosolic Ca2+ concentrations and the generation of reactive oxygen species (2, 3), activating a variety of genes (3–5), some with protective, beneficial effects (e.g., neurotrophins) (6) and others with deleterious ones (e.g., proinflammatory cytokines) (7–9). Currently, the only approved pharmacological treatment for stroke is the recombinant thrombolytic tissue plasminogen activator, which has a narrow time window (3–4.5 h) and is contraindicated in hemorrhagic stroke (10, 11). Identifying molecules that participate in the pathological mechanisms underlying ischemic injury will help elucidate potential therapeutic targets.
Prokineticins 1 (PK1) and 2 (PK2), also known as “endocrine gland vascular endothelial factor” and “Bombina varigata 8,” are a pair of secreted bioactive peptides that are highly conserved across species (12, 13). Since their initial discovery in 2001, multiple physiological roles for PKs have been discovered, including gastrointestinal motility (12), generation of circadian rhythms (14–16), angiogenesis (17, 18) and choroidal neovascularization (19), olfactory bulb neurogenesis (20), neuroexcitation (21–24), inflammation (25–28), and reproduction (29, 30). Recent evidence implicated PK2 in a human disease in which different point mutations in genes encoding PK2 or its receptor (PKR2) lead to a type of Kallmann syndrome (29), a disease characterized by abnormal olfactory function and underdeveloped gonads caused by a deficiency in hypothalamic gonadotropin-releasing hormones. Mice lacking PK2 or PKR2 also exhibited similar phenotype (20, 31).
Despite the versatile effects of PKs during homeostasis, the role of PKs in neuropathology is unclear. Analyses of the PK promoter revealed the existence of multiple copies of hypoxia-responsive elements (HREs) (17), suggesting that their transcription can be regulated during hypoxic conditions. Indeed, both PKs have been shown to be inducible by hypoxia in human adrenal carcinoma cells (17). However, it is unknown whether PK expressions are activated in neurological insults such as stroke and, if so, whether PKs play a protective or deleterious role. PK2 has been shown to protect against NMDA excitotoxicity in cerebellar cultures (32), to protect cardiomyocytes against oxidative stress (33), and to promote neurogenesis in the olfactory bulb (20). However, PK2 also induces monocyte and macrophage migration and stimulates LPS-induced production of proinflammatory cytokines such as IL-1 and IL-12 in mouse peritoneal macrophages (25). Thus, the current knowledge of PKs suggests intriguing possibilities for their role in neurological insults such as stroke.
In this study we investigated the role of PK2 in cerebral ischemic injury. Using primary cortical cultures, we investigated PK2 expression after various insults. We also examined the expression profile of PK2 in the ischemic rat brain after in vivo focal ischemia. Furthermore, we investigated the effects of enhancing or blocking PK2 on infarct volume, inflammatory infiltration, and behavioral outcome after stroke. Our data show that PK2 is a deleterious mediator for ischemic injury and identify PK2 as a potential therapeutic target for stroke.
Results
PK2 Expression Is Up-Regulated by Pathological Insults.
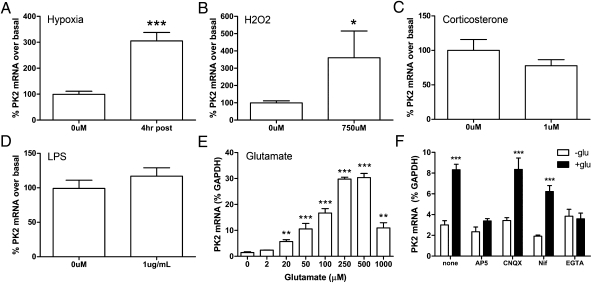
We first examined PK2 expression after exposure to various pathological insults (Fig. 1). We used primary cortical cultures, which provide a more controlled environment than in vivo, allowing us to assess the direct effects of various pathological insults. Using quantitative PCR (qPCR), we found that PK2 expression is up-regulated by 6 h of hypoxia (Fig. 1A) and reactive oxygen species (750 μM hydrogen peroxide) (Fig. 1B). Interestingly, treatment with corticosterone (1 μM) or lipopolysaccharide (10 μg/mL) did not induce PK2 expression (Fig. 1 C and D).
Fig. 1.
PK2 mRNA is up-regulated by various pathological insults in vitro. Primary cortical cultures were treated with 6 h of (A) hypoxia, (B) reactive oxygen species (hydrogen peroxide, 750 uM), (C) corticosterone (1 uM), (D) LPS (10 ug/mL), or (E) excitotoxic glutamate (2–1,000 μM). PK2 mRNA is induced by hypoxia, reactive oxygen species, and glutamate. n = 4–6. *P < 0.05, **P < 0.01, ***P < 0.001, Student's t test or one-way ANOVA with Bonferroni's post hoc test. (F) Glutamate-induced PK2 expression is dependent on NMDA receptor activation and extracellular calcium. Error bars represent SEM. n = 7–11. ***P < 0.001, two-way ANOVA, Bonferroni's post hoc test.
Treatment with excitotoxic glutamate also induced PK2 expression, with the greatest potency in the 250–500 nM range (Fig. 1E). NMDA (100 μM) also up-regulated PK2 expression, an effect blocked by the noncompetitive NMDA receptor antagonist MK801 (5 μM) (Fig. S1). Glutamate-induced PK2 expression was dependent on NMDA receptor activation, because treatment with 50 μM dl-2-amino-5-phosphonopentanoic acid, a competitive NMDA receptor antagonist) completely blunted glutamate's actions, whereas treatment with 50 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) or 10–20 μM nifedipine (a calcium-channel blocker) had no effect (Fig. 1F). The extracellular Ca2+ chelator EGTA (2 mM) also completely abolished this effect (Fig. 1F), indicating that extracellular Ca2+ is required for glutamate-induced PK2 expression.
Cellular Expression of PK2 and Its Receptors.
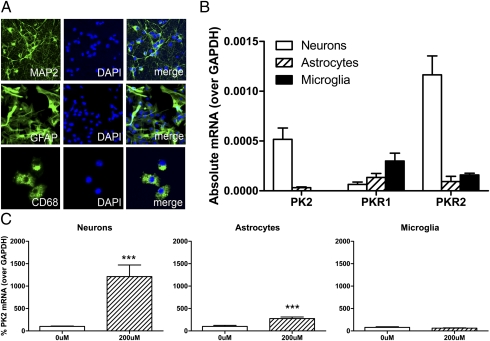
To determine the cell type(s) expressing PK2 and its receptors, we examined their mRNA expression in neuron-enriched, astrocyte-enriched, and microglia-enriched primary cultures using qPCR (Fig. 2 A and B). During normal conditions, PK2 mRNA was detected mainly in neurons (Fig. 2B). Interestingly, PK1 receptor (PKR1) mRNA expression was higher in microglia, whereas PK2 receptor (PKR2) mRNA expression was higher in neurons (Fig. 2B). After treatment with excitotoxic glutamate (200 μM), we found that glutamate-induced PK2 occurred mainly in neurons (Fig. 2C), although a small increase also occurred in astrocytes. PK2 mRNA was not altered by glutamate in microglia (Fig. 2C).
Fig. 2.
Cellular expression of PK2 and its receptors in primary cortical cultures. (A) Representative images of neuron-enriched (MAP2), astrocyte-enriched (GFAP), and microglia-enriched (CD68) cultures. (B) Cellular expression of PK2 and its receptors during normal conditions. PK2 mRNA is expressed mainly in neurons; PKR1 mRNA expression is highest in microglia; and PKR2 mRNA expression is highest in neurons. n = 4–7 per group. (C) Glutamate-induced PK2 is highly expressed in neurons, is induced slightly in astrocytes, and is not altered in microglia. n = 4–6 per group. Error bars represent SEM. ***P < 0.001; Student's t test.
PK2 mRNA Up-Regulation After Transient Intraluminal Middle Cerebral Artery Occlusion.
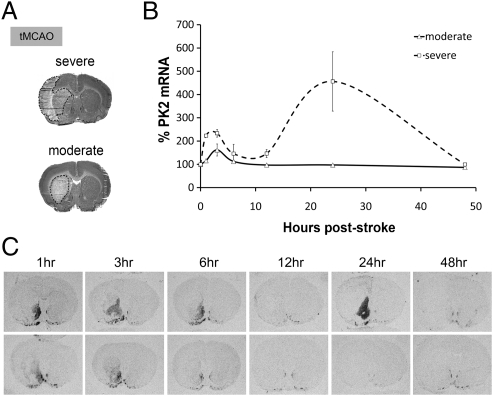
Next we examined whether PK2 mRNA is up-regulated after an in vivo stroke model. Using a model of transient intraluminal middle cerebral artery occlusion (tMCAO) (Fig. 3A), we examined PK2 mRNA at various time points after reperfusion. In situ hybridization showed that PK2 mRNA is induced specifically in the ischemic striatum as early as 1 h after reperfusion (Fig. 3 B and C). Time-course analysis indicated a biphasic pattern of PK2 mRNA in severely infarcted brains, with a small peak at 3 h followed by a larger peak at 24 h after reperfusion, whereas stroke brains subjected to moderate stroke exhibit only the first peak at 3 h (Fig. 3 B and C). We also used qPCR to verify PK2 expression after MCAO. In both experimental models of stroke, qPCR showed that PK2 mRNA is induced significantly in the ischemic striatum (Fig. S2B), ischemic core, and infarct regions (Fig. S2C). PK2 expression is dependent on the severity of stroke damage, because prolonged MCAO (2-h vs. 1-h tMCAO) causes a significantly higher expression of PK2 (Fig. S3). Sense control for PK2 showed undetectable levels, indicating that stroke-induced PK2 expression is specific (Fig. S4). Interestingly, PKR2 mRNA also is up-regulated in the ischemic cortex after MCAO (Fig. S5).
Fig. 3.
Time course of in situ of PK2 hybridization mRNA in a model of an in vivo stroke. (A) Cresyl-violet staining of brains subjected to moderate (striatum infarct only) and severe (striatum and cortex) stroke in the tMCAO model. (B) In situ hybridization showed that PK2 expression is induced biphasically in the brains subjected to severe stroke: A small peak at 3 h is followed by a larger peak at 24 h. (C) Representative autoradiographic images of PK2 expression 1, 3, 6, 12, 24, and 48 h after tMCAO. Error bars represent SEM. n = 5–6 per time point.
Enhancing PK2 Levels Worsens Infarct Volume.
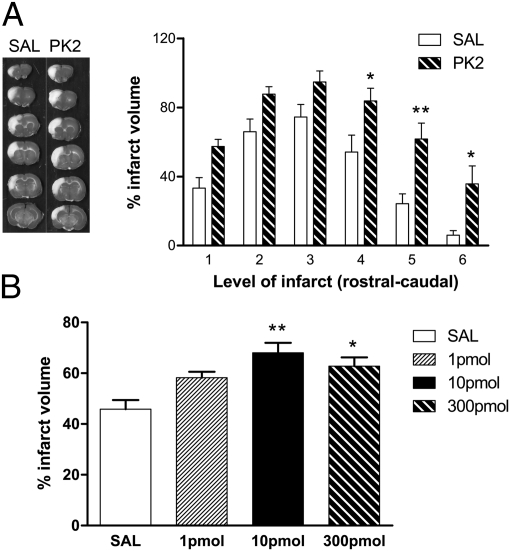
The induction of PK2 expression after stroke suggests a potential role of PK2 as a mediator of ischemic brain injury. To investigate whether the stroke-induced PK2 is beneficial or deleterious, we manipulated PK2 levels poststroke and examined its effects on infarct volume. To increase PK2 levels, we administered recombinant PK2 intracerebroventricularly (ICV) after stroke. This administration resulted in a worsened infarct volume (Fig. 4 A and B); 10 pmol PK2 caused the most dramatic effect on infarct volume, whereas no significant effect was observed with 1 pmol PK2 (Fig. 4B). Please note that the endotoxin level for all of the PK2 and control samples was below 0.25 endotoxin units (EU)/kg. The Food and Drug Administration/United States Pharmacopeial Convention standard for endotoxin limit is 5 EU⋅kg−1⋅ h−1 for parental drugs and 0.25 EU⋅kg−1⋅ h−1 for intrathecal injections (34). Levels below this limit are considered nonpyrogenic. Thus, the effects we observed in our in vivo studies of PK2 in stroke were not caused by endotoxin contamination.
Fig. 4.
ICV PK2 worsened ischemic injury. (A) (Left) Representative images of TTC-stained brain treated with ICV saline (SAL) or PK2. White area indicates infarct tissue. (Right) Quantitation of infarct volume in brains treated with 300 pmol saline or PK2 (from rostral to caudal). ICV PK2 increased infarct volume significantly. n = 6 per group. *P < 0.05, **P < 0.01. Two-way ANOVA with Bonferroni's post hoc test indicates a significant difference between levels 4, 5, and 6. (B) Quantitation of infarct volume in brains treated with 1, 10, or 300 pmol saline (open bar) or PK2 (black and hatched bars). Treatment with 10 pmol PK2 had the most severe effect on infarct volume. Error bars represent SEM. n = 6–7 per group. *P < 0.05, **P < 0.01, one-way ANOVA with Bonferroni's post hoc test.
Blocking PK2 Actions Reduces Infarct Volume and Central Inflammation.
To block PK2 actions, we used a small-molecule PK2 receptor antagonist (PKR-A) that blocks both PKR1 and PKR2. PKR-A is a morpholine carboxamide synthesized in the laboratory of Q.-Y. Z. (Fig. 5A) (35). PKR-A has an IC50 of 48.1 ± 4.6 nM for PKR2 (Fig. 5B), measured by an aequorin-based Ca2+ luminescent assay in CHO cells stably expressing the photoprotein aequorin and PKR2, as described previously (36). I.p. delivery of PKR-A (75 mg/kg) reduced infarct volume when given 30 min poststroke (Fig. 5C), compared with the polyethylene glycol (PEG)-treated control group. Treatment with PKR-A poststroke also decreased the number of CD68+ cells in the infarct (Fig. S6A). Similarly, a decrease in neutrophil infiltration in the infarct was observed in antagonist-treated animals, as indicated by myeloperoxidase immunostaining (Fig. S6B). In contrast, ICV PK2 significantly increased CD68+ cells in the ischemic striatum (Fig. S6C).
Fig. 5.
Blocking PK2 actions reduced infarct volume after stroke. (A) Chemical structure of PKR-A. (B) The PKR-A IC50 for PKR2 is 48.1 ± 4.6 nM, measured by an aequorin-based Ca2+ luminescent assay in CHO cells stably expressing the photoprotein aequorin and PKR2. RLU, relative luminescence unit. (C) I.p. delivery of PKR-A (75 mg/kg) significantly reduced infarct volume when given 30 min poststroke. Error bars represent SEM. n = 6–7 per group. *P < 0.05, Student's t test. (D) Central delivery of lentiviral-mediated RNAi against PK2 reduced ischemic injury. Diagram displays sequence of shRNAs against PK2 and Scrambled negative control (Scr). (E) Screening of shRNA candidates in HEK cells indicated that shRNA candidates OB1 and OB2 are the most effective in knockdown of PK2 expression. (F) Glutamate (250 uM) induced PK2 expression in primary striatal cultures. n = 5. **P < 0.01, Student's t test. (G) Lentiviral-mediated OB2 shRNA inhibited glutamate-induced PK2 expression as compared with Scrambled negative control. n = 4–5. *P < 0.05, Student's t test. (H) Confocal images of GFP, OB2, and Scrambled expression in the striatum. Lentivirus was delivered into striatum stereotaxically 1 wk before dMCAO. (I) Lentiviral-mediated OB2 shRNA reduced infarct volume. Error bars represent SEM. n = 11–13 per group. *P < 0.05, Student's t test. (Scale bar, 0.1 mm.)
Although i.p. delivery of PK2R-A reduced ischemic injury, it remains unclear whether this protection was secondary to other peripheral effects of the antagonist. To address this question, we designed shRNAs OB1 and OB2 and cloned them into a lentiviral vector to knockdown stroke-induced PK2 expression directly in the striatum and examined the effects of this knockdown on infarct volume (Fig. 5D). Initial screening of shRNA candidates in HEK 293 cells indicated that OB1 and OB2 shRNAs were most effective in PK2 knockdown (Fig. 5E). Using primary striatal cultures, we showed that excitotoxic glutamate (250 μM) induced PK2 mRNA (Fig. 5F), and OB2 shRNA inhibited glutamate-induced PK2 expression by ∼60% (Fig. 5G). We next tested the effects of lentivirus-OB2 shRNA in vivo. We delivered lentivirus-OB2 shRNA into the striatum and confirmed its transduction by GFP fluorescence (Fig. 5H). Using an in vivo model of stroke [permanent distal MCA occlusion (dMCAO) with 30-min bilateral common carotid artery occlusion that produces infarct only in the cortex], we showed that lentiviral-mediated knockdown of PK2 results in reduced infarct volume (Fig. 5I).
Blocking PK2 Action Improves Behavioral Functional Outcome.
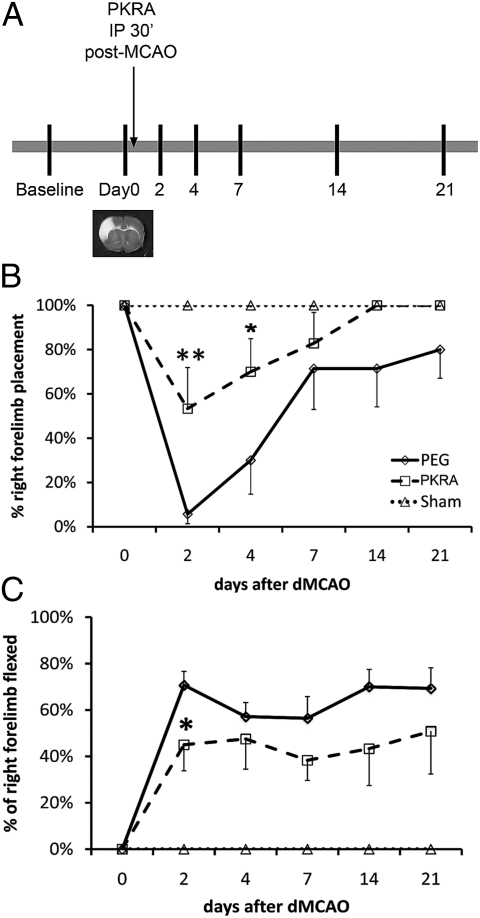
Although PKR-A reduced infarct volume and central inflammation, it is important to examine if this protection translates into an improvement in functional outcome. A single dose of PKR-A (75 mg/kg) was given i.p. 30 min poststroke, and behavioral tests were performed at days 2, 4, 7, 14, and 21 d after reperfusion (Fig. 6A). PKR-A treatment significantly improved behavioral outcome, especially at day 2 and day 4 poststroke, as shown by the vibrissa-elicited forelimb placing test (whisker test) (Fig. 6B). PKR-A treatment accelerated recovery; PKR-A–treated animals started to recover at day 4, and all PKR-A–treated animals reached 100% recovery by day 14 (Fig. 6B). In contrast, the control PEG-treated animals did not start to recover until day 7 and did not recover completely even by day 21. The forelimb flexion test also showed a significant improvement at day 2 in the PKR-A group (Fig. 6C).
Fig. 6.
Blocking PK2 actions improved functional behavioral outcome after stroke. (A) Diagram of the experimental design for behavior studies. A single dose (75 mg/kg) of PK2 receptor antagonist was given 30 min after stroke, and neurological tests were performed on days 2, 4, 7, 14, and 21 d after reperfusion. Baseline data were collected 7 d before stroke. PKR-A significantly improved neurological outcome in the whisker test (B) and in the forelimb flexion test (C). Error bars represent SEM. n = 10–12. *P < 0.05, **P < 0.01, two-way ANOVA with Bonferroni's post hoc test.
Discussion
Stroke is an acute neurological insult that disrupts brain function and causes neuron death. Understanding the molecular changes that occur during stroke will help identify potential therapeutic targets. This study provides evidence that PK2 mediates cerebral ischemic injury. We showed that PK2 mRNA can be up-regulated in primary cortical cultures by various pathological insults, including hypoxia, reactive oxygen species, and excitotoxic glutamate (Fig. 1 A, B, and E). Glutamate-induced PK2 expression is NMDA receptor-dependent (Fig. 1F) and occurs mainly in neurons (Fig. 2 B and C). Using two in vivo models of stroke, we showed that PK2 expression is induced in the ischemic striatum and cortex (Fig. 4 B and C), suggesting that stroke-induced PK2 expression is a general response to ischemic injury. Interestingly, PK2 expression is induced in a biphasic manner in brains that experienced severe stroke (Fig. 3), and the level of PK2 expression increases with severity of damage (Fig. S3). To determine the role of stroke-induced PK2, we examined the effects of enhancing or blocking PK2 poststroke. Exogenous delivery of PK2 poststroke worsened infarct volume (Fig. 4), whereas blocking PK2 with a PKR-A reduced infarct volume (Fig. 5C), decreased central inflammation (Fig. S6 A and B), and improved behavioral outcome (Fig. 6). Central inhibition of PK2 using RNAi also reduced ischemic injury (Fig. 5I). Together, these data suggest that injury-induced PK2 expression is deleterious and that blocking PK2 after stroke may be therapeutic.
Our data show that PK2 expression can be induced by a number of pathological insults, including hypoxia, reactive oxygen species, and excitotoxic glutamate (Fig. 1 A, B, and E). These results suggest that the stroke-induced biphasic expression of PK2 may be driven by a number of factors released during stroke (Fig. 2 B and C). Because glutamate can up-regulate PK2 expression potently (Fig. 1E), this initial expression of PK2 at 1–3 h may be driven predominantly by the rapid release of excitotoxic glutamate upon injury, whereas the larger peak of PK2 expression at 24 h after injury may be driven by other factors in addition to glutamate. A likely candidate is transcription factor hypoxia-inducible factor 1-α, which peaks around 12–24 h after hypoxia-ischemia (37) and in which the PK2 promoter contains an HRE (17). In addition to an HRE, a bioinformatic search using Biobase Match algorithm of the human and rat PK2 promoter predicted additional transcription factor binding sites relevant to stroke (Table S1). Thus, multiple transcription factors may contribute to the biphasic expression of PK2 after stroke. However, future studies are needed to elucidate further the molecular mechanisms by which stroke up-regulates PK2 expression.
PKs exert their actions by binding to two G protein-coupled receptors, PKR1 and PKR2 (36). Under normal conditions, activation of PK2 receptors has been shown to stimulate intracellular calcium and activate the Erk1,2/MAPK and Akt pathways (38–40). However, it is unclear whether these pathways also are activated by PK2 during insult. Although Erk1,2 and Akt signaling have been associated mostly with beneficial effects such as promoting cell survival and proliferation (41–43), they also can exert deleterious effects (44–46). For example, Erk1,2 generated from cytokines and free radicals can increase ischemic damage (44). Similarly, Akt phosphorylation in astrocytes can cause vascular leakage in ischemic brain (45), and Akt1 has been demonstrated to mediate leukocyte migration and acute inflammation (46). Interestingly, our data suggest that PK2 is a deleterious mediator during pathological insult such as ischemic stroke. To address whether PK2 activates differential Erk1,2 and Akt signaling during insult, we used primary neuron cultures that overexpressed PKR1 or PKR2 and examined the effects of PK2 on the levels of pErk1,2 and pAkt after excitotoxicity or oxygen glucose deprivation (OGD) (Fig. S7). In addition, we also examined levels of pSAP/JNK, a stress-activated MAPK that has been associated with inflammation and cell death (47). In normal neuron cultures, PK2 treatment increased pErk1,2 levels, but pAkt and pSAP/JNK levels did not change. Interestingly, after excitotoxicity or OGD, PK2 treatment increased both pErk1,2 and pSAP/JNK MAPK levels, but no changes were detected in pAkt levels. Because of the dual role of Erk1,2 and the endangering role of SAP/JNK (44, 47), these results suggest that PK2 activates differential signaling during insult conditions, and PK2's effects on pErk1,2 and pSAP/JNK may contribute in part to its deleterious effects in ischemic injury. Further studies are required to understand fully the mechanism by which PK2 exacerbates ischemic injury.
The biphasic stroke-induced expression of PK2 suggests that PK2 may participate in multiple cellular events that lead to neuron death. The smaller peak of PK2 may contribute to potentiating glutamate excitotoxicity, because PK2 is neuro-excitatory (21–23). The larger PK2 expression at 24 h may have an additional role; this time point also marks the period when up-regulation of a number of proinflammatory genes and infiltration of various inflammatory cells, such as monocytes and macrophages, occurs poststroke (3, 48). Inflammation is a key mechanism that occurs during ischemic injury, leading to edema, cellular necrosis, and tissue infarction (3, 48, 49). Because PK2 has been shown to induce cytokine expression and attract inflammatory monocytes and macrophages in mouse peritoneal macrophages (25), the second, larger phase of PK2 expression may promote the production of proinflammatory cytokines and augment migration of inflammatory cells. In agreement with this notion, ICV delivery of PK2 1 h poststroke increased CD68+ inflammatory cells in the ischemic infarct (Fig. S6C) and increased infarct volume (Fig. 4B); blocking the PK2 receptor had the opposite effects, lessening such inflammation (Fig. S6 A and B). However, because greater injury causes more inflammation, and vice versa, future studies are needed to understand fully whether the increased inflammation by PK2 is a direct contributor to the ischemic injury, is a consequence of such injury, or is independent of the increased infarct volume.
Since their discovery in 2001, prokineticins have been associated with central and peripheral functions (13, 30, 50). We now report that PK2 is an insult-inducible endangering mediator for cerebral ischemia. A recent transcriptome study by Choke et al. (51) also showed that PK2 expression is up-regulated after abdominal aortic ruptures in humans, further supporting a role of PK2 in pathological states. Our study identifies PK2 as an endangering mediator for ischemic brain injury and a compelling target for stroke treatment. Because PK2 can be activated by a number of pathological insults, these results suggest that PK2 may be a mediator in other neurological/neurodegenerative diseases.
Methods
All experiments were conducted in compliance with animal care laws and institutional guidelines and were approved by the Stanford Institutional Animal Care and Use Committee. In vivo models of stroke were used as described in SI Methods. In situ hybridization and qPCR were used to assess PK2 mRNA expression. Infarct volume was assessed by triphenyl tetrazolium chloride (TTC) staining as described in SI Methods. Details of materials and methods used are given in SI Methods.
Supplementary Material
Acknowledgments
We thank Rong Xie for technical help and Cindy Samos for editorial assistance. This work was supported by National Institutes of Health Grants 5R01 AG020633 (to R.M.S.) and 2P01 NS37520 (to G.K.S.) and by generous funds from John A. and Cynthia Fry Gunn Research Fund in Neuroscience (to R.M.S.). This work also was supported in part by American Heart Association Grants 855156F (to Q.-Y.Z.) and 10GRNT4200024 (to H.Z.). M.Y.C. is supported by National Institutes of Health National Research Service Award 5F32NS060480 Postdoctoral Fellowship.
Footnotes
Conflict of interest statement: A patent application has been filed with Q.-Y.Z., A.G.L., M.Y.C., and R.M.S. as inventors. The patent application was based in part on findings described in this paper.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113363109/-/DCSupplemental.
References
- 1.Lloyd-Jones D, et al. WRITING GROUP MEMBERS American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky RM. Neuroprotective gene therapy against acute neurological insults. Nat Rev Neurosci. 2003;4:61–69. doi: 10.1038/nrn1006. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael ST. Gene expression changes after focal stroke, traumatic brain and spinal cord injuries. Curr Opin Neurol. 2003;16:699–704. doi: 10.1097/01.wco.0000102621.38669.77. [DOI] [PubMed] [Google Scholar]
- 5.Lu XC, et al. Microarray analysis of acute and delayed gene expression profile in rats after focal ischemic brain injury and reperfusion. J Neurosci Res. 2004;77:843–857. doi: 10.1002/jnr.20218. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael ST, et al. Growth-associated gene expression after stroke: Evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C, Ross ME. Molecular pathology of cerebral ischemia: Delayed gene expression and strategies for neuroprotection. Ann N Y Acad Sci. 1997;835:203–217. doi: 10.1111/j.1749-6632.1997.tb48631.x. [DOI] [PubMed] [Google Scholar]
- 8.Kinouchi H, Huang H, Arai S, Mizoi K, Yoshimoto T. Induction of cyclooxygenase-2 messenger RNA after transient and permanent middle cerebral artery occlusion in rats: Comparison with c-fos messenger RNA by using in situ hybridization. J Neurosurg. 1999;91:1005–1012. doi: 10.3171/jns.1999.91.6.1005. [DOI] [PubMed] [Google Scholar]
- 9.Sairanen T, et al. Cyclooxygenase-2 is induced globally in infarcted human brain. Ann Neurol. 1998;43:738–747. doi: 10.1002/ana.410430608. [DOI] [PubMed] [Google Scholar]
- 10.Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: A metaanalysis. Stroke. 2009;40:2438–2441. doi: 10.1161/STROKEAHA.109.552547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lees KR, et al. ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: Recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 13.Kaser A, Winklmayr M, Lepperdinger G, Kreil G. The AVIT protein family. Secreted cysteine-rich vertebrate proteins with diverse functions. EMBO Rep. 2003;4:469–473. doi: 10.1038/sj.embor.embor830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng MY, et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 15.Li JD, et al. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maywood ES, et al. Genetic and molecular analysis of the central and peripheral circadian clockwork of mice. Cold Spring Harb Symp Quant Biol. 2007;72:85–94. doi: 10.1101/sqb.2007.72.005. [DOI] [PubMed] [Google Scholar]
- 17.LeCouter J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 18.LeCouter J, et al. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci USA. 2003;100:2685–2690. doi: 10.1073/pnas.0337667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka N, Ikawa M, Mata NL, Verma IM. Choroidal neovascularization in transgenic mice expressing prokineticin 1: An animal model for age-related macular degeneration. Mol Ther. 2006;13:609–616. doi: 10.1016/j.ymthe.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Ng KL, et al. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 21.Cottrell GT, Zhou QY, Ferguson AV. Prokineticin 2 modulates the excitability of subfornical organ neurons. J Neurosci. 2004;24:2375–2379. doi: 10.1523/JNEUROSCI.5187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingves MV, Ferguson AV. Prokineticin 2 modulates the excitability of area postrema neurons in vitro in the rat. Am J Physiol Regul Integr Comp Physiol. 2010;298:R617–R626. doi: 10.1152/ajpregu.00620.2009. [DOI] [PubMed] [Google Scholar]
- 23.Yuill EA, Hoyda TD, Ferri CC, Zhou QY, Ferguson AV. Prokineticin 2 depolarizes paraventricular nucleus magnocellular and parvocellular neurons. Eur J Neurosci. 2007;25:425–434. doi: 10.1111/j.1460-9568.2006.05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong YC, et al. Prokineticin 2 suppresses GABA-activated current in rat primary sensory neurons. Neuropharmacology. 2010;59:589–594. doi: 10.1016/j.neuropharm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Martucci C, et al. Bv8, the amphibian homologue of the mammalian prokineticins, induces a proinflammatory phenotype of mouse macrophages. Br J Pharmacol. 2006;147:225–234. doi: 10.1038/sj.bjp.0706467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mollay C, et al. Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur J Pharmacol. 1999;374:189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 27.Negri L, et al. Nociceptive sensitization by the secretory protein Bv8. Br J Pharmacol. 2002;137:1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giannini E, et al. The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain. Proc Natl Acad Sci USA. 2009;106:14646–14651. doi: 10.1073/pnas.0903720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitteloud N, et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2007;104:17447–17452. doi: 10.1073/pnas.0707173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maldonado-Pérez D, Evans J, Denison F, Millar RP, Jabbour HN. Potential roles of the prokineticins in reproduction. Trends Endocrinol Metab. 2007;18:66–72. doi: 10.1016/j.tem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto S, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melchiorri D, et al. The mammalian homologue of the novel peptide Bv8 is expressed in the central nervous system and supports neuronal survival by activating the MAP kinase/PI-3-kinase pathways. Eur J Neurosci. 2001;13:1694–1702. doi: 10.1046/j.1460-9568.2001.01549.x. [DOI] [PubMed] [Google Scholar]
- 33.Urayama K, et al. Prokineticin receptor-1 induces neovascularization and epicardial-derived progenitor cell differentiation. Arterioscler Thromb Vasc Biol. 2008;28:841–849. doi: 10.1161/ATVBAHA.108.162404. [DOI] [PubMed] [Google Scholar]
- 34.Malyala P, Singh M. Endotoxin limits in formulations for preclinical research. J Pharm Sci. 2008;97:2041–2044. doi: 10.1002/jps.21152. [DOI] [PubMed] [Google Scholar]
- 35.Balboni G, et al. Triazine compounds as antagonists at Bv8-prokineticin receptors. J Med Chem. 2008;51:7635–7639. doi: 10.1021/jm800854e. [DOI] [PubMed] [Google Scholar]
- 36.Bullock CM, Li JD, Zhou QY. Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol. 2004;65:582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, et al. Temporal and spatial differences of multiple protein expression in the ischemic penumbra after transient MCAO in rats. Brain Res. 2010;1343:143–152. doi: 10.1016/j.brainres.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 38.Lin DC, et al. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 39.Masuda Y, et al. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- 40.Soga T, et al. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- 43.Li B, Xu W, Luo C, Gozal D, Liu R. VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res Mol Brain Res. 2003;111:155–164. doi: 10.1016/s0169-328x(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 44.Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–1669. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- 45.An J, et al. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain. Blood. 2008;112:2787–2794. doi: 10.1182/blood-2008-02-141630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Lorenzo A, Fernández-Hernando C, Cirino G, Sessa WC. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc Natl Acad Sci USA. 2009;106:14552–14557. doi: 10.1073/pnas.0904073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Gelderblom M, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 49.Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs. 2003;4:522–529. [PubMed] [Google Scholar]
- 50.Zhou QY, Cheng MY. Prokineticin 2 and circadian clock output. FEBS J. 2005;272:5703–5709. doi: 10.1111/j.1742-4658.2005.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choke E, et al. Whole genome-expression profiling reveals a role for immune and inflammatory response in abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2009;37:305–310. doi: 10.1016/j.ejvs.2008.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.