Abstract
Rationale
Dronabinol (Δ9tetrahydrocannabinol) is approved for HIV-related anorexia, yet little is known about its effects in HIV-positive marijuana smokers. HIV-negative marijuana smokers require higher than recommended dronabinol doses to experience expected effects.
Objectives
Employing a within-subjects, double-blind, placebo-controlled design, we assessed the effects of repeated high-dose dronabinol in HIV-positive marijuana smokers taking antiretroviral medication.
Methods
Participants (N = 7), who smoked marijuana 4.2 ± 2.3 days/week, resided in a residential laboratory for two 16-day stays, receiving dronabinol (10 mg QID) in one stay and placebo in the other. Efficacy was assessed with objectively-verified food intake and body weight. Tolerability was measured with sleep, subjective, and cognitive assessments. For analyses, each inpatient stay was divided into two phases, Days 1–8 and 9–16; we compared dronabinol’s effects with placebo in each 8-day phase to investigate tolerance.
Results
Despite sustained increases in self-reported food cravings, dronabinol only increased caloric intake in the initial eight days of dosing. Similarly, sleep quality was improved only in the first 8 days of dosing. Dronabinol’s mood-enhancing effects were sustained across the 16-day inpatient stay. Dronabinol was well tolerated, causing few negative subjective or cognitive effects.
Conclusions
In HIV-positive marijuana smokers, high dronabinol doses safely and effectively increased caloric intake. However, repeated high-dose dronabinol appeared to result in selective tolerance to these effects. These findings indicate that HIV-positive individuals who smoke marijuana may require higher dronabinol doses than are recommended by the FDA. Future research to establish optimal dosing regimens, and reduce the development of tolerance, is required.
Keywords: HIV, dronabinol, THC, appetite stimulation, anorexia
INTRODUCTION
Although antiretroviral treatments have reduced the prevalence of anorexia and weight loss in individuals with HIV/AIDS (Abrams 2000), many patients continue to struggle to maintain weight (Wanke et al. 2000). The orally-administered cannabinoid agonist, dronabinol (Marinol®), is FDA-approved for HIV/AIDS-related anorexia. Dronabinol consists of synthetic Δ9tetrahydrocannabinol (THC), marijuana’s main psychoactive constituent. Dronabinol and smoked cannabis are both used medically to increase appetite; they may also reduce gastrointestinal side effects of antiretroviral treatments (Chubineh and McGowan 2008).
There has been substantial debate about medicinal cannabinoid use (Abrams 1998; Grinspoon and Bakalar 1997; Hall and Degenhardt 2003; Martin 2002), including the benefits of dronabinol versus smoked marijuana. It has been argued that smoked marijuana is more effective because its quick onset and short effect duration allow for better titration of therapeutic effects (Abrams 2000; Abrams et al. 2003). Further, patients experiencing nausea may find smoked THC preferable to oral medications such as dronabinol (Institute of Medicine 1999). Smoked marijuana also, however, has drawbacks: it is associated with substantial pulmonary risk (Ashton 2001; Mehra et al. 2006; Taylor et al. 2000; Taylor et al. 2002), its illegal status in most countries leads to difficulties distributing marijuana to patients (Hall and Degenhardt 2003), and precise THC doses are difficult to deliver via smoked marijuana (Institute of Medicine 1999). Although vaporizing devices may avoid the respiratory risks of smoked marijuana delivery (Hazekamp et al. 2006), legal difficulties and issues related to precise dosing remain.
Notably, the medical marijuana debate has occurred in the context of limited evidence about the relative efficacy of medical cannabinoids in people with HIV/AIDS (Haney et al. 2005; Hollister 2000). Early clinical trials, prior to widespread antiretroviral use, indicated that although low doses of dronabinol (2.5 mg daily to 5 mg BID) increased self-reported appetite relative to placebo in patients with HIV or AIDS (Beal et al. 1995; Beal et al. 1997), neither caloric intake (Struwe et al. 1993) nor weight (Beal et al. 1995; Struwe et al. 1993) increases reached significance. More recently, a clinical trial of the effects of dronabinol (2.5 mg TID) and smoked marijuana (3.95% THC cigarette TID) in HIV-positive participants found that both drugs increased body weight over the 21-day study (Abrams et al. 2003).
A limitation of these studies is that they excluded regular marijuana smokers. There is a high incidence of marijuana use among HIV-positive people, with one quarter (Prentiss et al. 2004) to one third (Dansak 1997; Furler et al. 2004; Ware et al. 2003; Woolridge et al. 2005) of HIV/AIDS patients estimated to be current marijuana users, many reporting using the drug for medicinal purposes. The absence of this group in cannabinoid trials may have important implications. In HIV-negative regular marijuana smokers, dronabinol doses substantially above those recommended by the FDA are necessary for clinical effect due to cannabinoid tolerance (Haney et al. 1999a; Hart et al. 2002a; Hart et al. 2002b; Kirk and de Wit 1999). For instance, whereas the initial recommended dose of dronabinol is 2.5 mg (BID) and the maximal recommended daily dose is 20 mg, daily doses of at least 80 mg are well-tolerated in HIV-negative, regular marijuana smokers (Haney et al. 1999a; Hart et al. 2002b). Thus, efficacy and tolerability data for medical cannabinoids obtained in non-marijuana-using HIV/AIDS patients may not generalize to the sizable proportion of people with HIV who already use marijuana.
To address this limitation, we compared effects of dronabinol and smoked marijuana in HIV-positive marijuana smokers (Haney et al. 2007; Haney et al. 2005). As expected, acute doses of up to 20 mg dronabinol were well tolerated. Individuals with clinically significant muscle mass loss demonstrated comparable increases in caloric intake after marijuana (1.8%, 3.9% THC) and dronabinol (10, 20 mg), with few negative effects (Haney et al. 2005). An inpatient study in HIV-positive participants showed that repeated dosing of dronabinol (5, 10 mg QID) and marijuana (2.0%, 3.9% THC QID) dose-dependently increased caloric intake and weight over four days. Effects of oral and smoked THC dosing were, again, similar (Haney et al. 2007). Thus, both smoked marijuana and high dronabinol doses appear to be efficacious and well-tolerated over short periods in HIV-positive marijuana smokers. What remains unclear is the efficacy and tolerability of longer-term cannabinoid administration in HIV-positive participants. Repeated dronabinol administration (20, 30 mg QID) results in attenuation of peak ratings of ‘high’ and ‘good drug effect’ over 3–4 days in HIV-negative individuals, without any change in the enhancement of food intake (Haney et al. 1999a), suggesting that tolerance selectively develops to dronabinol’s intoxicating, but not appetite increasing, effects. Such selective tolerance could have important, and perhaps desirable, implications for medical use.
Thus, the objective of this study was to assess the efficacy of high-dose dronabinol (10 mg QID; twice the maximal recommended daily dose), compared to placebo, over 16 days on food intake and weight in HIV-positive marijuana smokers on antiretroviral medication. Tolerability of repeated dosing was investigated by measuring subjective effects, sleep, social behavior, and cognition. To assess tolerance, we divided the 16-day stays into two phases, Days 1–8 and 9–16, and compared effects of dronabinol with placebo in each phase separately. Based on our studies and an early clinical trial indicating ongoing appetite improvement for up to 12 months (Beal et al. 1997), we hypothesized that dronabinol’s effects on food intake and weight would persist, while tolerance would develop to dronabinol’s mood effects.
METHODS AND MATERIALS
Participants
HIV-positive individuals were recruited with advertisements. Participants were required to 1) smoke marijuana at least twice/week, 2) be between the ages of 21 and 50, 3) be in stable physical and psychiatric health, 4) use at least two antiretroviral medications under physician supervision, and 5) indicate that they were not seeking treatment for marijuana use. Candidates were excluded based on 1) substance dependence except nicotine or marijuana (DSM-IV criteria; American Psychiatric Association 1994), 2) major Axis 1 psychiatric disorder (DSM-IV criteria) 3) use of steroids in the prior six weeks, 4) nutritional malabsorption or dementia, and 5) abnormal medical findings. Screening included medical and psychiatric examination, electrocardiogram, blood and urine toxicology, and clinical interview. Participants provided written informed consent. They were fully debriefed and compensated at study completion. The New York State Psychiatric Institute’s (NYSPI) Institutional Review Board approved all procedures, which were in accordance with the Helsinki Declaration of 1964, as revised in 2008.
Experimental Protocol
A within-subjects, double-blind design was used. The study consisted of two training sessions (3–4 hours/session) and one outpatient predosing session followed by two 16-day inpatient stays in a residential laboratory. Inpatient stays were separated by a 5-to-15 day outpatient phase for study medication clearance. In training, participants practiced laboratory procedures and assessments. In predosing, participants sampled dronabinol (5 mg) under medical supervision. Dronabinol was administered in one inpatient stay and placebo in the other; dose order was counterbalanced. During dronabinol treatment, participants received dronabinol (20 mg; 5 mg QID) for two days followed by 14 days of dronabinol (40 mg; 10 mg QID). During placebo (PBO) treatment, they received PBO capsules (QID) for 16 days.
Dronabinol/PBO (0, 5, 10 mg; Unimed Pharmaceuticals, Buffalo Grove, IL) was encapsulated by the NYSPI Pharmacy in size 00 capsules with lactose filler. The selected dose (10 mg QID) is eight times the recommended starting dose for appetite stimulation (2.5 mg BID) and double the maximal recommended dose. This dose was selected because smaller doses have little effect in marijuana smokers (Haney et al. 2005) who require substantially larger doses to show alterations in food intake (Haney et al. 2003; Haney et al. 2007).
Residential Laboratory
Participants completed inpatient phases in groups of 3 and 4 in the NYSPI Substance Use Research Center residential laboratory. The laboratory, designed to allow continuous behavioral observation, has four single participant rooms, a shared recreational area, two bathrooms, and two vestibules for supply exchanges (Haney et al. 1999a). Participants were observed continuously, except in the bathroom or private dressing areas, through video and audio monitors. Staff communicated with participants via a networked computer system linking private computers with the adjacent control room. Participants were not able to use telephones, the internet, television, or receive visitors while inpatient.
Daily schedule
Table 1 presents the schedule. Participants woke at 8:30AM. They completed the first subjective effects measures, were weighed, received a daily food box and were allowed to eat breakfast (Haney et al. 1999a). All participants received the same food box items, comprising a variety of meals, beverages, and snack foods. The food items, reflecting over 10 years experience conducting residential laboratory studies, were selected to provide a wide choice across individuals. Participants were provided with a list of the items available in the residential laboratory as part of the informed consent process. Participants received capsules at 9:00AM, 1:00PM, 5:00PM and 9:00PM. Between 9:15AM and 5:00PM, they completed repeated subjective effects and cognitive batteries, with a lunch/recreation break between 1:15PM and 2:00PM. The evening recreation period, when participants could watch films, play video games, socialize, or be alone, went from 5:00PM to11:30PM. Participants completed subjective effects measures before bed. Food boxes were returned prior to lights out. To minimize nicotine withdrawal (Parrott et al. 1996), participants smoked cigarettes ad libitum.
Table 1.
Residential Laboratory Daily Schedule
| Time | Event | Time | Event |
|---|---|---|---|
| 8:30AM | Wake | 2:15PM | Cognitive task battery |
| VAS, HSQ | 2:45PM | VAS, HSQ, DEQ | |
| Sleep Questionnaire | 3:00PM | Cognitive task battery | |
| Weigh-in | 3:30PM | VAS, HSQ | |
| Receive food box | 3:45PM | Cognitive task battery | |
| 9:00AM | Capsule 1 | 4:15PM | VAS, HSQ, FDQ |
| 9:15AM | Cognitive task battery | 4:30PM | End work period |
| 9:45AM | VAS, HSQ | 5:00PM | Capsule 3 |
| 10:15AM | Cognitive task battery | Evening recreation begins | |
| 10:45AM | VAS, HSQ, DEQ | 6:15PM | Film 1a |
| 11:00AM | Cognitive task battery | 6:45PM | DEQ |
| 11:30AM | VAS, HSQ | 7:30PM | VAS, HSQ |
| 11:45AM | Cognitive task battery | 9:00PM | Capsule 4 |
| 12:15PM | VAS, HSQ | 9:15PM | Film 2a |
| 12:30PM | Cognitive task battery | 10:45PM | DEQ |
| 1:00PM | Capsule 2 | 11:30PM | Evening recreation ends |
| VAS, HSQ | VAS, HSQ | ||
| 1:15PM | Lunch recreation begins | Food boxes collected | |
| 2:00PM | Lunch recreation ends | 12:00AM | Lights out |
VAS = Visual Analogue Subjective Effects Battery.
HSQ = Hunger-Satiety Questionnaire.
FDQ = Food Desirability Questionnaire.
DEQ = Drug Effects Questionnaire.
Optional.
Assessment Measures
Food Intake
Food consumption during waking hours was unrestricted. The food box was supplemented with frozen meals or extra items as required. Participants reported items eaten by scanning custom-designed bar codes that recorded time, calories, and macronutrient content for individual items. Self-reports were confirmed by observation, and daily examination of trash.
Body Weight
Participants were weighed each morning before breakfast.
Subjective Hunger and Satiety
The Hunger-Satiety Questionnaire (HSQ) is a 6-item Visual Analogue instrument that assesses self-rated hunger, fullness, nausea, thirst, and dry mouth. This scale has been used in prior studies of this type (Haney et al. 2007; Haney et al. 2005). Outcome measures were maximal daily ratings.
Food Cravings
Desire for particular food types was assessed with the Food Desirability Questionnaire (FDQ; Evans et al. 1999), a self-report instrument listing 38 food types; participants use a 5-point scale, ranging from 0 (‘not at all’) to 4 (‘extremely’), to rate the extent to which they would like each food type. FDQ items are grouped into six subscores: 1) savory carbohydrates/fats e.g. French fries, 2) sweet carbohydrates/fat e.g. cookies; 3) protein/fats e.g. cheese; 4) carbohydrates alone e.g. bread; 5) beverages; and 6) alcohol. We have previously used this measure to assess drug effects (Evans et al. 1999). Outcome measures were daily ratings.
Subjective Mood and Drug Effect
Assessments of self-reported mood and drug effects consisted of Visual Analogue Scales (VAS; Folstein and Luria 1973) and a Drug Effects Questionnaire (DEQ; Johanson and Uhlenhuth 1980). The VAS included 44 mood (‘mellow’), physical symptom (‘chills’) and drug effect (‘high’) descriptors; participants rated the extent to which each descriptor applied to them at that time. Based on previous cluster analyses (Haney et al. 2007), we employed arithmetic means of item scores to produce six subscales: Negative Affect (example item: ‘miserable’); Anxiety (‘on edge’); Somatic Symptoms (‘upset stomach’); Sedation (‘tired’); Positive Affect (‘content’); and Drug High (‘high’). The DEQ consisted of 5 items assessing the extent to which participants: felt a good drug effect; felt a bad effect; liked the effect; felt a strong effect; and would take the drug again. We have employed these questionnaires in previous studies of cannabinoids (Haney et al. 2003; Hart et al. 2002a; Hart et al. 2002b). Outcome measures for both the VAS and the DEQ were maximal daily ratings.
Sleep
Objective sleep quality was measured using a Nightcap sleep monitor (Respironics, Atlanta, GA), consisting of a portable amplifier attached to two leads, one attached to the forehead to measure body movement (participants were instructed to remain in bed overnight) and the second attached to eyelids to measure eye movement. Outcome measures included sleep latency, number of awakenings, and sleep efficiency (proportion of total bed time in Rapid Eye Movement [REM] and non-REM [NREM] sleep). Subjective sleep quality was measured with 7 Visual Analogue Scales assessing perceived quality of sleep that were completed every morning after waking. These measures are sensitive to the effects of dronabinol (Haney et al. 2007).
Cognitive Function
A computerized task battery measured basic and higher-level neuropsychological functions including psychomotor function, simple and divided attention, information processing, and verbal and working memory. The battery included a 3-minute Digit-Symbol Substitution Task, a 10-minute Divided Attention Task, a 10-minute Rapid Information Processing Task, a 3-minute Rapid Acquisition Task, and a 2-minute immediate and delayed Digit Recall task (Haney et al. 2007). We have employed this battery in previous drug studies (e.g. Haney et al. 2007; Hart et al. 2002b). Outcome measures were maximal scores per day.
Social Behavior
Social behavior was recorded during evening recreation hours as previously described (Haney et al. 2008; Haney et al. 2004; Haney et al. 1999a). Briefly, a computerized observation program prompted recording of each participant’s behavior every 2.5 minutes. Behaviors were classified into private (time spent in bathroom/bedroom) and social (time spent in the recreational area). Time spent in the social area was further divided into time spent talking and time spent in silence. Outcomes were total minutes spent engaging in each behavior per day.
Data Analyses
Within-subjects Analyses of Variance (ANOVAs) followed by planned comparisons assessed effects of drug and study day on food intake, hunger, food cravings, self-rated mood and drug effect, sleep, cognitive function, and social behavior. We tested two planned complex comparisons for each analysis: 1) Days 1–8 dronabinol versus Days 1–8 PBO; and 2) Days 9–16 dronabinol versus Days 9–16 PBO (Sheskin 2000). This a priori approach allowed investigation of the development of tolerance, while limiting the number of analyses. We chose phase lengths of 8 days because previous studies have suggested that tolerance to dronabinol’s effects of on caloric intake does not occur over shorter periods in healthy cannabis smokers (e.g. 3 days (Hart et al. 2002b) and 6 days (Haney et al. 2004)). We conservatively employed a significance threshold of p<0.01. Subjective and cognitive data comprised maximal ratings or scores each day. For body weight analyses, data were change scores (Days 8 – Day 1; Day 16 – Day 9). Analyses were undertaken with SPSS 16.0 and Microsoft Excel 2007.
RESULTS
Participants (N = 7) were male. They had been diagnosed HIV-positive 8.7 (± 6.4) years prior to participation and their most recent CD4 count was 510 (± 291). Further demographic and drug-use information is provided in Table 2.
Table 2.
Demographics and Drug-Use Details
| Mean | S.E.M | Range | |
|---|---|---|---|
| Age | 36.6 | 1.3 | 29.0–39.0 |
| Weight (kgs) | 81.4 | 4.9 | 65.9–102.3 |
| MJ | 4.2 | 0.9 | 2.0–7.0 |
| Days/Week | |||
| MJ | 4.8 | 1.8 | 1.0–10.5 |
| Joints/Daya | |||
| Alcohol Days/Weekb | 1.1 | 0.4 | 0.25–3.0 |
| Alcohol | 5.6 | 1.5 | 1.5–10.0 |
| Drinks/Occasionb | |||
| Cigarettes/Dayc | 15.1 | 4.9 | 0.3–20.0 |
| Participant | Race | Education | HIV Medications |
Cocaine Use? |
Heroin Use? |
Other Drug Use? |
|---|---|---|---|---|---|---|
| 1 | AA | HS, some college |
lopinavir ritonavir azithromycin sulfamethoxazole abacavir |
Occasional | No | No |
| 2 | Caucasian | HS, some college |
tenofovir ritonavir didanosine atazanavir |
No | Occasional | LSD, Ecstasy |
| 3 | Mixed | Technical schooling |
tenofovir lamivudine efavirenz |
No | No | No |
| 4 | Mixed | B.A. | nevirapine lamivudine zidovudine gemfibrozil |
No | No | No |
| 5 | AA | 8th grade | tenofovir lamivudine efavirenz |
Occasional | No | LSD |
| 6 | AA | HS, some college |
sulfamethoxazole ritonavir didanosine atazanavir loperamide tenofovir emtricitabine |
No | No | No |
| 7 | AA | 10th grade | nevirapine lamivudine zidovudine |
No | No | No |
Estimated based on a rate of 3 marijuana joints per marijuana blunt; N = 6 because 1 participant reported smoking 1.5 bowls of unknown size per day.
N = 6 who reported past month alcohol consumption.
N = 4 who reported past month cigarette smoking.
S.E.M = Standard Error of the Mean; kgs = kilograms; MJ = marijuana; AA = African American; HS = completed high school; B.A. = completed Bachelor of Arts; LSD = lysergic acid diethylamide.
Food Intake
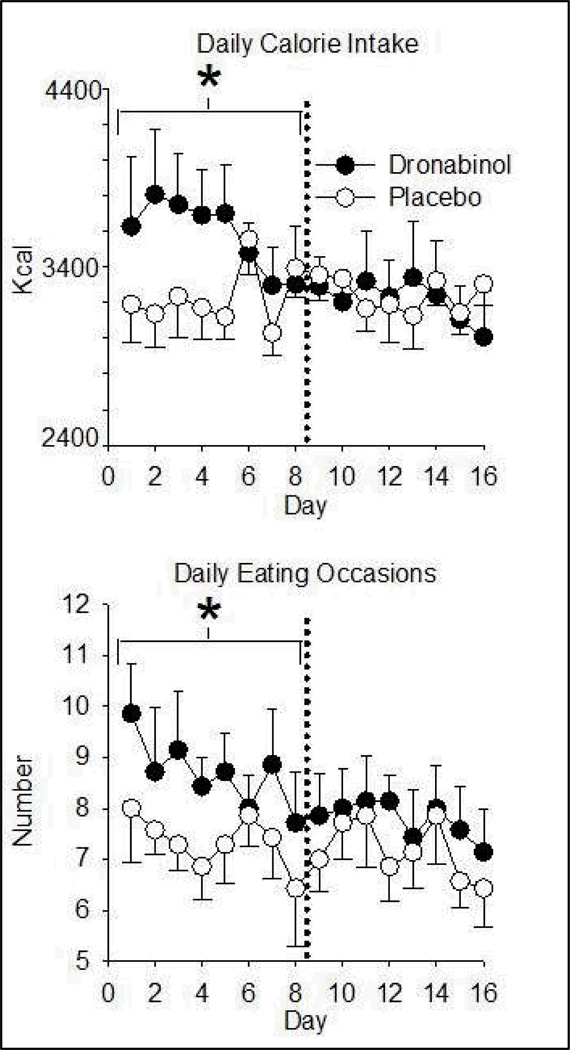
Dronabinol increased caloric intake on Days 1–8 relative to PBO, with an average daily consumption of 3579.9 (± 563) calories on dronabinol compared to 3227.6 (± 385) on PBO. Dronabinol did not affect daily caloric intake during Days 9–16 (Figure 1; Table 3). Higher caloric consumption over Days 1–8 during dronabinol administration was primarily due to an increased number of eating occasions (defined as beginning with food consumption onset, and ending at the first pause in food reporting > 10 minutes; Figure 1). There was no increase in the number of calories consumed per eating occasion during Days 1–8 or 9–16. On Days 1–8 participants consumed more calories in the morning on PBO, whereas they consumed more calories during the evenings when on dronabinol compared to PBO (Table 3). There were significant, although small, differences in macronutrients consumed between conditions (Table 3). Whereas dronabinol increased absolute consumption of fat and carbohydrates on Days 1–8, there was no effect on protein consumption during this time. On dronabinol, the amount of protein consumed was lower than on PBO over Days 9–16. Relative to PBO, protein consumption as a proportion of overall intake was therefore lower on dronabinol during both phases (Days 1–8 and 9–16).
Figure 1.
Dronabinol effects on daily caloric intake (top) and number of eating occasions (bottom) as a function of time. Data are daily means (± S.E.M.). Dronabinol/PBO was administered 4 times per day. Asterisks denote an increase from placebo (p< 0.01) in the indicated eight-day phase. PBO = Placebo
Table 3.
Efficacy of Dronabinol: Selected Food Consumption and Body Weight Measures
| PBO | Days 1–8 DRON |
PBO | Days 9–16 DRON |
|||
|---|---|---|---|---|---|---|
| Meana (S.E.M) |
Meana (S.E.M) |
Fb | Meana (S.E.M) |
Meana (S.E.M) |
Fb | |
| Total Daily Caloric Intake (kcal) |
3227.6 (145.3) |
3579.9 (212.8) |
16.0*⇑ | 3240.8 (87.5) |
3216.2 (215.3) |
0.1 |
| Daily Intake: | ||||||
| Morning (kcal) | 764.8 (55.2) |
669.4 (81.5) |
9.7*⇓ | 728.1 (71.5) |
679.3 (104.2) |
2.5 |
| Afternoon (kcal) | 955.2 (95.6) |
1120.8 (120.0) |
5.3 | 1048.4 (119.6) |
1015.9 (166.7) |
0.2 |
| Evening (kcal) | 1501.8 (164.8) |
1749.6 (157.2) |
11.4*⇑ | 1439.9 (169.7) |
1484.4 (211.7) |
0.4 |
| Daily Fat Intake (gm) | 112.9 (5.9) |
130.6 (12.4) |
12.6*⇑ | 113.9 (5.6) |
112.2 (12.1) |
0.1 |
| Daily Carbohydrate Intake (gm) | 456.2 (32.5) |
501.7 (34.9) |
13.0*⇑ | 456.3 (22.7) |
464.8 (34.0) |
0.5 |
| Daily Protein Intake (gm) | 96.9 (4.0) |
99.6 (7.1) |
0.9 | 97.8 (3.1) |
87.1 (5.7) |
14.2*⇓ |
| Daily Protein – % Total Intake | 12.0 (0.2) |
11.2 (0.4) |
8.4*⇓ | 12.1 (0.4) |
10.9 (0.4) |
18.1*⇓ |
| Change in Body Weight (kg)c | −0.2 (0.5) |
1.0 (0.4) |
2.7 df = (1,18) |
−0.4 (0.7) |
−0.1 (0.3) |
0.2 df = (1,18) |
| HSQ Thirst | 38.3 (6.8) |
51.7 (6.6) |
14.7*⇑ | 46.3 (5.6) |
58.1 (5.9) |
11.5*⇑ |
| HSQ Dry Mouth | 29.5 (7.6) |
51.6 (9.6) |
36.0*⇑ | 34.1 (9.3) |
50.7 (8.9) |
20.1*⇑ |
| FDQ Savoury | 0.8 (0.2) |
1.1 (0.3) |
26.5*⇑ | 0.7 (0.2) |
1.1 (0.3) |
49.5*⇑ |
| FDQ Sweet | 1.2 (0.3) |
1.6 (0.4) |
44.2*⇑ | 1.0 (0.3) |
1.6 (0.5) |
80.8*⇑ |
| FDQ Carbohydrates | 1.5 (0.3) |
1.6 (0.4) |
5.5 | 1.4 (0.4) |
1.6 (0.4) |
11.7*⇑ |
| FDQ Proteins/Fats | 1.2 (0.3) |
1.4 (0.3) |
16.9*⇑ | 1.3 (0.4) |
1.3 (0.3) |
0.0 |
| FDQ Non-Alcoholic Beverages | 1.1 (0.4) |
1.4 (0.4) |
13.1*⇑ | 1.2 (0.4) |
1.5 (0.5) |
13.1*⇑ |
Data are means per day.
Degrees of freedom = (1,186) except where otherwise noted.
Weight change between Days 1 and 8, and Days 9 and 16.
DRON = dronabinol (10 mg QID); PBO = placebo; S.E.M = Standard Error of the Mean; df = degrees of freedom;
= Difference between dronabinol and placebo, p < 0.0l;
= Dronabinol increased scores;
= Dronabinol decreased scores; HSQ = Hunger and Satiety Questionnaire; FDQ = Food Desirability Questionnaire.
Body Weight
Over Days 1–8, dronabinol increased weight by 1.0 kg (± 1.1) from baseline, however this effect was not significant; there was no drug effect on Days 9–16 (Table 3).
Subjective Hunger and Satiety
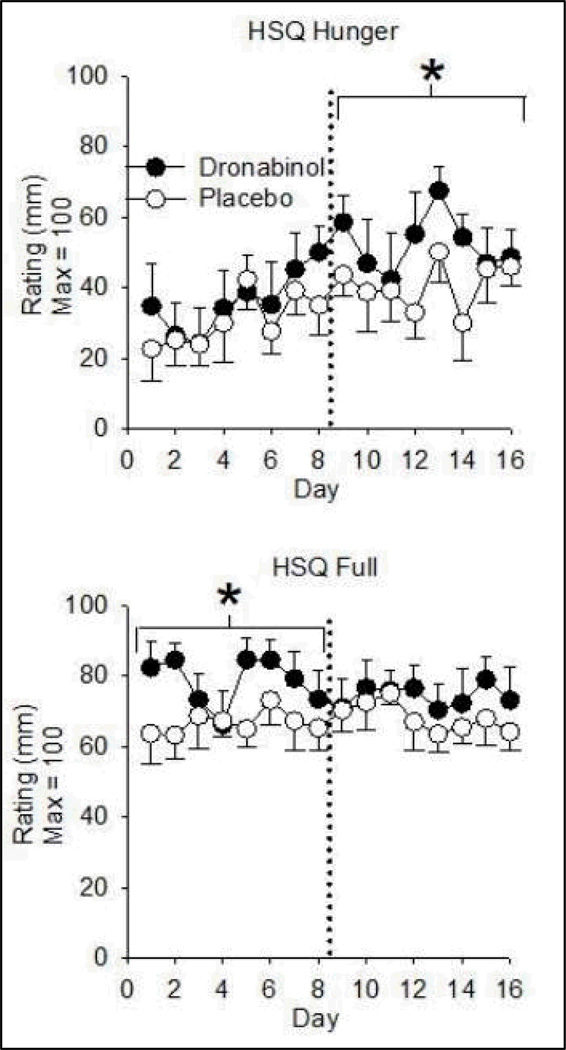
Relative to PBO, dronabinol increased HSQ Fullness ratings over Days 1–8 and increased HSQ Hunger ratings on Days 9–16 only (Figure 2). Participants endorsed higher HSQ Thirst and HSQ Dry Mouth levels across Days 1–8 and Days 9–16 on dronabinol (Table 3). There was no drug effect on HSQ Desire to Eat or Nausea ratings (nausea ratings were low across conditions).
Figure 2.
Dronabinol effects on subjective hunger (top) and fullness (fullness) ratings as a function of time. Data are means (± S.E.M.) of daily maximum scores. Dronabinol/PBO was administered 4 times per day. Asterisks denote an increase from placebo (p< 0.01) in the indicated eight-day phase. HSQ = Hunger and Satiety Questionnaire; PBO = Placebo
Food Cravings
Dronabinol increased FDQ ratings of desire for savory and sweet foods on Days 1–8 and 9–16. Dronabinol increased reports of wanting foods containing proteins and fats on Days 1–8 only, and wanting carbohydrate-based food on Days 9–16 only. Consistent with increased thirst and dry mouth (HSQ), dronabinol increased desire for non-alcoholic beverages on Days 1–8 and 9–16 (Table 3). There was no drug effect on FDQ ratings of alcohol cravings.
Subjective Mood and Drug Effect
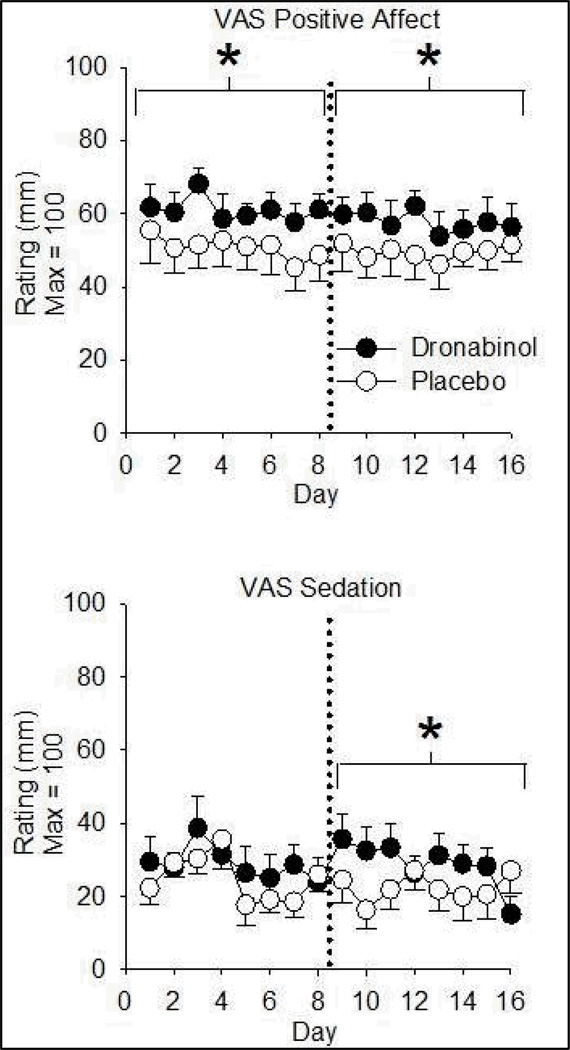
Dronabinol increased VAS Positive Affect (Figure 3) and Drug High (Table 4) ratings on Days 1–8 and 9–16. There was a small but significant dronabinol-related increase in VAS Sedation ratings on Days 9–16 only (Figure 3). There was no effect of drug on VAS Anxiety, Somatic Symptom, or Negative Affect subscale ratings. DEQ Good Drug Effect ratings were higher on dronabinol than PBO over both 8-day periods, as were DEQ Drug Liking scores and ratings of wanting to take the drug again (Table 4). Dronabinol increased DEQ Strong Drug Effect ratings over Days 9–16 only and did not affect DEQ Bad Drug Effect.
Figure 3.
Dronabinol effects on self-reported positive affect (top) and sedation (bottom) ratings as a function of time. Data are means (± S.E.M.) of daily maximum scores. Dronabinol/PBO was administered 4 times per day. Asterisks denote an increase from placebo (p< 0.01) in the indicated eight-day phase. VAS = Visual Analogue Scale; PBO = Placebo
Table 4.
Tolerability of Dronabinol: Selected Mood, Sleep, and Behavioral Measures
| PBO | Days 1–8 DRON |
PBO | Days 9–16 DRON |
|||
|---|---|---|---|---|---|---|
| Meana (S.E.M) |
Meana (S.E.M) |
Fb | Meana (S.E.M) |
Meana (S.E.M) |
Fb | |
| VAS Drug High | 10.8 (3.3) |
25.5 (4.5) |
25.4*⇑ | 11.2 (3.5) |
27.5 (9.0) |
31.0*⇑ |
| DEQ Good Drug Effect | 0.8 (0.2) |
1.6 (0.3) |
27.8*⇑ | 0.9 (0.3) |
1.7 (0.5) |
27.8*⇑ |
| DEQ Like Drug Effect | 0.7 (0.3) |
1.5 (0.3) |
20.5*⇑ | 0.7 (0.3) |
1.6 (0.5) |
25.1*⇑ |
| DEQ Strong Drug Effect | 1.1 (0.2) |
1.5 (0.2) |
5.8 | 1.1 (0.3) |
1.6 (0.4) |
11.8*⇑ |
| DEQ Take Drug Again | 1.1 (0.2) |
2.0 (0.3) |
37.2*⇑ | 1.3 (0.3) |
2.0 (0.4) |
21.5*⇑ |
| Minutes NREMc | 283.1 (18.5) |
325.3 (15.6) |
11.0*⇑ df =(1,155) |
316.5 (26.2) |
307.1 (25.6) |
0.4 df = (1,155) |
| VAS Slept Well | 52.1 (6.8) |
62.4 (9.5) |
7.5*⇑ | 56.5 (8.2) |
59.0 (9.9) |
0.4 |
| DAT Distracter Response Time (msecs) | 1211.0 (174.1) |
1310.5 (164.4) |
3.4 | 1126.9 (102.2) |
1289.1 (199.3) |
9.1*⇑ |
| DAT False Alarms | 3.5 (1.9) |
5.4 (3.5) |
8.9*⇑ | 3.3 (1.6) |
4.6 (3.1) |
4.3 |
| Digit Span | 7.7 (0.2) |
8.0 (0) |
27.4*⇑ | 8.0 (0) |
8.0 (0) |
0.0 |
| RAT Total Trials | 31.7 (8.3) |
26.1 (4.4) |
16.3*⇓ | 34.6 (7.7) |
30.4 (5.7) |
9.2*⇓ |
| RAT No. Errors | 57.4 (12.0) |
58.7 (11.8) |
1.0 | 56.7 (12.6) |
60.5 (11.4) |
9.6*⇑ |
Data presented are means per day.
Degrees of freedom = (1,186) except where otherwise noted.
As measured with a NightCap sleep monitor; N = 6 due to missing data.
DRON = dronabinol (10 mg QID); PBO = placebo; S.E.M = Standard Error of the Mean; df = degrees of freedom;
= Difference between dronabinol and placebo, p<.0l;
= Dronabinol increased scores;
= Dronabinol decreased scores; VAS = Visual Analogue Scale; DEQ = Drug Effects Questionnaire; NREM = Non-Rapid Eye Movement sleep; DAT = Divided Attention Task; RAT = Rapid Acquisition Task.
Sleep
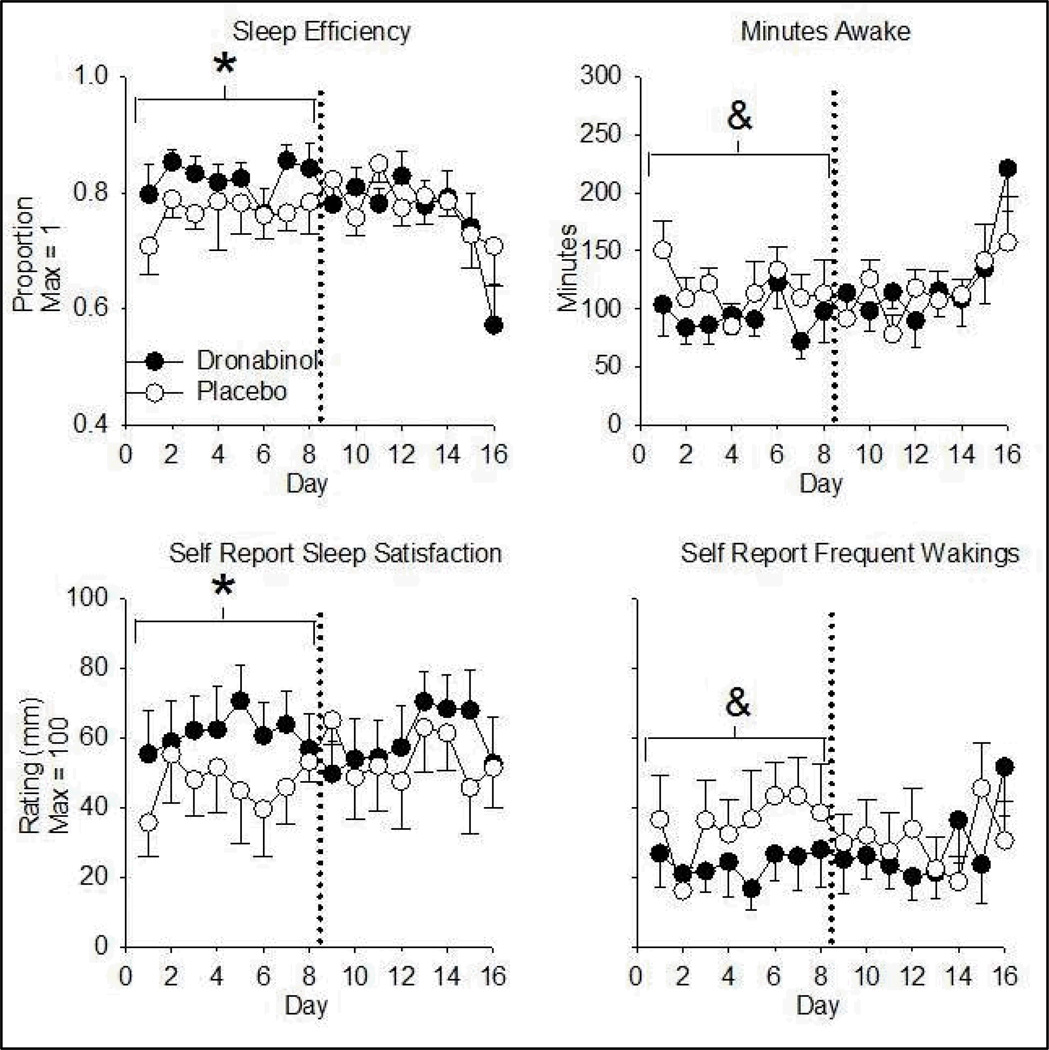
Nightcap data were not available for one participant (equipment failure). Dronabinol increased sleep efficiency over Days 1–8 only. The effect of dronabinol on Days 1–8 was due to an increase in minutes spent in NREM sleep and a decrease in minutes spent awake. Consistent with these objective indices, participants (N = 7) rated themselves as being more satisfied with their sleep during Days 1–8 in the dronabinol relative to the PBO condition. Dronabinol increased ratings of having slept well and having awoken less on Days 1–8 (Figure 4; Table 4).
Figure 4.
Dronabinol effects on objectively-measured sleep efficiency (REM + NREM sleep as a proportion of total time in bed; top left), objectively measured minutes awake (top right), self-rated satisfaction with sleep (bottom left), and self-ratings of frequency of waking (bottom right) as a function of time. Data are daily means (± S.E.M.). Dronabinol/PBO was administered 4 times per day. Asterisks denote an increase from placebo (p< 0.01) in the indicated eight-day phase. & denotes a decrease relative to placebo (p< 0.01). PBO = Placebo. N = 6 top left and top right panels due to equipment failure.
Cognitive Function
There were minor effects of dronabinol on performance on three of four cognitive tasks (Table 4). Consistent with slightly reduced processing speed, participants had a longer latency period when responding to distracter items in the Divided Attention Task, however this effect only reached significance on Days 9–16. Dronabinol also increased false alarms for distracter items on this task across Days 1–8. On the Rapid Acquisition Task, participants entered fewer sequences on dronabinol than PBO on Days 1–8 and 9–16, and made more errors on Days 9–16. There was no drug effect on performance on the Rapid Information Processing task, or delayed digit recall. Participants entered more numbers on dronabinol relative to PBO on immediate recall digit span, however the absolute difference was minor.
Social behavior
Dronabinol did not affect the social behavior indices assessed.
Over-the-Counter (OTC) Medication
In addition to formally analyzed outcome measures, we recorded requests for OTC medication. Of 16 total medication requests, 5 occurred on dronabinol and 11 occurred on placebo. On placebo, 4 requests related to gastrointestinal complains, whereas on dronabinol, there was one request for gastrointestinal OTC medications.
DISCUSSION
Consistent with findings in HIV-negative marijuana smokers (Haney et al. 1999a; Kirk and de Wit 1999), these results indicate that repeated high doses of dronabinol are safe and well-tolerated in HIV-positive marijuana users. Dronabinol produced sustained increases in self-reported food cravings as well as positive mood changes, without substantive negative side effects. Moreover, initial effects on caloric intake were consistent with previous findings that acute (Haney et al. 2005) and short-term (Haney et al. 2007) high doses of dronabinol increase caloric intake in HIV-positive marijuana smokers. Participants consumed some 350 more calories daily over the first 8 days of dronabinol dosing, consuming more fat and carbohydrates and showing a small, non-significant increase in body weight compared to PBO. During the initial eight days, objective indicators of sleep quality also improved. However, contrary to hypotheses, during the second eight-day period (Days 9–16) effects of dronabinol on food intake and sleep were no longer apparent. Indeed, during Days 9–16, participants’ absolute protein intake decreased on dronabinol. Body weight did not increase during the second week.
Also contrary to hypotheses, self-reported positive mood and psychoactive effects of dronabinol did not decrease, with sustained effects on ratings of positive affect, drug ‘high’, good drug effects, and wanting to take the drug again. However, these effects were modest; for instance, maximal drug ‘high’ ratings reached an average of around 40/100. The overall pattern of results - decreasing dronabinol effects on food intake and sleep in conjunction with stable mood-enhancing effects - suggests that the dosing regime employed produces selective tolerance to the clinically targeted outcome (food intake) in HIV-positive marijuana smokers.
There is a possible alternative explanation for these findings. Dronabinol did not increase food consumption in the second inpatient week, despite participants reporting sustained elevations in food cravings. Thus, rather than developing tolerance, participants may have became disinterested with the available food. Although this explanation cannot be completely discounted, there are indications that this was not the case. There was no corresponding decrease in intake in week two of placebo dosing, which would be expected if participants became bored with laboratory food over time. There was a range of food available (79 items). Moreover, 27 of 38 items comprising the Food Desirability Questionnaire were available. Given that dronabinol’s effects on sleep also attenuated after several days, it is likely that tolerance, rather than disinterest with available food, is the explanation for decreasing drug effects on food intake.
One possible confound in interpreting these data could be withdrawal from marijuana, which may have started in the early days of the placebo condition. However, there are a number of factors suggesting that withdrawal did not have substantial impacts on these results. First, we would anticipate that anorectic effects of marijuana withdrawal would peak around day 2, beginning to reverse by day 5 (Haney et al. 2005), a pattern that did not occur (see Figure 1). Second, marijuana withdrawal would be expected to produce increases in negative affect and anxiety (Haney et al. 1999b), which was also not the case. The absence of a demonstrable withdrawal syndrome in this study is likely due to the fact that participants were lighter marijuana smokers than those in which we previously demonstrated withdrawal (Haney et al. 1999b). Thus, differences between drug conditions observed in the first 8 days of treatment appear to be due to direct effects of dronabinol, rather than withdrawal amelioration.
Based on available evidence, it is unclear whether tolerance has occurred in other studies of cannabinoid effects in HIV/AIDS. In our previous laboratory studies with HIV-positive participants (Haney et al. 2007; Haney et al. 2005), treatment length was not sufficient to assess tolerance. Clinical studies cannot easily include objective measures of food intake, thus previous clinical studies have measured self-reported appetite and weight gain. Although these studies reported sustained increases in appetite, they did not report weight gain (Beal et al. 1995; Beal et al. 1997; Struwe et al. 1993). According to our present as well as previous findings (Haney et al. 2005), self-reported appetite and food cravings may not equate with actual consumption. Indeed, given the sustained increase in positive mood in the present study, self-reports of improved appetite reported in clinical studies might represent the effect of a more positive mood state. Therefore, in clinical studies tolerance may have developed, but remained undetected.
Tolerance to a range of cannabinoid effects has previously been suggested in HIV-negative participants. Light cannabis users demonstrate greater cognitive alterations after smoked THC administration than heavy users (D'Souza et al. 2008; Ramaekers et al. 2009). Light users also show more perceptual alterations after intravenous THC relative to heavy users, whereas positive mood effects are comparable (D'Souza et al. 2008). Cardiovascular tolerance and partial attenuation of subjective (‘high’) effects of both smoked and intravenous THC have been shown to develop after 12 days of concurrent high-dose oral THC (30 mg every four hours; Hunt and Jones 1980). The development of selective tolerance in this study is broadly consistent with earlier reports, although we are unaware of previous studies showing tolerance to the appetite enhancing effects of cannabinoids while mood effects are sustained.
There are a number of future directions. Having established that high, repeated dronabinol doses are well-tolerated in HIV-positive marijuana smokers, an important issue is whether different dosing regimes might improve the drug’s long-term clinical utility. Future studies could assess variable or intermittent dosing schedules to reduce tolerance. A second important direction will be to investigate if smoked marijuana produces objectively-observable, sustained increases in food consumption in regular users. Third, it remains to be seen whether tolerance to dronabinol’s effects also develops in individuals who do not smoke marijuana. The medical marijuana debate, as noted above, has relied on limited empirical evidence. Given substantial inter-individual variability (Davidson and Schenk 1994; Kirk and de Wit 1999; Sachse-Seeboth et al. 2009) in cannabinoid effects, further studies examining the efficacy of different delivery methods and doses of medical cannabinoids in clinically-relevant patient groups is critical to inform the debate over medical use of these drugs.
Some limitations should be noted. Participants recruited were not, at baseline, anorexic, which may have contributed to results. One outpatient study we conducted indicated that single dronabinol doses increase caloric intake over a seven-hour period only in individuals with significant muscle loss (Haney et al. 2005). However, in HIV-positive individuals participating in an inpatient study, repeated dronabinol administration increased food intake and weight over four days despite the fact that 80% of participants did not have anorexia or muscle wasting (Haney et al. 2007). Increases observed during initial dronabinol dosing in the present study were of comparable magnitude to those reported in the shorter-term inpatient study (Haney et al. 2007). Moreover, dronabinol increases food intake in HIV-negative individuals over 3–4 days (Haney et al. 1999a; Hart et al. 2002b), suggesting that the lack of anorexia in this population should not have influenced results substantially.
A related issue is that, on placebo, participants did not report substantial nausea. Thus, it is unclear whether HIV-positive individuals experiencing gastro-intestinal complications related to antiretroviral medications (Chubineh and McGowan 2008) might benefit more from dronabinol. For instance, reduced nausea might increase quality of life and enhance treatment compliance (Chubineh and McGowan 2008; Johnson et al. 2005). The present findings tentatively suggest that dronabinol may serve to reduce gastrointestinal complaints, given that of five requests for OTC medication for gastrointestinal discomfort, four occurred during the placebo phase. Future research could formally assess these possibilities. A further limitation is that the sample size of seven was small, however due to the within-subjects design, it was sufficient to allow the detection of a range of drug effects. A final limitation to this study is that all participants were male, limiting generalization.
Such limitations notwithstanding, the present results add substantially to existing knowledge about the utility of dronabinol in treatment of people with HIV. In HIV-positive marijuana smokers, repeated dronabinol doses double the recommended maximal dose safely and effectively increased caloric intake, improved sleep and had positive mood effects while producing few cognitive disruptions. After eight days, however, dronabinol no longer affected either food intake or sleep, suggesting that maintenance on this dosing regime results in development of selective tolerance. These findings indicate that HIV-positive individuals who smoke marijuana may require higher dronabinol doses than are recommended by the FDA. Future research to establish optimal dosing regimens, and reduce the development of tolerance, is required.
Acknowledgements
Thanks to Brooke Roe, Diana Paksarian and Michael Rubin for assistance in data collection, and to Jennifer Redman for input into interpretation of sleep measurements. This research was supported by the National Center for Complementary and Alternative Medicine (DA0126980). This study complies with the laws of the country in which it was performed (USA).
Footnotes
The authors have no conflict of interest to declare.
References
- Abrams DI. Medical marijuana: Trials and tribulations. J Psychoactive Drugs. 1998;30:163–169. doi: 10.1080/02791072.1998.10399686. [DOI] [PubMed] [Google Scholar]
- Abrams DI. Potential interventions for HIV/AIDS wasting: An overview. JAIDS. 2000;25:S74–S80. doi: 10.1097/00042560-200010001-00012. [DOI] [PubMed] [Google Scholar]
- Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Short-term effects of cannabinoids in patients with HIV-1 infection. Ann Intern Med. 2003;139:258–266. doi: 10.7326/0003-4819-139-4-200308190-00008. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association, American Psychiatric Association; 1994. [Google Scholar]
- Ashton CH. Pharmacology and effects of cannabis: A brief review. Br J Psychiatry. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995:89–97. doi: 10.1016/0885-3924(94)00117-4. [DOI] [PubMed] [Google Scholar]
- Beal JE, Olson R, Lefkowitz L, Laubenstein L, Bellman P, Yangco B, Morales JO, Murphy R, Powderly W, Plasse TF, Mosdell KW, Shepard KV. Long-term efficacy and safety of dronabinol for Acquired Immunodeficiency Syndrome-associated anorexia. J Pain Symptom Manage. 1997;14:7–14. doi: 10.1016/S0885-3924(97)00038-9. [DOI] [PubMed] [Google Scholar]
- Chubineh S, McGowan J. Nausea and vomiting in HIV: A symptom review. Int J STD AIDS. 2008;19:723–728. doi: 10.1258/ijsa.2008.008244. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetracannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansak DA. Medical use of recreational drugs by AIDS patients. J Addict Dis. 1997;16:25–30. doi: 10.1300/J069v16n03_03. [DOI] [PubMed] [Google Scholar]
- Davidson ES, Schenk S. Variability in subjective responses to marijuana: Initial experiences of college students. Addict Behav. 1994;19:531–538. doi: 10.1016/0306-4603(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW, Fischman MW. Food "cravings" and the acute effects of alprazolam on food intake in women with Premenstrual Dysphoric Disorder. Appetite. 1999;32:331–349. doi: 10.1006/appe.1998.0222. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Furler MD, Einarson TR, Millson M, Walmsley S, Bendayan R. Medicinal and recreational marijuana use by patients infected with HIV. AIDS Patient Care STDS. 2004;18:215–228. doi: 10.1089/108729104323038892. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB. Marihuana: The Forbidden Medicine. New Haven: Yale University Press; 1997. [Google Scholar]
- Hall W, Degenhardt L. Medical marijuana initiatives. Are they justified? How successful are they likely to be? CNS Drugs. 2003;17:689–697. doi: 10.2165/00023210-200317100-00001. [DOI] [PubMed] [Google Scholar]
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology. 2003;166:77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood and sleep. JAIDS. 2007;45:545–554. doi: 10.1097/QAI.0b013e31811ed205. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Rabkin J, Gunderson EW, Foltin RW. Dronabinol and marijuana in HIV+ marijuana smokers: Acute effects on caloric intake and mood. Psychopharmacology. 2005;181:170–178. doi: 10.1007/s00213-005-2242-2. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999a;141:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999b;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002a;67:301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Δ9-tetrahydrocannabinol in humans. Psychopharmacology. 2002b;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Hazekamp A, Ruhaak R, Zuurman L, Van Gerven J, Verpoorte R. Evaluation of a vaporizing device (Volcano®) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci. 2006;95:1308–1317. doi: 10.1002/jps.20574. [DOI] [PubMed] [Google Scholar]
- Hollister LE. An approach to the medical marijuana controversy. Drug Alcohol Depend. 2000;58:3–7. doi: 10.1016/s0376-8716(99)00076-9. [DOI] [PubMed] [Google Scholar]
- Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther. 1980;215:35–44. [PubMed] [Google Scholar]
- Institute of Medicine. Marijuana and medicine: Assessing the science base. Washington DC: Institute of Medicine; 1999. [Google Scholar]
- Johanson C, Uhlenhuth E. Drug preference and mood in humans: Diazepam. Psychopharmacology. 1980;71:269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Johnson M, Charlebois E, Morin SF, Catz SL, Goldstein RB, Remien RH, Rotheram-Borus MJ, Mickalian JD, Kittel L, Samimy-Muzaffar F, Lightfoot MA, Gore-Felton C, Chesney A, Team NHLP Perceived adverse effects of antiretroviral therapy. J Pain Symptom Manage. 2005;29:193–205. doi: 10.1016/j.jpainsymman.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Responses to oral tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav. 1999;63:137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- Martin BR. Medical marijuana: Moving beyond the smoke. The Lancet. 2002;360:4–5. doi: 10.1016/S0140-6736(02)09360-1. [DOI] [PubMed] [Google Scholar]
- Mehra R, Moore BA, K C, Tetrault J, Fiellin DA. The association between marijuana smoking and lung cancer: A systematic review. Arch Int Med. 2006;166:1359–1367. doi: 10.1001/archinte.166.13.1359. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: Comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol: Clin Exp. 1996;11:391–400. [Google Scholar]
- Prentiss D, Power R, Balmas G, Tzuang G, Israelski D. Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. JAIDS. 2004;35:38–45. doi: 10.1097/00126334-200401010-00005. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Sachse-Seeboth C, Pfeil J, Sehrt D, Meineke I, Tzvetkov M, Bruns E, Poser W, Vormfelde SV, Brockmoller J. Interindividual variation in the pharmacokinetics of Delta-9-tetrahydrocannabinol as related to genetic polymorphisms in CYP2C9. Clin Pharmacol Ther. 2009;85:273–276. doi: 10.1038/clpt.2008.213. [DOI] [PubMed] [Google Scholar]
- Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. 2nd edn. Boca Raton, FL: CRC Press LLC; 2000. [Google Scholar]
- Struwe M, Kaempfer SH, Geiger CJ, Pavia AT, Plasse TF, Shepard KV, Ries K, Evans TG. Effect of dronabinol on nutritional status in HIV infection. Ann Pharmacother. 1993;27:827–831. doi: 10.1177/106002809302700701. [DOI] [PubMed] [Google Scholar]
- Taylor D, Poulton R, Moffitt TE, Ramankutty P, Sears MR. The respiratory effects of cannabis dependence in young adults. Addiction. 2000;95:1669–1677. doi: 10.1046/j.1360-0443.2000.951116697.x. [DOI] [PubMed] [Google Scholar]
- Taylor DR, Fergusson DM, Milne BJ, Horwood LJ, Moffitt TE, Sears MR, Poulton R. A longitudinal study of the effects of tobacco and cannabis exposure on lung function in young adults. Addiction. 2002;97:1055–1061. doi: 10.1046/j.1360-0443.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- Wanke CA, Silva M, Knox TA, Forrester J, Speigelman D, Gorbach SL. Weight loss and wasting remain common complications in individuals infected with Human Immunodeficiency Virus in the era of Highly Active Antiretroviral Therapy. Clin Infect Dis. 2000;31:803–805. doi: 10.1086/314027. [DOI] [PubMed] [Google Scholar]
- Ware M, Rueda S, Singer J, Kilby D. Cannabis use by persons living with HIV/AIDS: Patterns and prevalence of use. J Cannabis Therapeutics. 2003;3:3–15. [Google Scholar]
- Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]