Abstract
Large cell neuroendocrine carcinoma (LCNEC) of the uterine cervix is a rare and aggressive malignancy with poor prognosis even in its early stage, despite multimodality treatment strategy. Here, we report a case of a woman with clinical polypoid stage IB LCNEC of the cervix, which was detected in her 6-week postpartum checkup. A literature review was also conducted to evaluate current therapeutic approaches and potential new strategies.
Key words: large cell neuroendocrine carcinoma, uterine cervix.
Introduction
Neuroendocrine tumors of the uterine cervix are a group of uncommon neoplasms characterized as highly aggressive and prone to early metastasis. They are classified as typical carcinoid, atypical carcinoid, small cell carcinoma (SCC) and large cell neuroendocrine carcinoma (LCNEC) based on mitotic activity, nuclear atypia and geographic type of necrosis.1 In contrast to SCC whose aggressive behavior and resistance to therapy have been well-established, cervical LCNEC was often under-recognized and misdiagnosed as poorly differentiated adeno- or squamous cell carcinoma until 1997 when Gilks et al.2 reported 12 cervical LCNEC cases. Since then, nearly 70 cases have been reported from either a clinical or pathological standpoint. However, prognosis for this population remains bleak despite multimodal treatments, and the majority of patients die within 2 to 3 years of diagnosis (Table 1). Here, we report a case of early stage LCNEC treated with surgery followed by adjuvant therapy with cisplatin and etoposide and review the literature on the effectiveness of current treatment strategies and examine new approaches that are being developed to treat LCNEC.
Table 1. A summary of 78 cases reported of large cell neuroendocrine cervical carcinoma (current case included).
| First author (No. of cases) |
Age | Stage | Treatment (No. of cases) |
Outcome (No. of cases) |
|---|---|---|---|---|
| Gilks (12) | 36–38 | IA2 | RH(1) | 36+mo (1) |
| RH with Chemo (1) | NA(1) | |||
| 21–36 | IB | RH (1) | 24 mo(1) | |
| RH+ Chemo (6) | 8–12mo(3), 6+−36+mo (3) | |||
| RH + Chemo +RT(2) | 18–24 mo(2) | |||
| 62 | IIA | RH | 6mo | |
| Tsou (1) | 35 | IIB | Chemo+RT | 18mo (1) |
| Yun (1) | 31 | IA1 | RH | 10+mo (1) |
| Krivak (2) | 25 | IB | RH; chemo for met | 35mo (1) |
| 36 | IIA | RH+ Chemo | 33mo (1) | |
| Cui (1) | 35 | NA | Neoadjuvant Chemo+ RH | NA |
| Rhemtula (5) | 55 | IIB | None (1) | 1+mo (1) |
| (South Africa) | 75 | IIIB | RT (1) | 3mo (1) |
| 51–65 | IVB | None (1) | 0.25mo (1) | |
| RT (1) | 1mo (1) | |||
| 42 | NA | None (1) | NA (1) | |
| Grayson (12) | 42–72 | NA | NA | NA |
| Wen (1) | 57 | IIB | TAHBSO+ RT | 41mo (1) |
| Dikmen (1) | 45 | IIB | TAHBSO+ RT+ Chemo | NA |
| Sato (6) | 27–51 | IB | TAHBSO+RT+ Chemo(5) | 12+−151+mo (2) |
| 16–19mo(3) | ||||
| 42 | IIA | TAHBSO+RT+ Chemo(1) | 6mo (1) | |
| Kumar (1) | 39 | IV | NA | NA |
| Baykal (1) | 38 | IB | TAHBSO+ Chemo+ RCT | 21+mo (1) |
| Tangjitgamol (6) | NA | I | NA (5) | NA (6) |
| II | NA (1) | |||
| Kawauchi (1) | 40 | IB | TAHBSO | 9mo+ (1) |
| Cetiner (1) | 47 | IIB | TAHBSO+ RT | 6mo+ (1) |
| Wang (4) | 42+/− | IA2 | RT+ Chemo (1) | NA |
| 11.3 | IB1 | RT+ Chemo (3) | ||
| Ko (1) | 45 | IB | RH+RT+ Chemo | 24mo+ (1) |
| Tangjitgamol (1) | 42 | III | Chemo | 44mo (1) |
| McCluggage (3) | 72 | I | RT+ Chemo | NA (1) |
| 32 | IIB | TAHBSO+RT+ Chemo | 17mo (1) | |
| 48 | IVB | RT+ Chemo | NA (1) | |
| Saavedra (2) | 25–42 | IB | RT+ Chemo (2) | 36+−60+mo (2) |
| Powell (1) | 31 | IIIB | TAHBSO+RT+ Chemo | NA |
| Kajiwara (2) | 55 | IIA | NA | 12mo (1) |
| 37 | IIIB | NA | 21mo (1) | |
| Li (1) | 30 | IIB | RT+ Chemo | 23mo+ (1) |
| Wang (7) | 37 | IA2 | RH+ Chemo (1) | 17.2mo (1) |
| 28–48 | IB1 | RH (1) | 114.3+mo (1) | |
| RH+ Chemo (2) | 3–17.2mo (2) | |||
| RH+ Chemo+ RT(1) | 39mo (1) | |||
| 41–62 | IB2 | RH (1) | 7mo (1) | |
| BSH+ Chemo+ RT(1) | 11.8mo (1) | |||
| Markopoulos (1) | 60 | NA | RH+ Chemo+ RT | 18mo (1) |
| Brown (1) | 40 | IVB | Chemo | NA |
| Embry (1) | 24 | IB2 | RH+ Chemo+ RT | 47mo+ |
| Yoseph (1) (current study) |
33 | IB | TAHBSO+Chemo | 24 months |
RT, radiotherapy; RCH, radio-chemotherapy; Chemo, chemotherapy; RH, radical hysterectomy; BSO, bilateral salpingo-oophorectomy; TAHBSO, Total hysterectomy with bilateral salpingo-oophorectomy; NA, not available; in outcome, + indicates being alive/censored, otherwise died.
Case Report
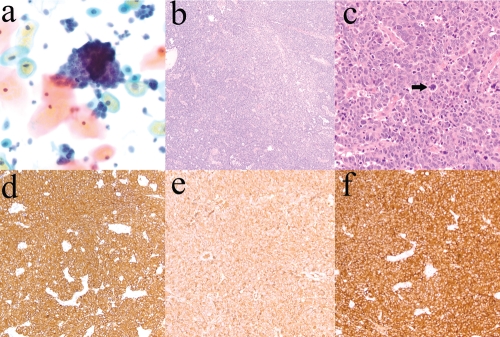
A 33-year-old Caucasian female (G4 P3-0-1-3) arrived for her 6-week postpartum checkup, and a speculum examination revealed a 1.2 cm cervical polyp. A liquid-based Pap specimen showed abundant clusters of overlapping tumor cells with scant cytoplasm, finely granular chromatin and conspicuous mitotic activity (Figure 1a). Upon reevaluation 4 weeks later, the polyp was still present, and the patient underwent polypectomy. An approximately 2×2 cm polyp was submitted for histopathological analysis.
Figure 1.
Large cell neuroendocrine carcinoma of the cervix. a) Liquid-based Pap specimen showing clusters of tumor cells. Notice a mitotic figure in the bottom half of the cluster (Pap stain, original magnification ×400). b) Tumor with a solid and trabecular architecture (hematoxylin and eosin, original magnification ×100). c) Large tumor cells with a high nuclear-to-cytoplasmic ratio, small nucleoli, finely granular chromatin and numerous mitotic figures, including atypical mitoses (arrow) (hematoxylin and eosin, original magnification ×400). d) Tumor cells with diffuse and strong positive immunoreactivity for cytokeratin AE1/AE3 (immunostain for cytokeratin AE1/AE3, original magnification ×100). e) Positive immunostaining of the tumor for chromogranin (immunostain for chromogranin, original magnification ×200). f) Positive immunostaining of the tumor for synaptophysin (immunostain for synaptophysin, original magnification ×200).
The specimen consisted entirely of tumor, a poorly differentiated carcinoma with a solid and trabecular architecture (Figure 1b). The tumor cells were large, with a high nuclear-to-cytoplasmic ratio, small nucleoli, finely granular chromatin and numerous mitotic figures, including atypical mitoses (Figure 1c). On immunohistochemical staining, the tumor showed diffuse positive immunoreactivity for cytokeratin AE1/AE3 (Figure 1d) and neuroendocrine markers, including chromogranin (Figure 1e) and synaptophysin (Figure 1f) and CD56. Immunostain for S-100, another neuroendocrine marker, was weakly positive. In addition, the tumor cells were focally weakly positive for P63, a marker of cervical basal cells, which is diffusely positive in squamous cell carcinoma of the cervix but is negative or only focally positive in cervical neuroendocrine carcinomas.3 These morphologic and immunohistochemical findings are consistent with LCNEC. The tumor had KRAS mutation (Gly12Asp), but no EGFR or BRAF mutations. The tumor also had low levels of EGFR expression, and high levels of thymidylate synthase, ERCC1, and RRM1 expression.
Radical hysterectomy with bilateral salpingo-oophorectomy and bilateral pelvic and periaortic lymph node dissection was performed, followed by baseline PET/CT and brain MRI scans, showing no evidence of regional or distant metastasis. The clinical picture was consistent with stage IB disease, as defined by the Federation of Gynaecologists and Obstetricians (FIGO) staging system for carcinoma of the uterine cervix.
Due to the aggressive morphology, adjuvant therapy with intravenous cisplatin and etoposide was started. A total of five 21-day cycles were given, with minimal toxicity consisting of grade 1 peripheral neuropathy, which resolved 3 months after the last cycle of treatment. At the 24-month follow-up, there was no evidence of disease recurrence.
Discussion
Knowledge on LCNEC of the uterine cervix has gradually accumulated over the past two decades, since a clear classification of cervical neuroendocrine-differentiated tumors was established in 1997. More than 70 LCNEC cases have been reported since then (Table 1). Neuroendocrine carcinomas comprise less than 5% of all cervical cancers, with SCCs being the most common and LCNECs being rare.4 Patients with LCNEC often present in the early stages with vaginal bleeding or are diagnosed during regular vaginal check-ups. They may also occasionally present with carcinomatous meningitis5 or mimic vaginitis.6 We did not perform molecular studies for human papillomavirus (HPV) on this tumor, but LCNECs of the cervix have been reported to be associated with high-risk HPV types 16 and 18 and, to a lesser extent, types 31 and 33.7
Due to the rarity of cervical LCNEC, no consensus has been reached on an optimal treatment plan, and current multimodal strategies that combine radical hysterectomy (with or without bilateral salpingo-oophorectomy), chemotherapy and radiation are mainly adapted from treatments used for neuroendocrine carcinomas of the lung.8 While the role of surgical intervention remains controversial with no sign of improving long-term survival so far, chemotherapy has become the mainstream of management, especially considering LCNEC's aggressive, early metastatic behavior.9 Among various chemotherapy options, the combination of cisplatin and etoposide is most common and was used for our patient as well. Three other commonly used combinations include vincristine, doxorubicin and cyclophosphomide, carboplatin plus paclitaxel and occasionally epirubicin, topotecan plus thalidomide. A recent systematic review of published cases10 has also indicated that, while the addition of chemotherapy at any point of initial treatment will offer survival benefits, platinum with (P=0.0027) or without etoposide (P=0.0034) in particular is associated with statistically significant improvement in survival compared to chemo-regimens without these agents.
However, regardless of great efforts invested, the majority of LCNEC patients do not survive more than two years after being diagnosed (Table 1). Accordingly, novel treatment strategies have been proposed that require further evaluation. Tangjitgamol et al.11 measured the prevalence of estrogen receptor and/or progesterone receptor among neuroendocrine tumors to evaluate the feasibility of applying hormonal treatment to cervical LCNEC patients, but unfortunately only a very small portion of recruited patients (3 out of 24) expressed these hormonal receptors. Another strategy was proposed by Kajiwara et al.12 using the somatostatin type 2A (SSTR2A) analog, octreotide, to treat neuroendocrine tumors, given that 3 out of 7 cases (2 out of 5 SCC and 1 out of 2 LCNEC) expressed SSTR2A receptors; however, this strategy has not been tested yet in a larger study.
In conclusion, chemotherapy is associated with improved survival and should be considered in resected cases.
References
- 1.Mannion C, Park WS, Man YG, et al. Endocrine tumors of the cervix: Morphologic assessment, expression of human papillomavirus, and evaluation for loss of heterozygosity on 1p,3p, 11q, and 17p. Cancer. 1998;83:1391–400. doi: 10.1002/(sici)1097-0142(19981001)83:7<1391::aid-cncr17>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Gilks CB, Young RH, Gersell DJ, Clement PB. Large cell neuroendocrine [corrected] carcinoma of the uterine cervix: A clinicopathologic study of 12 cases. Am J Surg Pathol. 1997;21:905–14. doi: 10.1097/00000478-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Houghton O, McCluggage WG. The expression and diagnostic utility of p63 in the female genital tract. Adv Anat Pathol. 2009;16:316–21. doi: 10.1097/PAP.0b013e3181b507c6. [DOI] [PubMed] [Google Scholar]
- 4.Rhemtula H, Grayson W, van Iddekinge B, Tiltman A. Large-cell neuroendocrine carcinoma of the uterine cervix--a clinicopathological study of five cases. S Afr Med J. 2001;91:525–8. [PubMed] [Google Scholar]
- 5.Kumar S, Nair S, Alexander M. Carcinomatous meningitis occurring prior to a diagnosis of large cell neuroendocrine carcinoma of the uterine cervix. J Postgrad Med. 2004;50:311–2. [PubMed] [Google Scholar]
- 6.Baykal C, Al A, Tulunay G, et al. High-grade neuroendocrine carcinoma of the cervix. A case report. Gynecol Obstet Invest. 2005;59:207–11. doi: 10.1159/000084259. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda N, Wada Y, Inoue K, et al. Smear cytology findings of large cell neuroendocrine carcinoma of the uterine cervix. Diagn Cytopathol. 2011 Oct 11; doi: 10.1002/dc.21834. doi: : 10.1002/dc.21834. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Li WW, Yau TN, Leung CW, et al. Large-cell neuroendocrine carcinoma of the uterine cervix complicating pregnancy. Hong Kong Med J. 2009;15:69–72. [PubMed] [Google Scholar]
- 9.Krivak TC, McBroom JW, Sundborg MJ, et al. Large cell neuroendocrine cervical carcinoma: A report of two cases and review of the literature. Gynecol Oncol. 2001;82:187–91. doi: 10.1006/gyno.2001.6254. [DOI] [PubMed] [Google Scholar]
- 10.Embry JR, Kelly MG, Post MD, Spillman MA. Large cell neuroendocrine carcinoma of the cervix: Prognostic factors and survival advantage with platinum chemotherapy. Gynecol Oncol. 2011;120:444–8. doi: 10.1016/j.ygyno.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Tangjitgamol S, Ramirez PT, Sun CC, et al. Expression of HER-2/neu, epidermal growth factor receptor, vascular endothelial growth factor, cyclooxygenase-2, estrogen receptor, and progesterone receptor in small cell and large cell neuroendocrine carcinoma of the uterine cervix: A clinicopathologic and prognostic study. Int J Gynecol Cancer. 2005;15:646–56. doi: 10.1111/j.1525-1438.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- 12.Kajiwara H, Hirabayashi K, Miyazawa M, et al. Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine carcinoma of uterine cervix. Arch Gynecol Obstet. 2009;279:521–5. doi: 10.1007/s00404-008-0760-y. [DOI] [PubMed] [Google Scholar]