Abstract
Despite decades of research on the etiology and treatment of depression, a significant proportion of the population is affected by the disorder, fails to respond to treatment and is plagued by relapse. Six prominent scientists, Aaron Beck, Richard Davidson, Fritz Henn, Steven Maier, Helen Mayberg, and Martin Seligman, gathered to discuss the current state of scientific knowledge on depression, and in particular on the basic neurobiological and psychopathological processes at play in the disorder. These general themes were addressed: 1) the relevance of learned helplessness as a basic process involved in the development of depression; 2) the limitations of our current taxonomy of psychological disorders; 3) the need to work towards a psychobiological process-based taxonomy; and 4) the clinical implications of implementing such a process-based taxonomy.
I am now the most miserable man living. If what I feel were equally distributed to the whole human family, there would not be one cheerful face on the earth. Whether I shall ever be better I cannot tell; I awfully forebode I shall not. To remain as I am is impossible; I must die or be better, it appears to me. (Lincoln, 1841/1953, p. 230)
The words that Abraham Lincoln pronounced at the height of his despair convey just how disabling depression is. Today, after decades of research on the etiology and treatment of depression, the disorder continues to affect approximately 17% of the United States population at some point during their lifetimes (Kessler et al., 2005) and remains one of the leading causes of disability in the world (World Health Organization, 2008). While several treatments, including medication and/or psychotherapy, can relieve the symptoms of depression (American Psychiatric Association, 2000a), as many as 20% of individuals afflicted with the disorder do not respond to treatment (Fava, 2003; Keller et al., 1992).
On June 1st 2010, six researchers, all experts on depression, gathered upon the invitation of Dr. Beck and Dr. Seligman to discuss the current state of scientific knowledge on depression. Dr. Beck, the father of Cognitive Therapy, is an emeritus professor of psychiatry and the director of the Psychopathology Research Unit at the University of Pennsylvania. Dr. Davidson, one of the world’s leading authorities in the neuroscience of emotion regulation, is the William James and Vilas professor of psychology and psychiatry and the director of the Laboratory for Affective Neuroscience at the University of Wisconsin-Madison. Dr. Henn, a leading expert on depression and neurodegenerative diseases, was the Associate Director of Life Sciences at Brookhaven National Laboratory in Brookhaven, New York, and is now a professor at Cold Spring Harbor Laboratory. Dr. Maier, one of the leading authorities on stress in general and the learned helplessness model of depression in particular, is a University of Colorado Distinguished Professor and the director of the Center for Neuroscience at the University of Colorado Boulder. Dr. Mayberg, one of the world’s most renowned experts on the neurocircuitry of depression, is a professor of neurology and psychiatry at Emory University. Finally, Dr. Seligman, the father of Positive Psychology and an expert on human helplessness and optimism, is the Zellerbach Family Professor of Psychology and the director of the Positive Psychology Center at the University of Pennsylvania.
Together, the six researchers reviewed many of the numerous scientific advances that have informed our current understanding of depression. They also delineated current problems in the field and offered suggestions for future research. Their conversation started with a discussion of the learned helplessness model of depression, and progressed towards a dialogue on the necessity of improving our current taxonomy of psychological disorders (including depression), as well as the importance of exploring a new taxonomy based on biologically informed psychological processes.
What is the Relevance of the Learned Helplessness Model to Depression?
The learned helplessness model has been considered to be one of the most useful animal models of depression. The phenomenon of learned helplessness has appeared to be one of the basic processes involved in the development of depression. In the first phase of the conversation, the six researchers attempted to answer the following questions: How is learned helplessness displayed in animals? Are some organisms more susceptible to developing learned helplessness than others? Is learned helplessness specific to depression or is it also at work in other disorders? What is the neurocircuitry underlying the development of learned helplessness? How does this neurocircuitry refine our understanding of the disorder and its treatment? Finally, how relevant is the learned helplessness model for today’s depression researchers? Dr. Maier, a leading authority on animal models of learned helplessness, opened the conversation by defining the contributions made by animal models (and in particular, the learned helplessness model) to clinical research on depression.
Steven Maier
To understand what the learned helplessness model is (as well as how it can be improved upon), it is first necessary to consider what a model (animal or human) is, and what it is that is being modeled. Currently, the disorder is “depression,” and the presence of depression is defined by a cluster of self-reported symptoms and/or clinical observations. An obvious question is whether this defines a unique “entity,” because if not, there would be no reason to expect to be able to find a set of biological changes that uniquely “underlies” this condition, or a delimited set of circumstances that produces it. For example, if individuals presenting with stomach pain were diagnosed as having “stomach pain disorder,” it would be futile to search for a coherent biological cause. While it is true that “pain regions” of the brain might be activated, the pain could be produced by cancer, ulcers, pancreatitis, obstructed bowels, etc., and these all have different biological causes and would be studied by totally different animal models. Furthermore, pain systems are not even fundamentally altered as in true pain disorders (e.g., disorders that involve pathology in spinal cord dorsal horn neurons in the pain pathway), but rather the pain is secondary to the true pathology (e.g., cancer). Pain is the self-reported experience that leads a diagnostician to search for the disorder, but it may not be the disorder and may not represent the biological system that is dysregulated or malfunctioning. It is true that a treatment, say morphine, might be able to suppress the pain experienced from many of these conditions, but this would be palliative - the underlying disorders would remain and pain would be again experienced as soon as the treatment is terminated. If the diagnostic category of depression, as currently defined by the DSM-IV-TR (American Psychiatric Association, 2000b), is more like “stomach pain disorder” than a coherent entity, then it would be unlikely that a set of critical genes or a discrete underlying neurobiology are to be found. In addition, a great deal of co-morbidity might be expected, further complicating the search for neurobiological mechanisms. For the same reasons, it would be unlikely that there would be a faithful animal model - consider developing an animal model of “stomach pain disorders,” as opposed to cancer, ulcers, etc. The value of animal models is that they allow the study of a particular mechanism in isolation (cancer, ulcers, bowel disease, etc.) in a reduced preparation. However, if depression is defined in such a way that there is unlikely to be a discrete and non-overlapping psychobiological mechanism, then no animal model may capture it.
This line of argument has recently been adopted by the Research Domain Criteria project (RDoC) at the NIMH (Insel et al., 2010). Its purpose is to find new ways of classifying psychopathology based on dimensions of observable behavior and neurobiological systems, rather than symptoms. The idea is that there are discrete neurobiological systems (e.g., those that mediate and regulate negative affect) that can become dysregulated, and that these cut across traditionally defined diagnostic categories. Thus, for example, altered functioning of a region of the medial prefrontal cortex has been implicated in both depression and posttraumatic stress disorder (e.g., Drevets, 2000; Phan, Britton, Taylor, Fig, & Liberzon, 2006; Shin et al., 2005). I would argue that it is the functioning and dysregulation of these defined systems, as well as the behavioral consequences of such dysregulation, that can be modeled in animals. This is not merely arguing that there are subtypes, it is rather arguing that the basis for diagnosis should be at a different level of analysis.
As to learned helplessness, the available data suggest that it, at least in part, models or reflects impaired medial prefrontal cortical inhibitory control over stress-responsive limbic and brainstem structures (see later), and models this very well. That is, exposure to uncontrollable relative to controllable aversive events interferes with the top-down inhibitory control over structures such as the dorsal raphe nucleus and the amygdala, normally exerted by the medial prefrontal cortex. The behavioral consequences of uncontrollable stress are then a product of exaggerated activity in the raphe and amygdala. If a process such as this is involved in the development of psychopathology, then this process can be studied within the learned helplessness model. However, this dysregulation is unlikely to be unique to depression, and so this does not model “depression.” It models a process that could be important in numerous disorders as defined by the DSM, including depression. It can be argued that it is these dysregulated processes that should be the target for treatment. No single treatment will ever be effective for “stomach pain disorder,” but there is hope for treatments of stomach cancer, ulcers, pancreatitis, etc. Having discussed what the learned helplessness model is and what is not, I now turn to outlining what has been found in this area of research.
Learned helplessness is defined by the occurrence of behavioral or physiological outcomes following exposure to aversive events that depend on the organism having had no behavioral control over the events (Maier & Seligman, 1976; Maier, Seligman, & Solomon, 1969; Seligman & Maier, 1967). That is, to qualify as a learned helplessness effect, some outcome must be shown to occur if the organism has had no control over the prior aversive events, but fails to occur if the organism did have control. When organisms have been previously exposed to aversive events over which they had no control (e.g., inescapable electric shocks), they later show inhibited fight or flight behavior (e.g., failure to escape), exaggerated fear or anxiety behavior (e.g., increased social avoidance), as well as behaviors suggestive of anhedonia (e.g., reduced self-administration of rewarding brain stimulation) (for a review see Maier & Watkins, 1998). Learned helplessness can be induced in an hour to an hour and a half, and the effects produced usually last for a few days (Overmier & Seligman, 1967). This timecourse has made the relationship between learned helplessness and psychopathology seem dubious. However, it has frequently been noted that humans “ruminate” (i.e., retrieve cognitive representations of past events) about negative events that they have experienced, and rumination has been argued to be critical in the etiology and duration of depression (e.g., Nolen-Hoeksema, 1991). Similarly, what has been called “reexperiencing” the precipitating event is a characteristic of PTSD (e.g., Ehlers, Hackmann, & Michael, 2004). It can be argued that animals such as rats require cues associated with an event to produce memory retrieval of the event. If this is so, the implication is that rats do not ruminate, that is, retrieve memories of the uncontrollable stressor in a learned helplessness experiment while in the home cage, but would need to be exposed to the context in which the stressor had taken place. Thus, Maier (2001) attempted to simulate rumination in rats. Rats received uncontrollable stress as in previous experiments and were tested for behavioral effects at various later times, and as typical, deficits persisted for two but not four days. However, if subjects were simply reexposed to the uncontrollable stressor apparatus for ten minutes two days after stress (Day 2), now behavioral deficits were still present on Day 4 after stress. Most importantly, reexposure every two days, with no further stressor exposure, was able to maintain behavioral effects indefinitely. The longest duration examined was reexposure every two days through Day 16, and now the behavioral effects were still present on Day 18 after uncontrollable stress.
Not all outcomes (including behavioral, physiological, or endocrine outcomes), however, are a function of behavioral control. For example, the amount of wheel-running a rat engages in does not vary according to behavioral control (Woodmansee, Silbert, & Maier, 1993), and so the reduced wheel running that follows inescapable shock is not a learned helplessness effect. Similarly, among other physiological outcomes, peripheral immune responses (Maier, Nguyen, Deak, Milligan, & Watkins, 1999) or hypothalamic-pituitary-adrenal (HPA) axis activation also do not always vary according to control (Maier, Ryan, Barksdale, & Kalin, 1986). If all outcomes varied as a function of behavioral control, the phenomenon of learned helplessness would be synonymous with stress and would become a meaningless concept (Maier & Watkins, 2005).
The neural circuitry involved in learned helplessness helps explain why only some outcomes are affected by behavioral control. In particular, the dorsal raphe nucleus (DRN) (a very small structure of approximately thirty thousand neurons in the rat located on the midline of the brainstem) constitutes the final common path for inputs coming from aversive stimulus - responsive structures (e.g., locus coeruleus, bed nucleus of the stria terminalis, lateral habenula, etc.) (Grahn et al., 2002). Such events activate all of these structures whether or not the animal has control, and all of these structures converge on the DRN (Amat et al., 2001). If the converging inputs are strong enough (i.e., if the event is aversive enough), they will potently activate the DRN. Serotonin (5-HT) neurons of the DRN, however, are differentially responsive depending on the controllability of the event because the medial prefrontal cortex (mPFC) inhibits them when there is behavioral control (Celada, Puig, Casanovas, Guillazo, & Artigas, 2001; Gabbott, Warner, Jays, Salway, & Busby, 2005; Hajos, Richards, Szekely, & Sharp, 1998). Excitatory glutamatergic projections descend from the mPFC and synapse onto GABAergic interneurons in the DRN (Jankowski & Sesack, 2004) that inhibit the serotonin (5-HT) neurons. We have been able to examine these projections by injecting a retrograde tracer into the DRN, thereby labeling the neurons in the mPFC that project to the DRN. Activation of these projecting neurons could then be assessed by measuring immediate early genes such as c-fos in the labeled cells, and control was shown to activate them—a direct demonstration that behavioral control produces top-down communication to the DRN (Baratta et al., 2009). Consequently, experimentally inhibiting 5-HT neurons in the DRN (i.e., mimicking the action of the mPFC) with a pharmacological agent during exposure to uncontrollable stress prevents learned helplessness from developing after exposure to inescapable shock (i.e., animals escaped without difficulty in a subsequent test) (Grahn et al., 2002). Conversely, if the DRN is stimulated without exposing the animals to inescapable shock, the animals later display learned helplessness by failing to escape (Maier, Busch, Maswood, Grahn, & Watkins, 1995; Schmitt, Sandner, Colpaert, & De Witte, 1983). Similarly, experimentally inactivating the mPFC during exposure to the initial shocks leads animals to fail to escape whether or not the initial shocks are escapable (Amat et al., 2005). Finally, experimentally activating the mPFC during stressor exposure, prevents inescapable shock from producing later escape deficits, (Amat, Paul, Watkins, & Maier, 2008), thus blocking the development of learned helplessness.
An initial experience with an uncontrollable stressor, therefore, produces learned helplessness, because the mPFC does not inhibit the DRN. In addition, Dr. Seligman and I noticed that the reverse - giving an organism an initial experience with behavioral control over an aversive event - will immunize it against the subsequent negative effects of not having control anymore (Maier & Seligman, 1976; Moye, Hyson, Grau, & Maier, 1983; Williams & Maier, 1977). At the neurochemical level, the initial experience with control blocks the effects of subsequent lack of control. In other words, the initial experience with control induces plasticity in the mPFC (Bland et al., 2007). The resulting semi-permanent changes in the mPFC result in the prolonged blocking of DRN activation, even when the animal does not have control anymore. This illusion of control is therefore observable at both the neurochemical and the behavioral level. We have, for example, given rats an initial experience with control over electric shocks. This initial experience blocked the negative effect of subsequent uncontrollable shocks (Maier, Amat, Baratta, Paul, & Watkins, 2006) or of social defeat (Amat, Aleksejev, Paul, Watkins, & Maier, 2010) the rats were exposed to one week later. The protective effect of this initial experience with control depends to some extent on the age of the animal: Rats that are given that experience at a young age (i.e., during or before adolescence) are protected for the longest.
One question of significant importance for our discussion is whether this phenomenon of learned helplessness uniquely corresponds to depression in humans. I do not think that it does. In fact, I am not sure one would want to relate learned helplessness to depression any more than to posttraumatic stress disorder (PTSD) or anxiety (Foa, Zinberg, & Olasoo-Rothbaum, 1992; Maier & Watkins, 1998). Consistent with our findings regarding the role of the mPFC in the development of learned helplessness, abnormalities of mPFC function have been reported in both depression (Drevets, 2000) and PTSD (Bremner et al., 1999; Phan et al., 2006; Shin et al., 2005). Learned helplessness is also affected by both antidepressant (Petty, Davis, Dabel, & Kramer, 1996) and anxiolytic (Drugan, Ryan, Minor, & Maier, 1984) medications. So while the phenomenon of learned helplessness can help us understand some aspects of depression, it should not be treated as synonymous with, or unique to depression. Learned helplessness may be one process that is relevant to depression among many others, and it is probably also involved in other disorders. This is what would be expected from the discussion that began this section.
Fritz Henn
Learned helplessness is probably relevant to disorders other than depression, but I want to argue - going beyond what Dr. Maier said - that it has some elements that are really useful in modeling depression in particular, as opposed to other disorders. Experimental inductions of learned helplessness produce the kinds of behavioral and neurochemical changes that are seen in depressed patients. Phenomenologically, animals in our studies display the same changes in REM sleep (Adrien, Dugovic, & Martin, 1991), and the same responsiveness to antidepressant medication (e.g., Henn, Edwards, Anderson, & Vollmayr, 2002; Schulz, Mirrione, & Henn, 2010) than individuals suffering from depression as a result of being exposed to uncontrollable aversive events. In contrast with Dr. Maier’s results, we have also found that lack of control results in changes in HPA axis activation (e.g., Vollmayr, Faust, Lewicka, & Henn, 2001).
Interestingly, not all animals display the same degree of helplessness as a result of lack of control. When we experiment with a hundred rats, we almost always find that 10% of them become completely helpless, whereas another 10% will not become helpless at all following exposure to uncontrollable aversive events. The remaining 80% fall somewhere in the middle. So we decided to breed the two extreme kinds of rats, and we created both a line of spontaneously resilient rats (in which uncontrollable aversive events fail to induce learned helplessness) and a line of spontaneously helpless rats (that are helpless even when aversive events are controllable from the very beginning). The spontaneously helpless rats display behaviors that are reminiscent of depression such as reduced sucrose preference, which is akin to anhedonia in humans (Henn & Vollmayr, 2005; Vollmayr et al., 2004). Using the susceptibility to the behavioral induction of helplessness or resistance to helplessness induction as the basis for breeding allows us to model genetic lines of helpless and resilient animals. This might let us get clues as to the genetic or anatomic differences between the lines and then go back to patient samples and compare these results.
We utilized these lines to examine the differences in the circuits mediating the response to stress and found unique aspects of the circuit that could be tested in patients cohorts. Spontaneously helpless animals displayed unusually high levels of activation in the lateral habenula (Shumake, Edwards, & Gonzalez-Lima, 2003), which appears to be another key area in the neural circuitry of depression. What the lateral habenula does is bring in the reward/disappointment system, including the Ventral Tegmental Area (VTA). Matsumoto and Hikosaka (2007) have conducted a study with primates which showed that high activation of the lateral habenula leads to shutting off the reward system. Using c-fos, we found that the activation of this habenular reward circuit is also controlled by the mPFC, which therefore modulates several key regions involved in the neural circuitry of depression. We were able to show that the lateral habenula receives limbic and cortical input, and, in the case of congenitally helpless animals, is 15-fold more active in regulating the VTA and DRN than it is in resilient animals (Li et al., 2011). Thus, combining the use of behavioral paradigms which utilize a stressor with breeding studies to attempt to define genetically susceptible populations may give us models which yield new information that can be tested in patient populations.
If this rat model of learned helplessness has anything to do with depression, we should be able to find the same results with human participants. Several research groups have independently conducted tryptophan depletion studies, a procedure known to induce depression in human participants (for a review see Reilly, McTavish, & Young, 1997). Participants were formerly depressed individuals who had responded to SSRI medication (but were off drugs at the time of the experiment). Paralleling the results found in animal studies, experimentally depressed human participants displayed increased habenular activation. The changes in mood induced by tryptophan depletion (which usually last a few hours) tracked the changes in habenular activation (Morris, Smith, Cowen, Friston, & Dolan, 1999; Roiser, et al., 2009). The experimentally depressed participants furthermore reported that their subjective experience was similar to that of their previous depressive episodes. Interestingly, whereas the rapid changes in habenular activation can be detected, we have failed to observe corresponding activation changes in the mPFC, using a variety of imaging techniques. So the most immediate neural correlate of mood changes appears to be habenular activation. Taking all of these findings together, I believe that learned helplessness (and its effect on the habenula) constitutes one useful model for depression.
Helen Mayberg
I have more questions than answers about learned helplessness as a model of depression, but I think that there a few common threads here. This model is very enticing, as it has a certain face validity in clinical patients and stress responses more generally. First, there is clear evidence that uncontrollable stress even in healthy people can results in negative effects on brain, behavior and physical functioning, and that these effects are correlated with changes in limbic, cortical and brainstem regions we have already discussed. Second, it is also well documented that life stressors are potent risk factors for depression and anxiety disorders, as well as triggers of full-blown depressive episodes in vulnerable people. So parallel to the animal studies, human depression is not merely a response to stress but rather an aberrant or nonadaptive response to stress. As with the animal studies, normal coping seems to depend on the efficiency of one’s ventral medial prefrontal cortex (ventral mPFC) to regulate the cascade of events that follow negative events. Interestingly, abnormal functioning of this region has been repeatedly observed in depressed patients studied with various imaging strategies, and changes in this region are seen with response to various antidepressant treatments (Mayberg, 2009). Variability in the anatomy and function of ventral mPFC have been further linked to genetic polymorphisms of the serotonin transporter (e.g., Pezawas et al., 2005) which in turn have been associated with behavioral differences in emotional reactivity and cortisol responses to experimental stressors and to depression vulnerability. This however, does not mean that learned helplessness is synonymous with clinical depression. Rather it may be a behavioral process that is part of the depressive syndrome, at least as we now define it. A related question is how is learned helplessness associated with anhedonia, an undisputed core symptom of major depression? Does learned helplessness precede or follow anhedonia? From an adaptation perspective, I could hypothesize that the subcortical activity that mediates the helpless behaviors overrides those systems that might respond to other stimuli. Said another way, excessive negative behavior is likely appropriately co-opting all of the organism’s attentional resources (Mayberg, 2004; Roy-Byrne, Weingartner, Bierer, Thompson, & Post, 1986) so that potentially rewarding, but less novel stimuli have reduced salience. So in this sense, learned helplessness could be a precursor to anhedonia, because it forces one to give priority to the negative and, as a result, forego the potential positive.
Richard Davidson
The findings described by Dr. Maier and Dr. Henn are extremely interesting and raise several new questions about the relationship between the neural circuitry involved in learned helplessness and depression. Research from our laboratory has recently shown that depressed individuals have difficulty maintaining ventral striatal activation (Heller et al., 2009). Is this also the case in learned helplessness? We do not have an answer yet. Research on the neural correlates of tryptophan depletion (causing depressive symptoms) and the resulting increase in habenular activation also offer important new avenues for research (Morris et al., 1999; Roiser et al., 2009). We should probably look more sensitively at the behavioral changes that accompany tryptophan depletion, and examine how they relate to both learned helplessness and depression. We need more accurate tools to look at changes in both mood and behaviors, as existing measures may not capture the full range of symptoms that we are looking at.
Martin Seligman
We need to better understand how learned helplessness accounts for the depression-like symptoms that are displayed by animals and humans. For instance, if an animal has an initial immunization experience (during which it is given control over an aversive experience) two phenomenological things could be going on when it is later presented with an uncontrollable aversive experience. On the one hand, the animal may have the illusion that it is still in control. On the other hand, the animal may realize that it does not have control in this particular situation, but that control may come back in the future. The fact that immunized animals who are placed in a social defeat situation (as described earlier by Dr. Maier) continue to fight suggest that they may be under the illusion that they are still in control (Amat et al., 2010). We know a bit more about what happens in this case with humans. First of all, we found that people become passive and stop trying after being exposed to inescapable bad events such as uncontrollable bursts of noise (e.g., Alloy, Peterson, Abramson, & Seligman, 1984), and as a result show greater levels of anxiety and depression. When presented with unsolvable problems, they will stop trying even when they are later presented with solvable ones (Klein, Fencil-Morse, & Seligman, 1976). To explain why humans show learned helplessness, we looked at how they explained the uncontrollable bad events, and found that those who showed learned helplessness developed what we called a “pessimistic explanatory style,” which explains why people stay helpless long after the event has stopped. In particular, they tend to believe that negative events are stable (“it will always be around in the future”), have global consequences (“my whole life is ruined”). More often than not, they also blame themselves for the bad events (“it’s my fault”) (Abramson, Seligman, & Teasdale, 1978; for a review see Peterson, Maier, & Seligman, 1995). Conversely, resilient individuals who do not develop learned helplessness even after having been exposed to uncontrollable aversive events tend to believe that negative events are unstable and specific to a particular situation (Seligman, 1991). So humans who are resilient in the face of uncontrollable adversity tend to believe that they will regain control soon.
But the central question that we should come back to is the following: What does this have to do with depression? One concern that has been raised is that the clinical syndrome of depression requires a duration of symptoms lasting at least two weeks, while learned helplessness is usually thought to last only a few days. While that was true of the original helplessness studies (e.g., Overmier and Seligman, 1967), a chronic form of learned helplessness is found in both rats and dogs. Repeated helplessness training and helplessness training given at weaning produces non-transient learned helplessness (Hannum, Rosellini, and Seligman, 1976; Rosellini and Seligman, 1976; Seligman and Groves, 1970). So learned helplessness, either repeated, or occurring in childhood, has the feature, like depression, of being non-transient. However, as Dr. Maier mentioned, learned helplessness seems to be at play not only in depression, but also in other disorders such as PTSD (see also Overmier, 2002). I personally do not take these categories very seriously. So learned helplessness seems to be a process at play in several diagnostic categories. This is the question that we will discuss next.
Aaron Beck
The central issue we have tried to address here is whether learned helplessness, as it has been studied in animals and humans, is relevant to depression. Dr. Mayberg mentioned earlier that one of the consequences of learned helplessness is that individuals are compelled to focus on the negative and they pay little attention to positive experiences (thus resulting in anhedonia). Experimental studies have shown that depressed individuals accentuate their reaction to negative stimuli and decentuate their reaction to positive stimuli (e.g., Gotlib, McLachlan, & Katz, 1988). This finding has also been corroborated using physiological methods (see for example Elliott, Rubinsztein, Sahakian, & Dolan, 2002).
I agree with others that the learned helplessness model should not just be considered to be a model of depression. In fact, when Dr. Seligman conducted his first experiment, we both thought that learned helplessness might be an excellent model for the negative symptoms of schizophrenia. We have been studying the negative symptoms of schizophrenia (Rector, Beck, & Stolar, 2005) and here is the typical profile we find: An adolescent that already has various cognitive impairments is subjected to a series of very negative experiences. Because he or she does not perform adequately according to cultural standards, he or she is deprecated by his parents and peers, and he or she does not develop adequate social skills as a result of his or her impairment. In the end, the adolescent becomes socially ostracized and withdrawn. A variety of factors, including early experiences with uncontrollable and aversive social events, therefore converge to produce the negative symptoms of schizophrenia. In addition to social withdrawal, individuals suffering from schizophrenia also tend to display some of the same cognitive distortions seen in depression. These distortions are actually quite treatable, using some of the same methods we use for depressed individuals (Rector & Beck, 2001). Individuals with schizophrenia will tend to build up negative attitudes toward performance, including beliefs such as “If I try something I will fail, so I shouldn’t even try” (Grant & Beck, 2009). Equating failing at one thing to being a total failure is something that we also see in depression. The negative beliefs held by individuals suffering from schizophrenia will in turn affect their motivation and effort. Studies of individuals suffering from schizophrenia have for example shown an inhibition of pupillary response (this response indicates effort) even when they say that they want to try (e.g., Minassian, Granholm, Verney, & Perry, 2004). So insofar as individuals with negative symptoms of schizophrenia have completely withdrawn and given up in part as a result of early exposure to uncontrollable aversive experiences, they fit the learned helplessness model (for a review of the relationship between childhood adversity and schizophrenia see Read & Bentall, 2010). Learned helplessness therefore appears to be highly relevant to several disorders including both depression and schizophrenia.
Conclusions
The phenomenon of learned helplessness develops when animals first exposed to uncontrollable aversive events later behave as though they still have no control over subsequent (controllable) aversive events. Learned helplessness appears to be mediated in the brain by the medial prefrontal cortex (mPFC), which exerts a differential influence on the dorsal raphe nucleus (DRN) according to the degree of control perceived by the organism. Activity of the DRN seems to be necessary and sufficient for learned helplessness. Whenever the DRN is activated, learned helplessness is observed. Whenever it is inhibited by the mPFC, learned helplessness is not observed. This casts doubt on whether the expectation of no control, as opposed to the activation of the DRN, is the cause of learned helplessness, at least in animals. The learned helplessness model may explain how uncontrollable life experiences lead to depression-like behaviors, but the model may not be specific to depression. It may also be a useful model for disorders such as PTSD or schizophrenia. The nonspecificity of learned helplessness suggests basic psychopathological processes that transcend present diagnostic categories.
It must be pointed out here that although the conversation between the six researchers was limited to the learned helplessness model, many other animal models using a variety of approaches (e.g., genetic, pharmacological, environmental, etc.) have been instrumental in allowing researchers to examine different aspects of depression. An exhaustive discussion of these other models is beyond the scope of the present paper, but readers are referred to other comprehensive reviews highlighting the specific contributions and limitations of a wide array of such animal models of depression (e.g., Anisman & Matheson, 2005; Canavello et al., 2010; Cryan & Slattery, 2007; Nestler & Hyman, 2010).
Towards a New Taxonomy of Psychological Disorders
In the second phase of the conversation, the six researchers discussed current flaws within the existing diagnostic system. They addressed the following questions: What are some of the current problems with the DSM-IV-TR’s (American Psychiatric Association, 2000b) classification of disorders? Is our current taxonomy helping or hindering our efforts to find the most effective treatments for individuals suffering from depression? How can recent advances in our understanding of biological processes be integrated within a new taxonomy of psychological disorders?
Martin Seligman
I think our diagnostic categories are the wrong ones. We are stuck with categories such as depression, PTSD, or schizophrenia, in which there are nonspecific pathological processes that are common to all of them – the process of learned helplessness is one example. Automatic thoughts are another such ubiquitous process. So it might be productive to focus on processes as the basis of taxonomy. The history of smallpox (Riedel, 2005) offers a good example of the adoption of a process-driven (as opposed to a symptom-driven) approach in medicine. Two hundred years ago, when someone had pustules on their face preceded by fever and backache (all symptoms), they were diagnosed with smallpox. But a germ theory of smallpox emerged, such that some of the cases involving similar symptoms were not smallpox, whereas some cases that did not have those symptoms were still smallpox, because the criterion for smallpox shifted from symptoms to the smallpox virus. Color blindness is another example. There once were symptom-driven categories of color-blindness. But then people discovered the basic processes involved in color-blindness, the different receptors each of which could be separately affected by disorder, and this led scientists to replace symptom-driven with several process-driven categories. I think psychopathology should now try to move from symptom-based categories to more refined and more useful process-based ones.
There are currently three features in the landscape of clinical psychology that could be unified. First, there are processes – psychological ones like learned helplessness, or automatic thoughts, and a large variety of neurological processes that can go awry. Second we have treatments, such as cognitive therapy, drugs, and deep brain stimulation. Third, we have the many DSM categories that relate poorly to the first or second features.
If disordered processes are more basic than disordered symptom clusters, then it follows that outcome studies of treatments based on symptoms will have mediocre results at best. It is commonly found that treatments only help half of participants. This is no surprise if the trial is based on the current symptom-cluster categories, because only those patients whose disorder corresponds to the relevant underlying (and undetermined) process so treated are helped. Outcome studies seem to have reached a very strange dead end. A good outcome study in depression, for example, typically gets a 65% response, in comparison to a 40–50% placebo response (e.g., Kirsch, Moore, Scoboria, & Nicholls, 2002).
What we need to do is to tailor treatments into the suspected underlying processes and ignore the DSM category. Ideally, we should strive for an exhaustive and exclusive set of pathological processes, and we would ask how different treatments map on to these disordered processes. For starters we do not need an exhaustive list of processes – all we need are a few processes where we know how the behavior or the cognition map onto neurocircuitry processes. If outcome studies were not about heterogeneous diagnostic categories, but about basic processes, we may be able to come closer to a 100% response rate.
Steven Maier
The whole idea for the DSM-V was to use newfound biological knowledge in order to refine our diagnostic categories so that they would map on to basic biological processes. DSM categories are unrelated to biological processes, and it appears that this will probably remain the case with the next edition (Hyman, 2007). Like Dr. Seligman, I agree that we need a process-driven nosology. That is why I have always refused to argue that learned helplessness is a model of some disorder – it is a process that can be seen in several of our so-called diagnostic categories. And understanding basic processes can help us figure out exactly what is going on with individuals who suffer from psychological disorders. For instance, animals that display learned helplessness by failing to escape aversive events have not “given up.” Studies have shown that they fail to escape because their escape behaviors are being inhibited (Maier et al., 2006). This means that by removing the inhibition, we can get them to escape again. So the cognitive process that leads to hopelessness (“giving up”) is quite different from the process of learned helplessness. We need to get rid of our current categories, because they do not inform us about the best way to treat people. When we say someone is depressed, all we can say is that their brain is dysregulated, because the types of dysregulations involved are far too heterogeneous to map on to our vague construct of “depression.” If someone goes to a physician complaining of stomach pain they are not typically diagnosed as having a pain disorder, and the treatment may not focus on treating pain per se. The search would be for the disturbed process that underlies the pain, and there are many possible. If the same treatment were applied to all instances of stomach pain, no treatment would work very well. The symptom called pain might be suppressed, but many of the diverse underlying disorders would remain untouched.
Helen Mayberg
We clearly need to better define these basic processes. Also, as we further consider clinical nosology and targets of different treatments, it would also be helpful to determine which processes are primary, and which are secondary. Take anhedonia versus social withdrawal, for example. Are depressed individuals anhedonic first, and as a result, withdraw? Is anhedonia an epiphenomenon of resource allocation misappropriated to resolve negative events? Or is a third process causing both behaviors? Similarly, when we think about depressive cognitions, are these primary or secondary adaptive cortical responses to some underlying non-cortical process?
Researchers have support for at least two primary processes in depression, and these appear to map onto known brain systems. One emphasizes top-down or cortical systems—i.e., negative bias, depressive cognitions and the like. The second takes a bottom-up approach, emphasizing distress, autonomic or circadian dysregulation implicating limbic-subcortical systems including the brainstem, amygdala/hippocampus and hypothalamus. One problem of course is that we cannot tell which process is primary and which is secondary, because the two processes appear to be yoked. It is very clear that negative life events can precipitate normal stress responses that if sustained, can lead to pathological functioning of these various brain systems. On the flip side, one can similarly imagine that these same stress-response systems may decompensate without a clear external precipitant. In such circumstances patients will still try to modulate their distress in the context of ongoing external events. It is difficult to disambiguate external (cortical) versus internal (subcortical) precipitants in the absence of measurements that avoid patient self-report and interpretation.
I agree with the concerns Dr. Seligman raised about how we run trials to determine the efficacy of treatments. At present, trials use definitions of syndromes based on a taxonomy that minimizes obvious heterogeneity and established metrics that assume treatments will affect all symptoms equally. Efficacy for many treatments has been demonstrated over the years despite these obvious deficiencies. But perhaps disregarding these issues accounts for the fact that remission rates are less than 50% for many treatments, and we continue to contend with residual symptoms and commonly, relapse even in those patients who initially show recovery. An improved taxonomy, or better still, treatments tested against primary processes rather than syndromal diagnostic criteria, might lead to treatments matched to individual symptoms. If we identify and disambiguate primary from secondary symptoms we may find that we not only get patients well but that they relapse less. In our ongoing experiments with deep brain stimulation (DBS) (see Lozano et al., 2008; Mayberg et al., 2005), we find that individuals who recover do not seem to relapse as they did with previous treatments, except of course when the battery running their stimulator runs down. While we would like to argue that we are somehow targeting a primary process with DBS, our analyses of standard depression rating scales do not capture this.
Uncovering the basic biological markers and processes has become a priority among researchers (Drevets, 2000; Mayberg, 2007). But changing the culture of clinical diagnosis, particularly when it is defined by approaches such as the DSM, is a slow process. We need to work using the current diagnostic categories to come up with more refined and useful process-driven ones. We still have a lot of questions to answer regarding the basic processes contributing to what we call depression. Certainly we know that processes like negative cognitive bias, anhedonia, HPA axis dysregulation, learned helplessness (with its circuitry involving the DRN and the mPFC) all contribute to depression. We need to determine, however, if some processes are more critical than others. We need to investigate whether some processes can be compensated for by others, and under what circumstances. My personal conceptualization is that when an organism encounters a stressor, it tries to adapt. Based on various preexisting conditions (genetics, early trauma, past experience, etc.), it may or may not be able to adapt and compensate for the perturbation introduced by the stressor. If it cannot adapt and compensate, then the response is illness (Mayberg, 2003). This seems to be a parsimonious way of explaining the heterogeneity we find in the presentations of the disorder, as well as why some individuals carrying the same genetic risk develop a disorder or not.
So most of us agree on the necessity to better define these basic processes. There in fact has always been an interest in looking for similarities in such basic processes across disorders - such as psychomotor slowing and apathy in depression, Parkinson’s disease and schizophrenia. But where we fall short is on agreeing on what the most pertinent and critical basic processes are. At this stage, I do not know which are most critical for depression although there are certainly top candidates. And beyond single processes, we need to look at the interactions between processes (Mayberg, 2003; Mayberg, 2006).
An alternative strategy is to ignore specific processes for the time being, and to let the brain classify itself, using treatment response as the independent measure. Identification of treatment-specific, brain subtypes would provide a novel classification schema whereby the relative contribution of various processes (genetic, epigenetic, immune, neuroendocrine, temperament, cognitive, behavioral, etc.) could be re-considered.
Richard Davidson
Every time I struggle with the problem of the basic, primary, process involved in depression, I am left with the conviction that there have to be different routes. Depression is a heterogeneous disorder, and our task is to better account for that heterogeneity based on the different components of the circuitry, the different basic neural processes, that are primary for each individual (Davidson, Pizzagalli, Nitschke, & Putnam, 2002). In turn, we need to explain how these basic neural processes are reflected at a psychological or behavioral level. The work we have done at our laboratory has illuminated the role of the basic process of emotion regulation in depression. Looking at neural circuitry, we have been able to show that the typical pattern of inverse coupling between the prefrontal cortex and amygdala during the voluntary down-regulation of negative affect using cognitive reappraisal strategies is reversed in depression (Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007). Behaviorally, this neurocircuitry maps on to two important basic processes: the capacity to sustain positive affect, and the capacity to down-regulate negative affect, both of which appear to be impaired in depression (Larson, Nitschke, & Davidson, 2007; Shestyuk, Deldin, Brand, & Deveney, 2005). Moreover, which of these impairments is predominant may define important depression behavioral and neurophenotypes (Davidson et al., 2002). And other research groups have also shown that measures of within-subjects connectivity are far better predictors of all kinds of outcomes than activation in any group of foci (e.g., Büchel, Coull, & Friston, 1999; Buckner, 2010). So as Dr. Mayberg pointed out, understanding the interactions between various neural processes will be a central endeavor if we want to uncover the basic processes at play in depression. The capacity to disengage is, I agree, a crucial process in depression: There appears to be hysteresis in the system (Davidson et al., 2002). We have shown recently that context-appropriate flexibility in connectivity reflecting emotion regulation is an important index that provides better information than data collected from situations where the context does not shift (e.g., Johnstone et al., 2007; Urry et al., 2006; see also Davidson, Jackson, & Kalin, 2000).
One way to study variations in basic processes is to start with nonclinical populations. Starting with a community-based sample ensures that we do not restrict the range of the variation in processes we are looking at. We are working with a sample of 350 families in a community-based sample, recruited when the mother was in her third trimester of pregnancy. The offspring, now adolescents, have been followed since birth. Many of these families have been subjected to various kinds of adversities but not explicit psychopathology. We are collecting data on various behavioral and biological processes at regular intervals in time (see for example Essex, Klein, Slattery, Goldsmith, & Kalin, 2010). This kind of research strategy will allow us to better describe and understand basic psychological processes and how they map onto basic biological processes.
Fritz Henn
Let me illustrate the problem we are faced with when doing clinical work, and why we need to look at processes rather than the present diagnostic categories. We did a genetics study of schizophrenia several years ago, and found a gene on chromosome 13 that seemed to be reliably associated with the disorder. Four centers replicated our finding (Abou-Jamra et al., 2006). We then replicated our finding with a sample of individuals suffering from bipolar disorder – the same gene appeared to be associated with both disorders (Schulze et al., 2005). We were then able to look at the histories of both samples to look at their phenomenological presentations, and found that this gene was really associated with some form of irrational fear, very similar to what Kraepelin described as paranoia in his very first textbook (Kraepelin, 1919). We then decided to look at panic disorder, another disorder involving irrational fear, and the same gene turned out to be associated (Schumacher et al., 2005)! Finally, we did structural imaging work and found that, across diagnostic categories, patients showing this type of basic process showed differences in the dorsal prefrontal area (Corlett et al., 2007; Tost et al., 2010). If important processes transcend our current diagnostic categories, we would be better off using a taxonomy based on such processes.
Aaron Beck
I do believe the current nosology is effective as it provides a phenomenology which is needed in order to anchor the processes we are describing. I think depression is a meaningful category, but I agree that there is considerable heterogeneity (Clark, Beck, & Alford, 1999). There may be several types of depression which at the surface level seem fairly similar, but at the process level may differ. Here is an analogy: Two individuals with breast cancer may look similar on imaging slides. But if you look at the microRNA involved in the cancer of these two individuals, you find that they are in fact completely different and respond differently to the same drugs (Lowery, Miller, McNeill, & Kerin, 2008). I think that this is also true of depression: We see similar symptoms, but the basic processes may be completely different, and may predict who will respond to what treatment.
There are different process levels – the behavioral, the cognitive, the physiological, the genetic levels, for instance – and we all have different investments in different levels. I study the cognitive level and I believe that a cognitive bias process is involved in various disorders (Mansell, Harvey, Watkins, & Shafran, 2008). With respect to depression, latent depressive schemata, once activated by stressors (Beck, 2008) trigger self-referential negative thoughts about the self, the personal world and the future (Clark et al., 1999). Activated depressogenic schemata bias the encoding of information and lead to impairments in interpretation, attention, and memory. When information is processed in schema-congruent ways negative and pessimistic interpretations, evaluations, and appraisals about one’s self and context become pervasive and lead to avoidance and withdrawal characteristic of depression (Clark et al., 1999). Faulty information processing also leads to selective biases for negative information (for a review see Gotlib & Joorman, 2010). Similarly there is strong support for cognitive biases in memory processes in depression. In particular, depressed individuals tend to exhibit a preferential recall of negative compared to positive material (Mathews & MacLeod, 2005).
Since the introduction of the cognitive model of depression, a proliferation of research has found support for the application of the cognitive model to a variety of mental health concerns. So we can look at the basic process that is common to all of these disorders, rather than focus on how these processes vary according to each person. The literature on cognitive behavioral processes across diagnoses has identified several likely transdiagnostic process candidates that fall under the following categories: attention, memory, reasoning, thought and behavior (Harvey, Watkins, Mansell, & Shafran, 2004). Dr. Haigh and I are currently developing a model called the Generic Cognitive Model, which is a framework for capturing the specific psychopathology of each disorder as well as the processes common to all clinical and subclinical suffering (Haigh & Beck, 2011). In this model, we theorize that the various diagnoses can be understood in terms of common cognitive processes; however, concomitantly these disorders can be differentiated by specific cognitive content (Haigh & Beck, 2011). For example, depression is characterized by themes of loss while anxiety is characterized by threat. One of the most important practical implications of the Generic Cognitive Model is that we can use the same treatment, with some modifications, to try to modify cognitive bias (Haigh & Beck, 2011).
Conclusions
Recent findings on the neurobiological processes at play in psychological disorders (and in particular, in depression) have highlighted that these processes transcend diagnostic categories. The six researchers agreed that the existing classification of disorders has important flaws and does not appear to carve nature at its joints. More importantly, the existing taxonomy impedes the development and outcome testing of effective treatments. Treatments targeting the heterogeneous construct of depression often fail to help all of those falling in this heterogeneously caused diagnostic category. A more useful taxonomy would include a list of basic processes (including underlying neurobiological mechanisms) that can be specifically targeted. The development of such a taxonomy would increase the efficacy of treatments, by pairing individuals presenting specific behavioral or biological markers of disordered processes with more appropriate interventions.
Research on the feasibility and usefulness of such a process-based approach is already underway. For example, the Research Domain Criteria Project (RDoC; Insel et al., 2010) at the NIMH is currently investigating the pathophysiological/neurobiological processes at play in various disorders. The main goals of the RDoC project are to identify the most useful target processes for treatment, facilitate the detection of subgroups that may respond differentially to treatment, and overall improve clinical decision-making. The conversation between the six researchers supported Insel et al.’s (2010) assertion that “in 2010, we do not know how many different disorders are embedded in the current diagnosis of schizophrenia or autism or other current categories that share clinical features. Our expectation, based on experience in cancer, heart disease, and infectious diseases, is that identifying syndromes based on pathophysiology will eventually be able to improve outcomes” (p. 749). The possibility that the DSM-V will incorporate dimensional information (Regier, Narrow, Kuhl, & Kupfer, 2007; Widiger, 2005) may also allow researchers and clinicians to better identify and target transdiagnostic processes.
Towards Convergence among Psychological and Neurobiological Processes
In the third phase of the conversation, the six researchers explored the possibility of developing a new taxonomy that is based on biologically-informed psychological processes. Dr. Seligman opened this section by raising a number of thought-provoking questions regarding how a biologically-informed process-based approach would translate to a clinical setting. The group also considered the two most promising process-based candidates - learned helplessness and emotion regulation - that to date appear to demonstrate convergence across psychological, neurobiological and treatment levels.
Martin Seligman
It occurs to me that one could make a good start at developing a process-based system if you had a potential candidate where you were able to link the phenomenology, the circuitry and the treatment. What is the possibility that “giving up” would correlate highly with activity in the DRN and habenula, and would be treatable by deep brain stimulation? How would you advertise for patients? What are the best candidates? What would this process-based system look like in a clinical setting? Let’s say that we were going to open a “medial frontal clinic” or a “posterior insula clinic,” and the reason we would do that is because there is a link between psychological processes, neural circuitry and treatment. How would people self-identify? Would they continue to say “I am depressed”? Or would they use a category which did not exist 60 years ago: “I am medial frontal”?
Richard Davidson
I think someone who had the “medial frontal syndrome” might describe him or herself as being the pawn of their emotions and cognitions. In terms of promising areas of convergence, we now have correlations in the .70 range over a two-year interval showing that prefrontal amygdala connectivity predicts objective measures of emotion regulation success two years later (unpublished data). This is a relatively large (n = 70) fMRI study where we are looking at corrugator EMG during and after a negative emotional stimulus. The variance is carried by the corrugator EMG in the post stimulus period, which reflects persistence of negative affect beyond the period where the negative affect elicitor was present.
Helen Mayberg
Currently we are in fact seeing points of convergence on a circuitry level from people to animals. There is more concordance on the circuitry and elements of behavior than at the syndromal level. I am encouraged when I see that Dr. Davidson gets similar findings looking at connectivity and emotion regulation to what we see in our resting state depression studies. He is not necessarily thinking about disease, yet he found the same regions we did, coming at it from a messy disease with different treatments. Such examples of behavioral and anatomical overlap between animal and humans give us a reasonable starting point to test some of these hypotheses.
Dr. Seligman asked earlier about the likelihood that “giving up” might correlate highly with a particular brain system. Well we know from Dr. Maier’s experiments that a critical region for control (the opposite of giving up) is the mPFC and its impact on the DRN and amygdala. These regions further interact with the ventral striatum and habenula as articulated by Dr. Henn. The ventral mPFC is similarly implicated in Dr. Davidson’s studies of emotion regulation and is targeted with our DBS procedure. It also overlaps with areas of change identified with infusions of ketamine, currently being tested as a rapid acting antidepressant (Deakin, Lees, McKie, Hallak, Williams, & Dursun, 2008; Zarate, 2006). While I am oversimplifying the similarities, these studies demonstrate anatomical, functional, biochemical and behavioral findings that speak to a potential common model to understanding control and its pathological correlate - giving up.
Fritz Henn
I think that the possibility that “giving up” would correlate highly with a brain system and be treatable with DBS is good. I am reminded of the rats that we bred over time who give up instantly, and the problem is that the DRN and the habenula are highly overactive. Now the question is, if you had a group of people like that, would they respond to DBS? We are asking that question experimentally now (e.g., Sartorius et al., 2010), and we are hoping that the answer is yes. Another, even better question is: Can we do this with a less stringent psychological and/or biochemical intervention? We might need to develop ways to stimulate the mPFC to have people learn that they can get control.
Steven Maier
In terms of candidate processes, the main one appears to be the medial prefrontal inhibitory regulation of other structures such as the DRN or the amygdala. And beyond inhibitory control, the mPFC is also involved in recruiting the dorsal striatum, which I think might be a separate but significant process. If we want to increase prefrontal cortical inhibitory control over some of these subcortical structures that have gone wild, there are lots of different routes to do that.
Something like depression is too big and heterogeneous and amorphous and does not fractionate the way the brain fractionates. It has joints in different places because our understanding of depression has been set up to be symptom-based, whereas the neurobiology of depression is dependent upon anatomy. In this sense, the two ways of understanding depression (symptom-based versus biology-based) are never going to correspond.
Aaron Beck
Although there does not seem to be much incentive to explore the surface manifestations of depression, specifically at the symptom level, there is considerable value in elucidating the psychological mechanisms involved in depression. Considerable research has supported the cognitive model of depression and the next step is to elucidate the relationship between neural circuitry and cognitive bias. So far, there have been a number of very interesting findings regarding the neural circuitry that maps on to the cognitive model of depression (Beck, 2008). Several lines of research suggest that cognitive biases in depression are due to maladaptive bottom-up processes (i.e. patterns of activation starting in subcortical brain regions that are lower along the cognitive hierarchy, which proceed sequentially to connected cortical areas higher up) that are generally perpetuated by attenuated cognitive control (i.e. failure of regions higher on the cognitive hierarchy to effectively regulate activity in those lower regions; Disner, Beevers, Haigh & Beck, 2011).
Specifically, researchers have found interactions among the amygdale, anterior cingulated gyrus, the medial prefrontal lobe, and the dorsolateral prefrontal lobe that correlate with basic aspects of the cognitive model (Clark & Beck, in press). For example, when depressed individuals process negative stimuli, they demonstrate more intense (by up to 70%) and longer lasting (up to three times as long) amygdala reactivity than healthy controls, even when an emotional task is immediately followed by a non-emotional task (Drevets, 2001; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002). Recent research has also shown that hyperactivity of the amygdala in the short 5-HTTLPR variant carriers is associated with increased sensitivity to negative stimuli (Munafò, Brown, & Hariri, 2008).
Cognitive processing biases, such as the inability to disengage from negative stimuli is thought to exacerbate symptoms of dysphoria and perpetuate the positive feedback loop of depressive symptoms (Hasler, Drevets, Manji, & Charney, 2004). Some evidence suggests that impaired attentional disengagement from negative stimuli is associated with decreased activity in the right ventrolateral prefrontal cortex and right superior parietal cortex for depressed individuals compared to healthy controls (Beevers, Clasen, Stice, & Schnyer, 2010).
Genetic factors also appear to have an important role in the cognitive model, as the short allele of the 5-HTTLPR polymorphism has been repeatedly associated with biased attention for emotional stimuli. The presence of the 5-HTTLPR short allele in children of mothers with a history of MDD during their children's lives exhibited greater attentional avoidance of sad faces (Gibb, Benas, Grassia, & McGeary, 2009). Further, among children with negative inferential styles, the presence of carriers of two copies of 5-HTTLPR short allele moderated the relationship between maternal criticism and depressive symptoms (Gibb, Uhrlass, Grassia, Benas, & McGeary, 2009).
This emerging literature has not only served to isolate putative neurobiological processes, but perhaps more importantly, has permitted us to make sense of these processes within a theoretical model such as the cognitive model. In keeping with this, Disner et al. (2011) proposed an Integrated Cognitive Neurobiological Model of Depression to summarize current knowledge on the ways in which the cognitive mechanisms underlying depressive symptoms map onto neurobiological processes. The model specifies, for example, how the activation of schemas and resulting biases in attention, processing, and memory map onto patterns of hyperactivity in the limbic system, as well as attenuation of cortical control.
Conclusions
An optimal understanding of psychopathology and its treatment depends on a multidisciplinary approach that synthesizes converging psychological and biological processes. Significant advances have been made towards identifying brain regions concordant with psychological processes (emotion regulation and learned helplessness). The identification of these concordant processes has led to outcome research examining the targeted use of new treatment approaches (e.g., DBS).
Dr. Beck discussed his ongoing work that involves expanding the cognitive model of depression to incorporate the neural correlates of negative cognitive processing biases (Beck, 2008). The Integrated Cognitive Neurobiological Model emphasizes the critical role of prefrontal inhibitory control on subcortical structures in depression. Dr. Beck’s work demonstrates how multidisciplinary research can inform existing theoretical models of psychopathology. The Integrated Cognitive Neurobiological Model (Disner et al., 2011) may serve as an important prototype for how other models of depression might proceed toward integrating biological and psychological processes. For example, the Integrated Cognitive Neurobiological Model (Disner et al., 2011) can also be modified to reflect the fact that many of the psychological and neurobiological processes at play in depression are also active in other disorders. Accordingly, Haigh and Beck’s (2011) Generic Model proposes that various disorders share common cognitive processes (e.g., negativity bias), but differ in terms of specific cognitive content. The Integrated Cognitive Model and the Generic Model can be synthesized to form a comprehensive model that effectively summarizes the six researchers’ key ideas regarding the relationship between transdiagnostic cognitive and neurobiological processes.
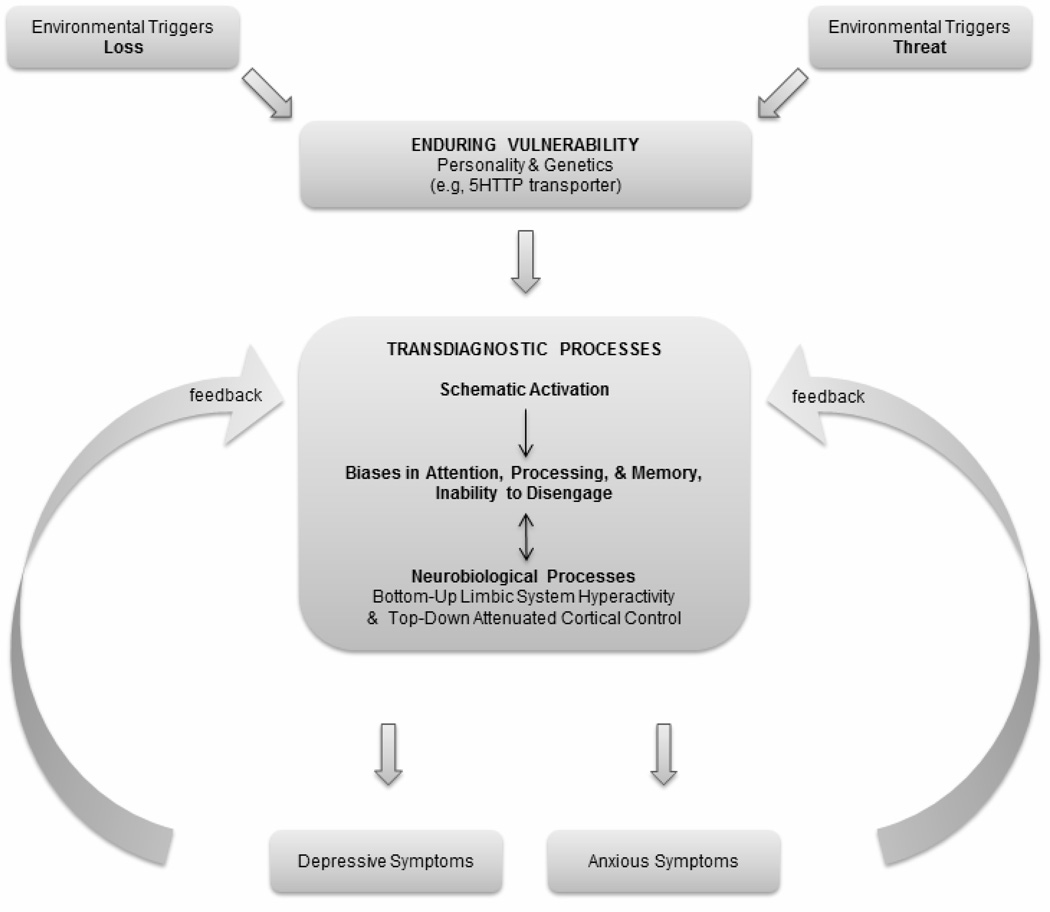
The process-based model depicted in Figure 1 proposes that the experience of environmental stressors by at-risk individuals leads to the activation of particular schemas and transdiagnostic biases in attention, processing, and memory. The schematic activation and cognitive biases may vary in content depending on the nature of the person’s vulnerability and type of environmental triggers experienced. The products of these transdiagnostic processes will play an important role in determining surface-level symptomatology. For example, individuals who selectively focus on threat-related information (as a result of particular environmental experiences, and/or as a result of a specific predisposition) will be most likely to develop an anxiety disorder. In contrast, individuals who selectively attend to information related to themes of loss or personal inadequacy will be most likely to develop a depressive disorder.
Figure 1.
The process-based model presented here integrates Disner, Beevers, Haigh, and Beck’s (2011) Integrated Cognitive Neurobiological Model of Depression with Haigh and Beck’s (2011) Generic Model. The model represents the sequence of events that leads to depressive and/or anxious symptomatology, highlighting the contribution of transdiagnostic cognitive biases in attention, processing, and memory, as well as corresponding neurobiological processes.
The process-based model highlights how particular psychopathological processes (here, biases in attention, processing, and memory) transcend current diagnostic categories. These transdiagnostic processes can be examined at different levels (behavioral, cognitive, neurobiological), which may reveal different routes for treatment. In their conversation, the six researchers agreed that targeting transdiagnostic processes may be more effective than basing treatment on current diagnostic categories. While the process-based model presented here focuses on cognitive and biological biases in attention, processing, and memory, it is possible to envision how a similar model could focus on other psychobiological transdiagnostic mechanisms such as learned helplessness or emotion regulation, two other important examples discussed earlier.
Implications of a New Psychobiological Process-based Taxonomy for Diagnosis and Treatment
In the fourth phase of the conversation, the researchers discussed the implications of creating a new biologically informed process-based taxonomy. The following questions were raised: How do we communicate a new biologically informed process-based taxonomy to patients and providers? Should we include subclinical suffering in a new taxonomy? Would a new process-based taxonomy lead to increased treatment response?
Richard Davidson
Our discussion on developing a new taxonomy based on biologically informed psychological processes might represent an opportunity to change how we view mental health and illness. For example, there is a lot of subclinical suffering that goes on in this world. A lot of the suffering has to do with our minds being out of control in ways that are not flagrant, but are sufficient to place a drag on our productivity and well-being. I think that it would be helpful if the nomenclature were reframed in a way that actually made it more accessible to people who are dealing with subclinical suffering.
Helen Mayberg
An important aspect of implementing a new taxonomy will involve how we communicate these biologically informed psychological processes to patients. What will become the new vocabulary for patients’ complaints? How do you learn that you need the “medial frontal doctor” as opposed to the “insula doctor” for your problem? We have conditioned patients to know how to complain to different people about the same thing. So people talk to a surgeon about depression in a very different way than they would to a psychiatrist. I personally do not like the term “depression” because complaining of depression is like complaining of a headache: The symptom could have all sorts of causes requiring very different treatments (e.g., brain surgery, taking an aspirin, or simply getting new glasses). So the symptom is helpful as a starting point, but the differential diagnosis is long and the work-up will require a variety of different tests to identify the underlying cause. The good news is that people now report symptoms improving the likelihood that a depression will be recognized and diagnosed by a primary caregiver. The problem is that generally results in a prescription for a medication, which often does not work. Are we doing people a disservice by treating all depressions the same way, even as a first pass? Perhaps not, if we are assured that a second treatment will be offered in a timely manner and that the risk of an inappropriate first treatment with sustained nonresponse or adverse effects is low.
Dr. Davidson raised an interesting point about subclinical suffering, which made me think about the difference between the ways we approach physical versus mental health. For example, a person might go to a physical trainer because they are out of shape but not ill. You engage someone that is an expert to help you become physically healthier. Using a trainer for physical exercise appears to be a good model for reducing subclinical physical suffering (i.e., being out of shape). This brings up what I think is the fundamental problem about what depression is, which is that our working definitions are profoundly overinclusive. From a clinical point of view, we need better ways to define subgroups so that patients who do not need medicine do not receive it, and those that need it are not stigmatized for taking them. A testable hypothesis is that by establishing biomarkers and a new nosology based on such biological processes would help to better match people with appropriate treatments (Mayberg, 2003). This can be explicitly tested by building on the well-established observation that while medication and CBT have equivalent clinical efficacy rates, a given treatment is not necessarily effective for an individual patient. By acquiring baseline measures (genetic, imaging, immune, endocrine or other potential biomarkers) and then randomizing patients to one of several evidenced based treatments, one can examine if any measures predicts recovery and/or nonresponse to a given intervention. Such findings will lay the necessary foundation to work backwards from such biomarkers to clinical symptoms that might index said biological differences. Such studies are currently ongoing.
Martin Seligman
My view of cognitive behavioral therapy generally is that with the present nosology, it has hit a 65% barrier (e.g., Kirsch et al., 2002). What factors contribute to this mediocre outcome rate? Let’s say we have this “medial frontal syndrome” or “insula syndrome” and we have advertised for these people, and they exhibit convergence of psychological symptoms and neurobiological markers, what are the chances that we are going to get better than a 65% response rate with such a discreet presenting symptom?
Consider another example. When a person suffering from schizophrenia comes into a clinic, they might suffer from both cognitive distortions and blunted affect. We would want to send them to one specialized treatment for the cognitive component, and another specialized treatment center for the affective component. So treatment would not be based on our current taxonomy, but on treating the basic processes. Similarly, we could open clinics specializing in the treatment of a “dorsal raphe syndrome” – a crucial neural process in learned helplessness (as described earlier by Dr. Maier). If two individuals, one diagnosed with schizophrenia, and one diagnosed with depression, both have similar cognitions such as “nothing I do matters,” and both have dysregulated DRN, shouldn’t we have treatments specialized for this particular process that transcends diagnostic categories?
Conclusions
Research examining the interaction between psychological and neurobiological processes is underway and suggests that significant consideration should be given to the development of a process-based taxonomy. Implementing a psychobiological process-based taxonomy represents a complex endeavor that will require extensive scrutiny. For example, how will a process-based deficit be communicated to and understood by patients? Should we implement a new process-based taxonomy one process at a time or wait until we have identified and organized at least several processes underlying each of the original diagnostic categories before proceeding? What timeframe should we expect to have identified and organized a process-based taxonomy? Would important knowledge or skills be lost by implementing a process-based taxonomy? Would an emphasis on identifying and treating putative neurobiological pathways stifle research examining other pathways? How can we ensure that we do not replicate some of the pitfalls inherent in our current classification system? The answers to these and other questions require careful deliberation and it is our hope that the present paper will serve as a springboard for these important conversations that are critical to our understanding and treatment of psychopathology.
Concluding Remarks
The complexities of implementing a new taxonomy represent a critical opportunity to incorporate new perspectives that will foster significant advances toward the understanding and treatment of mental illness. Progress toward a new taxonomy will require iterative working relationships across disciplines that will ultimately enable researchers to transcend their current understanding of psychopathology and lead to significantly improved clinical outcomes. To summarize and conclude their conversation, the six researchers offered the following remarks.
Fritz Henn
We have some understanding and see some similarities between animal models and the circuitry of depression in humans. This circuitry may mediate certain kinds of destructive behaviors, and those behaviors are amenable in general to psychotherapeutic modulation. Circuits that get out of whack may be reversed by DBS but we are not sure yet about the mechanism or the time course. It may well be that we could do a much better job of predicting success by understanding the phenotype of persons who have responded to these treatments.
Steven Maier
If one is interested in refining treatments and/or understanding the underlying neurobiology, then the DSM approach is making us look in wrong directions. The DSM may be useful for what it was designed to be, but it is not useful if you want to develop more refined tools for treatment as opposed to blunt instruments which apply to huge categories. We should rely on the equivalent of the endophenotype approach (Flint & Munafò, 2007; Gottesman & Gould, 2003) in order to understand these categories: We should look at components that might be nonspecific with regard to traditional DSM categories. It makes sense to figure out the neurobiology of these components and then to develop treatments that directly affect these components rather than developing treatments for something as heterogeneous as depression. As to what these components might be, we have been focusing on the mPFC. Although I am sure that this is not the only one, we focused on the idea that under some conditions the mPFC is unable to shut off underlying limbic and brain stem processes resulting in, for example, emotional perseveration.
Richard Davidson
To add to what Dr. Maier said, I think it will be an interesting task for us to think about the processes. We talked about two primary ones 1) emotion regulation, perseveration and negative emotion, and 2) the phenomenon of giving up. One of the things that I have been excited about is the idea of neurally-inspired behavioral interventions. Developing behavioral interventions based upon our knowledge of brain circuits can help refine those interventions in ways that are not focused on categories but are focused on processes. I think that the future of clinical psychology depends on our ability to focus on processes.
Aaron Beck
We can understand distress in a person on three different levels. One is the manifest level, which is the symptom level. The second is the intermediate cognitive-affective or psychological level. The third is the neural level. A composite of each of the three levels is what is important. Now the way symptoms have been arranged and organized according to DSM is not satisfactory. Everybody seems to be dissatisfied by only looking at symptoms because the same symptom can be caused by a variety of different causes. However, the next level down - the psychological level, which includes different biases, may actually be more fruitful because the biases themselves are actually more informative than the symptoms. Finally, I believe the biases have some representation in the various parts of the brain.
In terms of developing treatments we can think of discovering what the neural/psychological categories are, and then direct our attention to those. Or we can take a more global approach, which is that there is a dysfunction in the whole operation of the brain. Any one instrument in an orchestra can upset the functioning of the entire orchestra, and a more global rather than specific approach would get at the bad violinist and work with the orchestra itself to increase its integration. One of the reasons I believe in a global approach is that people get better just by knowing that they are going for treatment, and I do not believe that there is a specific region of the brain that doctors target when they see a patient. I think the whole brain itself starts functioning better in a warm climate just like a plant would do better in the sun. So in terms of treatment, there are going to be two aspects. One aspect is specific, which is going to be the direct beam that will go at the specific problem. The second aspect focuses on the more general problem, which is to get the brain better integrated.
Helen Mayberg
The thing that I take away from this meeting is that while we represent two different perspectives (cognitive and neurobiological), we can make the most progress if we combine the approaches. I think that we have to change the bar: We need to not just define a brain system or a cognitive process and match a patient’s brain to the most appropriate treatment, but to figure out how to optimally use different approaches synergistically. We cannot expect that a pure cognitive intervention no matter how well designed and executed can regulate all forms of depression. But equally unlikely is to expect that one can optimally treat severely and chronically ill patients with just a biological intervention, as we have learned with our studies of DBS. Patients once on the road to recovery need a rehabilitative strategy to retrain their brain in its new and now more normally responsive state. I was excited to hear about Dr. Davidson’s work with emotional reactivity and sustaining of positive affect (Heller et al., 2009). Conceptually speaking, Dr. Davidson’s work is getting at the idea that mental health is not state A or state B. It is rather about being able to move gracefully in and out of different states so when one encounters an emotional stimulus, one is able to express a full dynamic range of emotions and then move on. Not moving on, not being able to disengage, appears to be a central problem.
Martin Seligman
Psychopathology as we know it has asymptoted both clinically and scientifically. The DSM (and the science that follows from it) has done what it is ever going to do because it does not cut nature to the joints. What will replace it or what will come next? I think it is the convergence of biological and psychological processes, which is as close to what I think carving nature at the joints might be. To the extent that we can identify biologically-informed psychological processes and map them onto treatments, we can develop a better nosology based on a more accurate scientific understanding and ultimately a much higher hit rate with treatment. In this conversation, we have identified two possible candidates for biologically-informed psychological processes: 1) emotion dysregulation - the inability to dampen emotions, and 2) and giving up. If we can show that the phenomenology and the psychological processes converge with the circuitry and there is an effective treatment for that pathology, then I think that the future becomes clear.
Contributor Information
Marie J. C. Forgeard, Department of Psychology, University of Pennsylvania
Emily A. P. Haigh, Department of Psychiatry, University of Pennsylvania
Aaron T. Beck, Department of Psychiatry, University of Pennsylvania
Richard J. Davidson, Departments of Psychology and Psychiatry, University of Wisconsin-Madison
Fritz A. Henn, Department of Neuroscience, Cold Spring Harbor Laboratory
Steven F. Maier, Department of Psychology and Neuroscience, University of Colorado at Boulder
Helen S. Mayberg, Department of Psychiatry, Emory University
Martin E. P. Seligman, Department of Psychology, University of Pennsylvania
References
- Abou Jamra R, Schmael C, Cichon S, Rietschel M, Schumacher J, Nothen MM. The G72/G30 gene locus in psychiatric disorders: A challenge to diagnostic boundaries? Schizophrenia Bulletin. 2006;32:599–608. doi: 10.1093/schbul/sbl028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson LY, Seligman MEP, Teasdale JD. Learned helplessness in people: Critique and reformulation. Journal of Abnormal Psychology. 1978;87:49–74. [PubMed] [Google Scholar]
- Adrien J, Dugovic C, Martin P. Sleep-wakefulness patterns in the helpless rat. Physiology & Behavior. 1991;49:257–262. doi: 10.1016/0031-9384(91)90041-l. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Peterson C, Abramson LY, Seligman MEP. Attributional style and generality of learned helplessness. Journal of Personality and Social Psychology. 1984;46:681–687. doi: 10.1037//0022-3514.46.3.681. [DOI] [PubMed] [Google Scholar]
- Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–1038. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and DRN. Nature Neuroscience. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of DRN serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Research. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision) American Journal of Psychiatry. 2000a;157:1–45. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000b. [Google Scholar]
- Anisman H, Matheson K. Stress, depression and anhedonia: Caveats concerning animal models. Neuroscience and Biobehavioral Reviews. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. European Journal of Neuroscience. 2009;30:1111–1116. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165:969. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beevers C, Clasen P, Stice E, Schnyer D. Depression symptoms and cognitive control of emotion cues: A functional magnetic resonance imaging study. Neuroscience. 2010;167:97–103. doi: 10.1016/j.neuroscience.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, Maier SF. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 2007;144:1219–1228. doi: 10.1016/j.neuroscience.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biological Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning. Science. 1999;283:1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Human functional connectivity: New tools, unresolved questions. Proceedings of the National Academy of Sciences. 2010;107:10769–10770. doi: 10.1073/pnas.1005987107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavello PR, Egan RJ, Bergner CL, Hart PC, Cachat JM, Kalueff AV. Genetic animal models of depression. Chapter 10. In: Kalueff AV, Bergner LC, editors. Transgenic and mutant tools to model brain disorders, Neuromethods. Vol. 44. New York, NY: Humana Press; 2010. [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A GABAA, and glutamate receptors. Journal of Neuroscience. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Beck AT. Cognitive theory and therapy of anxiety and depression: A convergence with neurobiological findings. Trends in Cognitive Science. doi: 10.1016/j.tics.2010.06.007. (in press) [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Alford BA. Scientific foundations of cognitive theory of depression. New York, NY: Wiley & Sons; 1999. [Google Scholar]
- Corlett PR, Murray GK, Honey GD, Aitken MR, Shanks DR, Robbins TW, Fletcher PC. Disrupted prediction-error signal in psychosis: Evidence for an associative account of delusions. Brain. 2007;130:2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: Recent developments. Current Opinion in Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Deakin JFW, Lees J, McKie S, Hallak JEC, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: A pharmaco-Magnetic Resonance Imaging study. Archives of General Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, Beck AT. Towards an integrated cognitive neurobiological model of depression. Philadelphia, Pennsylvania: University of Pennsylvania; 2011. Manuscript under review. [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Ryan SM, Minor TR, Maier SF. Librium prevents the analgesia and shuttlebox escape deficit typically observed following inescapable shock. Pharmacology, Biochemistry and Behavior. 1984;21:749–754. doi: 10.1016/s0091-3057(84)80014-3. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Hackmann A, Michael T. Intrusive re-experiencing in post-traumatic stress disorder: Phenomenology, theory, and therapy. Memory. 2004;12:403–415. doi: 10.1080/09658210444000025. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood congruent processing biases in depression. Archives of General Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic inhibition and social anxiety disorder in adolescence. American Journal of Psychiatry. 2010;167:40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M. Diagnosis and definition of treatment-resistant depression. Biological Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychological Medicine. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E, Zinberg RE, Olasoo-Rothbaum B. Uncontrollability and impredictability in PTSD: An animal model. Psychological Bulletin. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children's attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child & Adolescent Psychology. 2009;38:415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb B, Uhrlass D, Grassia M, Benas J, McGeary J. Children’s inferential styles, 5-HTTLPR genotype, and maternal expressed emotion-criticism: An integrated model for the intergenerational transmission of depression. Journal of Abnormal Psychology. 2009;118:734–745. doi: 10.1037/a0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, MacLachlan A, Katz A. Biases in visual attention in depressed and nondepressed individuals. Cognition and Emotion. 1988;2:185–200. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Hammack SE, Will MJ, O’Connor KA, Deak T, Sparks PD, Maier SF. Blockade of alpha-1 adrenoreceptors in the DRN prevents enhanced conditioned fear and impaired escape performance following uncontrollable stressor exposure in rats. Behavior and Brain Research. 2002;134:387–392. doi: 10.1016/s0166-4328(02)00061-x. [DOI] [PubMed] [Google Scholar]
- Grant PM, Beck AT. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophrenia Bulletin. 2009;35:798–806. doi: 10.1093/schbul/sbn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh EAP, Beck AT. The Generic Cognitive Model. Philadelphia, Pennsylvania: University of Pennsylvania; Manuscript in preparation. [Google Scholar]
- Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Hannum RD, Rosellini RA, Seligman MEP. Retention of learned helplessness and immunization in the rat from weaning to adulthood. Developmental Psychology. 1976;12:449–454. [Google Scholar]
- Harvey AG, Watkins E, Mansell W, Shafran R. Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn FA, Edwards E, Anderson D, Vollmayr B. Psychotherapy and antidepressant treatment of depression: Evidence for similar neurobiological mechanisms. World Psychiatry. 2002;1:115–117. [PMC free article] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B. Stress models of depression: Forming genetically vulnerable strains. Neuroscience & Biobehavioral Reviews. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Can neuroscience be integrated into the DSM-V? Nature Reviews Neuroscience. 2007;8:725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn, Wang P. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat DRN: Ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. Journal of Comparative Neurology. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, Shea T. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5 year prospective follow-up of 431 subjects. Archives of General Psychiatry. 1992;49:809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Moore TJ, Scoboria A, Nicholls SS. The emperor’s new drugs: An analysis of antidepressant medication data submitted to the U.S. Food and Drug Administration. Prevention and Treatment. 2002 July 15; Retrieved from http://psycnet.apa.org/journals/pre/5/1/23a.html. [Google Scholar]
- Klein DC, Fencil-Morse E, Seligman MEP. Learned helplessness, depression, and the attribution of failure. Journal of Personality and Social Psychology. 1976;33:508–516. doi: 10.1037//0022-3514.33.5.508. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. In: Robertson GM, Livingstone S, editors. Textbook of Psychiatry. Chicago, IL: Medical Book Company; 1919. [Google Scholar]
- Larson CL, Nitschke JB, Davidson RJ. Common and distinct patterns of affective responses in dimensions of anxiety and depression. Emotion. 2007;7:182–191. doi: 10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx C, Schultz D, Malinow R. Synaptic potentiation onto the lateral habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln A. To John T. Stuart. In: Basler RP, Pratt MD, Dunlap LA, editors. The Collected Works of Abraham Lincoln. Volume 1. New Brunswick, NJ: Rutgers University Press; (1841/1953). [Google Scholar]
- Lowery AJ, Miller N, McNeil RE, Kerin MJ. MicroRNAs as prognostic indicators and therapeutic targets: Potential effect on breast cancer management. Clinical Cancer Research. 2008;14:360–365. doi: 10.1158/1078-0432.CCR-07-0992. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biological Psychiatry. 2001;49:763–773. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins P. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues in Clinical Neuroscience. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Busch C, Maswood C, Grahn RE, Watkins LR. The DRN is a site of action mediating the behavioral effects of the benzodiazepine receptor inverse agonist DMCM. Behavioral Neuroscience. 1995;106:759–766. doi: 10.1037//0735-7044.109.4.759. [DOI] [PubMed] [Google Scholar]
- Maier SF, Nguyen KT, Deak T, Milligan ED, Watkins LR. Stress, learned helplessness, and brain interleukin-1. In: Dantzer R, Wollmann BB, Yirmiya R, editors. Cytokines, stress, and depression. New York, NY: Plenum; 1999. pp. 235–250. [Google Scholar]
- Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behavioral Neuroscience. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman ME. Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General. 1976;105:3–46. [Google Scholar]
- Maier SF, Seligman MEP, Solomon RL. Pavlovian fear conditioning and learned helplessness. In: Campbell BA, Church RM, editors. Punishment. New York, NY: Appleton-Century-Crofts; 1969. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability, anxiety, and serotonin. Cognitive Therapy and Research. 1998;6:595–613. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and Biobehavioral Reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Mansell W, Harvey A, Watkins ER, Shafran R. Cognitive behavioral processes across psychological disorders: A review of the utility and validity of the transdiagnostic approach. International Journal of Cognitive Therapy. 2008;1:181–191. [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Depression: A neuropsychiatric perspective. In: Panksepp J, editor. Textbook of biological psychiatry. Hoboken, NJ: Wiley; 2004. pp. 197–229. [Google Scholar]
- Mayberg HS. Defining neurocircuits in depression. Psychiatric Annals. 2006;36:259–268. [Google Scholar]
- Mayberg HS. Defining the neural circuitry of depression: Toward a new nosology with therapeutic implications. Biological Psychiatry. 2007;61:729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. The Journal of Clinical Investigation. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Minassian A, Granholm E, Verney S, Perry W. Visual scanning deficits in schizophrenia and their relationship to executive functioning impairment. Schizophrenia Research. 2004;74:69–79. doi: 10.1016/j.schres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- Moye TB, Hyson RL, Grau JV, Maier SF. Immunization of opioid analgesia: Effects of prior escapable shock on subsequent shock-induced and morphine-induced antinociception. Learning and Motivation. 1983;14:238–251. [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nature Neuroscience. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Overmier JB. On learned helplessness. Integrated Physiological and Behavioral Science. 2002;37:4–8. doi: 10.1007/BF02688801. [DOI] [PubMed] [Google Scholar]
- Overmier B, Seligman MEP. Effects of inescapable shocks upon subsequent escape and avoidance learning. Journal of Comparative and Physiological Psychology. 1967;63:28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Peterson C, Maier SR, Seligman MEP. Learned helplessness: A theory for the age of personal control. New York, NY: Oxford University Press; 1995. [Google Scholar]
- Petty F, Davis LL, Dabel D, Kramer GL. Serotonin dysfunction disorders: A behavioral neurochemistry perspective. Journal of Clinical Psychiatry. 1996;57:11–16. [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant E, Verchinski BA, Munoz KE, Kolachana BS, Weinberger D. F-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Read J, Bentall R. Schizophrenia and childhood adversity. American Journal of Psychiatry. 2010;167:717–178. doi: 10.1176/appi.ajp.2010.10010105. [DOI] [PubMed] [Google Scholar]
- Rector NA, Beck AT. Cognitive-behavioral therapy for schizophrenia: An empirical review. Journal of Nervous and Mental Disease. 2001;189:278–287. doi: 10.1097/00005053-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Rector NA, Beck AT, Stolar N. The negative symptoms of schizophrenia: A cognitive perspective. Canadian Journal of Psychiatry. 2005;50:247–257. doi: 10.1177/070674370505000503. [DOI] [PubMed] [Google Scholar]
- Regier DA, Narrow WE, Kuhl EA, Kupfer DJ. The conceptual development of DSM-V. American Journal of Psychiatry. 2009;166:645–650. doi: 10.1176/appi.ajp.2009.09020279. [DOI] [PubMed] [Google Scholar]
- Reilly JG, McTavish SF, Young AH. Rapid depletion of plasma tryptophan: A review of studies and experimental methodology. Journal of Psychopharmacology. 1997;11:381–392. doi: 10.1177/026988119701100416. [DOI] [PubMed] [Google Scholar]
- Riedel S. Edward Jenner and the history of smallpox and vaccination. Proceedings (Baylor University Medical Center) 2005;18:21–25. doi: 10.1080/08998280.2005.11928028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Levy J, Fromm SJ, Nugent AC, Talagala SL, Hasler G, Drevets WC. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biological Psychiatry. 2009;66:441–450. doi: 10.1016/j.biopsych.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosellini RA, Seligman MEP. Failure to escape shock after repeated exposure to inescapable shock. Bulletin of the Psychonomic Society. 1976;7:251–253. [Google Scholar]
- Roy-Byrne PP, Weingartner H, Bierer LM, Thompson K, Post RM. Effortful and automatic cognitive processes in depression. Archives of General Psychiatry. 1986;43:265–267. doi: 10.1001/archpsyc.1986.01800030083008. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biological Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Schmitt P, Sandner G, Colpaert FC, De Witte P. Effects of dorsal raphe stimulation on escape induced by medial hypothalamic or central gray stimulation. Behavior and Brain Research. 1983;8:289–307. doi: 10.1016/0166-4328(83)90175-4. [DOI] [PubMed] [Google Scholar]
- Schulz D, Mirrione MM, Henn FA. Cognitive aspects of congenital learned helplessness and its reversal by the monoamine oxidase (MAO)-B inhibitor deprenyl. Neurobiology of Learning and Memory. 2010;93:291–301. doi: 10.1016/j.nlm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Ohlraun S, Czerski PM, Schumacher J, Kassem L, Deschner M, Rietschel M. Genotype-phenotype studies in bipolar disorder showing association between the DAOA/G30 locus and persecutory delusions: A first step toward a molecular genetic classification of psychiatric phenotypes. American Journal of Psychiatry. 2005;162:2101–2108. doi: 10.1176/appi.ajp.162.11.2101. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Abou Jamra R, Becker T, Klopp N, Franke P, Jacob C, Nöthen MM. Investigation of the DAOA/G30 locus in panic disorder. Molecular Psychiatry. 2005;10:428–429. doi: 10.1038/sj.mp.4001598. [DOI] [PubMed] [Google Scholar]
- Seligman MEP. Learned optimism. New York, NY: Knopf; 1991. [Google Scholar]
- Seligman MEP, Groves D. Non-transient learned helplessness. Psychonomic Science. 1970;19:191–192. [Google Scholar]
- Seligman ME, Maier SF. Failure to escape traumatic shock. Journal of Experimental Psychology. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Shestyuk AY, Deldin PJ, Brand JE, Deveney CM. Reduced sustained brain activity during processing of positive emotional stimuli in major depression. Biological Psychiatry. 2005;57:1089–1096. doi: 10.1016/j.biopsych.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Research. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Tost H, Ruf M, Schmal C, Schulze TG, Knorr C, Vollmert C, Rietschel M. Prefrontal-temporal gray matter deficits in bipolar disorder patients with persecutory delusions. Journal of Affective Disorders. 2010;120:54–61. doi: 10.1016/j.jad.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely couples during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Bachteler D, Vengeliene V, Gass P, Spanagel R, Henn FA. Rats with congenital learned helplessness respond less to sucrose but show no deficits in activity or learning. Behavioural Brain Research. 2004;150:217–221. doi: 10.1016/S0166-4328(03)00259-6. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Faust H, Lewicka S, Henn FA. Brain-derived neurotrophic factor (BDNF) stress response in rats bred for learned helplessness. Molecular Psychiatry. 2001;6:471–474. doi: 10.1038/sj.mp.4000907. [DOI] [PubMed] [Google Scholar]
- Widiger TA. A dimensional model of psychopathology. Psychopathology. 2005;38:211–214. doi: 10.1159/000086094. [DOI] [PubMed] [Google Scholar]
- Williams JL, Maier SF. Transituational immunization and therapy of learned helplessness in the rat. Journal of Experimental Psychology: Animal Behavior Proceedings. 1977;3:240–253. [Google Scholar]
- Woodmansee WW, Silbert LH, Maier SF. Factors that modulate inescapable shock-induced reductions in daily activity in the rat. Pharmacology, Biochemistry and Behavior. 1993;45:553–559. doi: 10.1016/0091-3057(93)90505-n. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global burden of disease: 2004 update. 2008 Retrieved from http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf.
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]