Abstract
Alzheimer’s disease (AD) and age-related macular degeneration (AMD) are complex and progressive inflammatory degenerations of the human neocortex and retina. Recent molecular, genetic and epigenetic evidence indicate that at least 4 micro RNAs (miRNAs) - including the NF-кB-regulated miRNA-9, miRNA-125b, miRNA-146a and miRNA-155 - are progressively up-regulated in both AD and AMD. This quartet of up-regulated miRNAs in turn down-regulate a small brain- and retinal-cell-relevant family of target mRNAs, including that encoding complement factor H (CFH), a major negative regulator of the innate immune and inflammatory response. Together miRNA-146a and miRNA-155 recognize an overlapping miRNA regulatory control (MiRC) region in the CFH 3’-untranslated region (3’- UTR; 5’-TTTAGTATTAA-3’) to which either of these miRNAs may interact. Progressive, pathogenic increases in specific miRNA binding to the entire 232 nucleotide CFH 3’-UTR appears to be a major regulator of CFH expression down-regulation, and the inflammatory pathology that characterizes both AMD and AD. The data presented in this report provides evidence that up-regulation of brain- and retinal- abundant miRNAs, including miRNA-9, miRNA-125b, miRNA-146a and miRNA-155, are common to the pathogenetic mechanism of CFH deficiency that drives inflammatory neurodegeneration, and for the first time indicates multiple, independent miRNA-mediated regulation of the CFH mRNA 3’-UTR.
Keywords: Brain, complement factor H, evolution, miRNA regulatory control (MiRC) region, micro RNA, miRNA-9, miRNA-125b, miRNA-146a, miRNA-155, retina, small non-coding RNA
Complement factor H (CFH) in AD and AMD
Alzheimer’s disease (AD) and age-related macular degeneration (AMD) and are multifactorial, late onset, progressive neurodegenerative diseases of the human brain and retina, and represent the leading cause of cognitive and visual impairment in aging populations of the industrialized world [1-6]. Despite considerable research efforts, the causes of AD and AMD are not well understood, and currently there are no effective treatments for these progressive degenerations of human cognition and vision. There are many shared pathological characteristics of AD and AMD, including (a) the appearance of dense, insoluble, polymorphic amyloid beta (Aβ) peptide-, oxidized lipid- and complement factor-enriched pathological lesions known as senile plaques (SP) in AD and drusen in AMD, (b) elevated cholesterol, 24S-hydroxycholesterol and other oxidized lipid metabolites, (c) amyloidogenesis, (d) heightened inflammatory and pro-apoptotic signaling, and (e) decreases and/or dysfunction in complement factor H (CFH), a major regulator of the innate immune and inflammatory response [8-30]. For instance, it has recently been shown that in AD, amyloiodgenic, pro-inflammatory and pro-apoptotic gene expression spreads from the limbic system into the primary visual sensory cortex (Brodmann area A17) as AD advances, rostrally through the thalamus/superior colliculus to the retina, along the entire anteroposterior axis of the retinal-primary visual cortex pathway. This spreading correlates with progressive visual disturbances reported in late-stage AD patients [7-9]. Further, transgenic models of AD, such as the 5xFAD model (that contains 5 familial amyloid transgenes regulated by neural-specific elements of the mouse Thy1 promoter to drive human amyloid over-expression) show age-related deposition of amyloid peptides, innate immune and inflammatory defects and CFH-associated pathology in both the brain and retina [9-13]. As discussed more fully below, at the molecular-genetic level pathogenic up-regulation of miRNA-9, miRNA-125b, miRNA- 146a and miRNA-155 appear to be involved in a comparable progressive, CFH-mediated inflammatory degeneration that is characteristic of both AD and AMD.
Consistent observations concerning changes in CFH signaling have been described in both AD and AMD [1-12,16-25]. CFH (also known as AC3bINA, adrenomedullin binding protein-1, AMBP-1, AM binding protein-1factor H, β1H globulin, C3b inactivator accelerator, H factor, HF, H factor-1, HF1) is an important member of the regulator of complement activation (RCA) group of proteins encoded within the RCA gene locus spanning chromosome 1q21-1q32. In the human brain and retina CFH is transcribed from a single copy gene that generates one major ~4100 nucleotide mRNA transcript [23, 25; ~95% of the CFH mRNA signal in a single band on Northern gels; Genbank BC142699; unpublished observations]. A major amount of CFH is also normally generated in, and secreted by, the liver, and CFH reaches blood plasma concentrations of 500-800 ug/ml in the systemic circulation [3,15,25]. Hence CFH is the second most abundant serum plasma protein, after human serum albumen, and normally performs a systemic sentinel function against unscheduled or spontaneous activation of the innate immune system [18-20]. Systemic CFH deficits are conducive to excessive and pathogenic complement pathway activation associated with increased complement activity on otherwise healthy host cells, autoimmunity, host tissue damage and a sustained and often chronic inflammatory response [7,9,10,18-20]. At the current time it is not clear if CFH, a soluble, hydrophilic 155 kDa glycoprotein, is permeable to the blood-brain barrier; such large glycosylated serum proteins are usually prevented from access to CNS compartments, and the brain and retina may have an independent CFH supply secreted by neurons, astroglia, microglial and/ or endothelial cells [2,12,19]. Otherwise, CFH may contribute to SP and drusen formation via a diseased or leaky neocortical or retinal vasculature [47-50]. CFH expression is significantly down-regulated in the degenerating brain and retina, suggesting that insufficient quantities of this RCA regulator lead to excessive activation of the innate immune response and pro-inflammatory signaling [5-8,18,20-25,32,79]. In both of these tissues CFH functions as a cofactor in the inactivation of its C3b ligand by factor 1 in the alternative complement pathway [2-5]. As a molecular constituent of the SP and drusen that accumulate in AD and in AMD, CFH co-localizes with its ligand C3b in sub-structural spherules within drusen that also contain Aβ42 peptide and additional related toxic βAPP-derived fragments [14-17,31,32]. Extensive inverse abundance interrelationships between high Aβ42 levels and low CFH levels have been described in degenerative disorders of retinal and brain cells [9,16]. Moreover, the significant statistical relationship between the CFH Y402H “risk” variant and AMD further suggests that either a defective ‘mutant’ CFH, or low CFH abundance may have analogous pro-inflammatory effects in activation of the innate immune response [2-5,20,33].
Pathogenic, pro-inflammatory micro RNAs (miRNAs) in the brain and retina
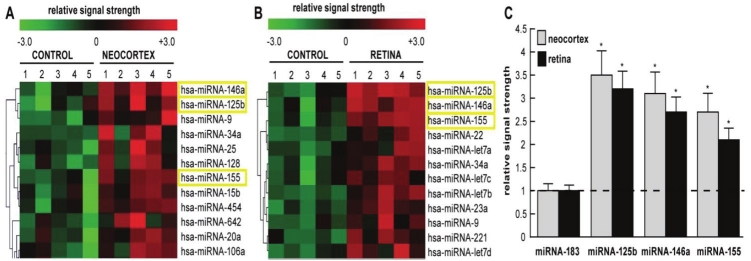
Micro RNAs (miRNAs) constitute a family of ~22 nucleotide (nt), non-coding, single-stranded labile RNAs [14,21-25,34,42,55,60,63,76-79] The major mode of biological action of miRNA is to bind to complimentary RNA sequences in the 3’ un-translated region (3’-UTR) of messenger RNA (mRNA), and thereby act as a repressor of that mRNA’s expression. It is now widely accepted that up-regulated miRNAs predominantly act to decrease their target mRNA levels, and hence down-regulate genetic information encoded by that target mRNA [14,21-25,34]. Of the approximately 1898 human miRNAs currently identified, less than 102 miRNAs are abundantly expressed in brain and retina [40-42; unpublished]. Interestingly, while up-regulated miRNAs have the potential to reduce the transcript levels of their target mRNAs on a genome-wide scale, in disease processes only certain mRNAs are preferentially affected. Figure 1A and 1B shows expression of a family of pathogenic miRNAs significantly up-regulated in aging human neocortex and in AMD retina. As discussed more fully below, these results were independently confirmed using RT-PCR and/or Northern dot-blot techniques (Figure 1C) [5-8,24,25]. Common to aged degenerating brain and retina are up-regulated miRNA-9, miRNA- 125b, miRNA-146a and miRNA-155, and up-regulation these 4 miRNAs has been shown to be involved with an up-regulation of inflammatory signaling in large part due to their specific targeting of the CFH mRNA 3’UTR and down-regulation of CFH expression [7,9,16,21-25,31,42,45,64,68,76]. Interestingly, certain of these up-regulated miRNAs also target other pathology-relevant mRNAs including tetraspanin 12 (TSPAN12; also known as NET-2 or transmembrane 4 superfamily member 12), a membrane spanning protein that associates with ADAM10, a membrane secretase responsible for the cleavage of beta amyloid precursor protein (βAPP) into neurotrophic sAPPα [25,30,31,37,38]. Hence miRNA-125b-, miRNA-146aand miRNA-155-mediated down-regulation of TSPAN12 impairs ADAM10 activity, shunting βAPP processing into amyloidogenic (Aβ42 generating) pathways [9,10,25,31,37,38]. As further discussed below, several recently published reports indicate a significant up-regulation of miRNA-146a and CFH and TSPAN12 down-regulation in human primary cells and tissues [16,25,31].
Figure 1.
Color-coded cluster analysis of significantly up-regulated miRNAs in (A) in the neocortex of AD versus age-matched controls and (B) in the retina of AMD versus age-matched controls; relative expression levels for miRNA- 125b, miRNA-146a and miRNA-155 the brain neocortex and retina are shown in (C) relative to levels for an unchanging control miRNA-183 which was set to 1.0 and marked by a dashed horizontal line. In this selective sampling, all control (N=5) and AD (N=5) neocortical samples were obtained from the superior temporal neocortex (Brodmann area A22). Retinal samples including controls (N=5) and AMD (N=5) included whole retina; all control, AD and AMD samples had post-mortem intervals (PMI; death to brain freezing interval) of 2 h or less [4-8,11,12,43]. Controls were age-matched to moderate-to-late stages of AD or AMD; increases in specific miRNAs increased as disease stage advanced [7, 23; data not shown]; further details on the pathology of these samples have been recently published [7,9,23,45]. There were no significant differences in age, PMI, or RNA yield or quality between either the brain or retinal groups. Of the 12 different homo sapien micro-RNAs (hsa-miRNAs) shown, miRNA-146a, miRNA-125b and miRNA- 155 exhibited the greatest up-regulation compared with age-matched controls (p<0.01, ANOVA); in some samples miRNA-9 also showed a trend for up-regulation; miRNA-9 can also target the CFH mRNA 3′-UTR (see below) [22; unpublished observations]. We cannot exclude the participation of other human brain-enriched miRNAs or other small non-coding RNAs (sncRNAs) which may additionally contribute to the neuropathological mechanisms which define the AD or AMD process; *p<0.01 (ANOVA).
Mathematical considerations of brain and retinal miRNA complexity
As previously mentioned, degenerating human neocortical and retinal cells display an intriguing overlap in the speciation and complexity of a small family of specific miRNA’s expressed within those tissues (Figure 1). Using the most advanced MRA-1001 miRNA microfluidic chip analytical platform currently available (LC Sciences, Houston TX) to date only 1898 miRNAs have been detected in the cells of all human tissues. However, depending on cell type, nervous tissues only contain a small fraction of all known miRNAs and some are far more abundant than others [39,40-42]. Bioinformatics and sequence analysis further indicates that a 22 nucleotide single stranded RNA composed of 4 different ribonucleotides can have over 1013 possible sequence combinations, so the fact that there typically only about 2 x 103 abundant miRNAs in all types of human cells suggests a very high developmental and evolutionary selection pressure to utilize only specific miRNA oligonucleotide sequences that will yield biologically useful miRNA-mRNA interactions. Put another way, miRNA-mRNA pairings have been strongly selected over evolution to maximize their efficiency in regulating brain and retinal gene expression as well as expression in other tissues. Further, miRNAs are highly developmental stage-, tissue- and cell-specific, even in adjacent cell types, and in human brain neocortical and retinal cells high abundance miRNAs number probably less than 102 individual species [31, 39-42, 45; unpublished observations]. The fact that out of the top 12 changed miRNAs in degenerating neocortex and retina that 5 are common to each tissue was the impetus for these studies, which collectively suggest that certain of these miRNAs might be significant in genetic regulatory mechanisms common to inflammatory neurodegenerative mechanisms characteristic of both AD and AMD.
AD and AMD; pathological similarities - senile plaques (SP) and drusen
AD and AMD are characterized pathologically by the progressive appearance within the brain parenchyma and retinal macular region of pro-inflammatory, highly insoluble, amyloid beta (Aβ) peptide enriched pathological lesions known, respectively, as neocortical senile plaques (SP) and retinal drusen. Although there are several other disease-related components and lesions associated with AD and AMD, SP and drusen represent major pathological hallmarks of these age-related degenerative disorders of the neocortex and retina [44-50,75]. SP are roughly spherical 20-30 μm in diameter, non-crystalline, argyrophilic globular masses composed of a diffuse-to-dense core of various amyloid peptides and other oxidized protein and lipid components; drusen are approximately 5- 15 μm diameter, circular, yellow-white lesions located throughout the fundus of the retina but more so in the macular region, around the optic disc or the periphery [46-50]. The biological environment in which these lesions initiate and grow may be their only major difference in establishing their final size and ultimate morphology. In AD SP usually form exterior to the neuron in an ‘unrestricted’ brain parenchyma free of connective tissue, allowing these lesions to form into globular aggregations considerably larger than drusen [47]. In AMD drusen form in a far more restricted and delineated region usually between the basement membrane of the retinal epithelial (RPE) cell layer and Bruch’s membrane [47-50]. In the study of neuropathology and pathobiology, strongly similar constituents of both SP and drusen are apparent; SP and drusen each contain upwards of about 100 different end-stage protein, lipid and other constituents including toxic trace metals [32,46-51]. Major common components of SP and drusen include beta-amyloid precursor protein (βAPP), the α-, β- and γ-secretases which cleave βAPP to generate a wide range of βAPP catabolites including self-aggregating Aβ peptides 37- 42 amino acids in length (Aβ37-Aβ42) [17,32]. Other important, common constituents of SP and drusen include α- and β-crystallin, serum related molecules such as amyloid P, clusterin, collagen, complement proteins such as C3, C5, complement factor H (CFH) and the C3b and C5b-9 complex, apolipoproteins B and E, esterified and non-esterified cholesterol, oxidized lipids and phospholipids, 24S-hydroxycholesterol, neutral vitronectin, neprilysin, vasogenic factors such as VEGF, vimentin, and the secretase-accessory proteins which further process βAPP into multiple amyloid peptides [47,48]. Interestingly, the major neurotrophic βAPP metabolite sAPPα attains some of its highest concentrations in the retina, the RPE and particularly in the vitreous humor located just adjacent to the retina [9, 30; unpublished observations]. SP and drusen also contain trace metals such as aluminum and zinc which are thought to assist in the aggregation of the constituents contained within these insoluble lesions [46-48; unpublished observations]. In both cases, waste products from neurons, astroglia, photoreceptors, RPE and retinal ganglion or blood plasma components via the highly vascularized neocortex and retinal choriocapillaris provide a steady source of extracellular serum-derived material for SP and drusen formation that over time support their enlargement. Indeed oxidative and immune-mediated modifications appear to further support their progressive aggregation, growth and expansion [46-50]. Interestingly, both highly insoluble pathological lesions are progressively deposited as a function of age, and many of their components represent ‘end-stage’ or metabolic waste products highly resistant to degradation by the endoplasmic-reticulum-associated protein degradation (ERAD) and ubiquitin-proteasome systems [46-52].
AD and AMD; similarities in specifically up-regulated miRNAs
Tissue- and cell-specific families and sub-families of miRNAs are emerging as extremely important post-transcriptional regulators of messenger RNA (mRNA) complexity and in shaping the transcriptome of a cell in development, health, aging and disease [16,23-26,34-36]. The human brain and retina rely on a specific subset of these miRNAs to shape their gene expression patterns and it is remarkable that the total number of abundant miRNAs they utilize to do this are probably less than 102 [53, 54; unpublished observations]. Recently, using miRNA arrays, DNA arrays, Northern micro-dot blots, RT-PCR, Western analysis, ELISA-based assays, bioinformatics and miRNA-mRNA pairing algorithms it has been shown that diseased brain and retina share a number of abundant pathology-relevant miRNAs, and related families of expressed pro-inflammatory and amyloidogenic genes are similarly targeted (Figure 1) [16,23,38-42]. As previously mentioned these include at least four inducible miRNAs known to be up-regulated by NF-кB that include miRNA-9, miRNA-125b, miRNA-146a and miRNA-155 (Figure 1) [22-25,31]. These up-regulated miRNAs in turn appear to down-regulate multiple target mRNAs and thus regulate a family of potentially pathogenic genes including 15- lipoxygenase (15-LOX), synapsin-2 (SYN-2), tetraspanin- 12 (TSPAN12) and complement factor H (CFH) [7,9,12,14,16,21-25]. These data further indicate that as few as four abundant NF -кB-mediated miRNAs have tremendous potential to contribute to many interrelated aspects of brain and retinal cell function and dysfunction, including non-homeostatic neurotrophic support (15-LOX), synaptogenesis (SYN-2), amyloidogenesis (TSPAN12) and innate immune signaling and inflammation (CFH) [21-25,28,30,31].
miRNA control of complement factor H (CFH) expression
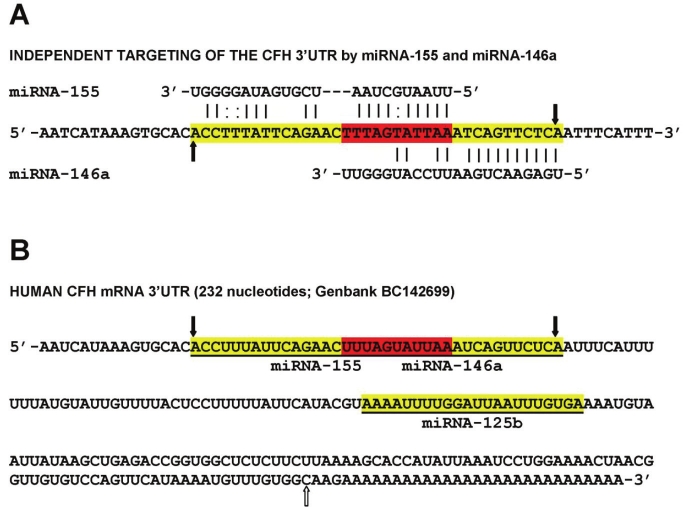
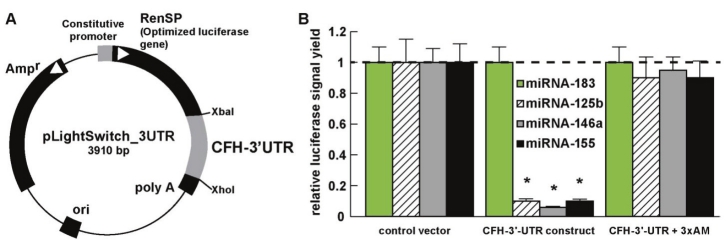
Importantly, up-regulated miRNA-125b, miRNA- 146a, miRNA-155 and perhaps miRNA-9, and subsequent down-regulation of CFH gene expression may in part explain the association of CFH deficiencies with innate immune system dysfunction and inflammatory degeneration in both AD and AMD (Figure 2) [2-5,7-12,14-16,42,45]. As previously mentioned, a mutant, defective or ‘loss of function’ CFH, as generated by the Y402H polymorphism, may have analogous pathophysiological effects as insufficient CFH [2-5, 33, 41, 45; unpublished observations]. Targeting of the CFH mRNA 3’-UTR by miRNA-125b, miRNA-146a and miRNA-155, either separately or as a group within the MiRC region, has recently been demonstrated; a MiRC region is a miRNA regulatory control region found within a 3’-UTR to which multiple miRNAs may bind (Figure 2) [14,16,22-25,45]. Note that miRNA-9 also has a binding site in the CFH mRNA 3’-UTR however its elevation in AD and AMD was much more variable than miRNA- 125b, miRNA-146a and miRNA-155. An overlap of the miRNA-155 and miRNA-146a binding sites in the CFH mRNA 3’-UTR suggests evolutionary relationships or different, or overlapping, modes of CFH expression control in the brain and retina (Figure 2) [14,16,23,73,74]. Additional experiments using a 3’UTR-luciferase expression assay that utilizes a luciferase gene fused to the 232 nt human CFH 3’UTR (pLightSwitch-3’UTR Luciferase assay system; Cat # S801178; Switchgear Genomics, Menlo Park CA) shows that indeed the CFH mRNA 3’- UTR is highly sensitive to miRNA-125b, miRNA- 146a and miRNA-155 inhibitory actions and that these may be restored using anti-miRNA strategies using nanomolar cocktails of anti-miRNAs (antagomirs; Figure 3) [23,25,45]. Lastly, the small size of miRNAs and the recent identification of miRNA-protective protein and miRNA-containing vesicles suggests that miRNAs may be a novel means for paracrine and related forms of inter-cellular and inter-tissue communication which may in part explain the propagation of the AD and AMD process over time [31,55,56].
Figure 2.
Overlapping miRNA-155 and miRNA-146a high affinity binding sites in the CFH mRNA 3’-UTR (energy of association of less than -22 kcal/mol) defines an exceptionally stable miRNA-mRNA interaction and a potential CFH mRNA 3’-UTR miRNA regulatory control (MiRC) region 5’-TTTAGTATTAA-3’ (see text) [23,31,25,45]; (A) entire nucleotide sequence of miRNA-155 and miRNA-146a interaction is highlighted in yellow; overlapping recognition for both miRNA-155 and miRNA-146a is highlighted in red; vertical black arrows indicate boundaries of miRNA-146a-miRNA- 155-CFH mRNA 3’-UTR potential region of interaction; (B) shows entire 232 nucleotide sequence of the human CFH mRNA 3’-UTR; as in (A) entire region of miRNA-155 and miRNA-146a interaction is highlighted in yellow; overlapping MiRC region is highlighted in red; black vertical arrows indicate boundaries of miRNA-mRNA potential region of interaction; the single miRNA-9 site is upstream to this site; the miRNA-125b recognition region is distal to this site; white arrow = polyadenylation site; a 28 nucleotide polyadenylation feature is shown.
Figure 3.
Functional validation of miRNA-125b-, miRNA-146a- and miRNA-155 CFH-3’UTR interaction using a 3’UTR-luciferase expression assay (luciferase gene fused to the human CFH 3’UTR; pLightSwitch-3’UTR Luciferase assay system; Cat #S801178; Switchgear Genomics, Menlo Park CA); (A) RPE-1 or primary RPE cells [44] transfected with either a co-transfection control vector carrying a scrambled 232 bp 3’UTR, or the entire human 232 bp CFH-3’UTR, were treated exogenously with either stressors (IL-1β+TNFα) and/or LNA-protected miRNA-183, miRNA-125b, miRNA- 146a or mRNA-155 [23,45,46]; (B) while neither the control vector, miRNA-183-treated nor 4 other sequence-related human miRNAs showed any effect on relative luciferase signal yield in transfected RPE-1 cells (dashed horizontal line set to 1.0), miRNA-125b, miRNA-146a or miRNA-155 reduced luciferase signal to 8% or less of control; a cocktail containing 5 nM each of anti-miRNA-125b, anti-miRNA-146a and anti-miRNA-155 (3xAM) restored luciferase expression, as did individual AM-125b, AM-146a, AM-155 (data not shown) [23,45]; N=5; *p<0.01 (ANOVA).
Up-regulated miRNAs, their mRNA targets and effects on gene expression in the brain and retina
miRNA-9
Although miRNA-9 was easily detected in both the neocortex and retina it was found to be one of the most variably abundant miRNA in these tissues, perhaps indicative of its highly inducible nature [59, 60, unpublished observations]. With a half-life of only 30-60 minutes in both cultured human primary brain cells and human brain tissues, miRNA-9 is a brain and retinal abundant miRNA possessing the shortest half-life of any miRNA studied to date [42,57]. Interestingly, miRNA-9 exerts significant control of neural progenitor cell proliferation and differentiation in the developing telencephalon by regulating the expression of multiple transcription factors including Foxg1, Nr2e1 and Pax6 [58]. We further note that miRNA-9 is developmentally regulated and abundant in ARPE-19 cells, and decreases in expression as human brain and retinal cells age in primary culture, in accordance with its established role as an age-related miRNA [43,59,60]. Knockdown of miRNA-9 in neural progenitor cells, results in an inhibition of neurogenesis along the anterior-posterior axis of the CNS, and miRNA-9 is also significantly down-regulated in the tissues of fetuses with severe congenital abnormalities, including anencephaly [61,62]. In a recent miRNA analysis of whole murine globes using both microarray and RNA in situ hybridization procedures, miRNA-9 expression was restricted to the retina and not seen in the cornea, lens, iris or ciliary body [76-80].
miRNA-125b
One of the most human brain and retinal abundant, if not the most abundant CNS miRNA and intensively studied miRNA is the inducible miRNA-125b, first shown to be up-regulated in differentiating mouse and human neurons, and since implicated in mammalian neuronal development and function in the brain [63]. Both miRNA-125b and miR-9 play central roles in neuronal differentiation during retinal development [60]. The abundance of miRNA-125b has been shown to be significantly induced by neurotoxic metal sulfates that generate robust levels of oxidative stress, and miRNA-125b is also up-regulated in brain cancers where it apparently targets and down-regulates CDKN2A, a negative regulator of cell growth [41,42,64,65]. Up-regulated miRNA-125b further associates with glial cell proliferation and astrogliosis in inflammatory neurodegenerative conditions such as AD and Down’s syndrome, as well as in glioma and glioblastoma multiforme [64]. Interestingly up-regulation of miRNA-125b is associated with down-regulation of both the 15- lipoxygenase (15-LOX) and the synaptic vesicle-associated phosphoprotein synapsin-2 (SYN-2) [21-25]. The 15-LOX enzyme is essential in the conversion of the essential omega-3 fatty acid docosahexaenoic acid (DHA) into the potent neuroprotectin D1 (NPD1), and deficits in 15- LOX correlate with NPD1 deficits in AD brain and other human tissues [46,66]. The neuronal -enriched phosphoprotein SYN-2 that associates with the cytoplasmic surface of synaptic vesicles is also a miRNA-125b target [67]. MiRNA- 125b up-regulation is further associated with SYN-2 down-regulation in inflammatory neurodegeneration and CFH down-regulation in human primary astroglial cells [16,22,64,67].
miRNA-146a
miRNA-146a was first described as an NF-кB-regulated pro-inflammatory miRNA that was found to target signaling proteins of innate immune responses, and more specifically the 3’- UTR of complement factor H (CFH) in human monocytes [68]. Elevated miRNA-146a in AD brain was subsequently shown to also target CFH and the interleukin-1 associated kinase 1 (IRAK-1) mRNAs, and is believed to contribute to altered innate immune responses and neuroinflammation in degenerating human brain cells and tissues in inflammatory neurodegenerative diseases including AD, AMD, prion disease, in experimental and human temporal lobe epilepsy and in experimental diabetes in retinal microvessel endothelial cells [14,21,23,25,41,45,69-71]. Although CFH was classically regarded as highly abundant human serum protein of hepatic origin, abundant CFH presence in brain and retinal tissues suggests CFH involvement in the innate immune response and inflammatory regulation within the ‘privileged immunology’ of these tissues [2-5,14,23]. While miRNA-146a is the least basally abundant miRNA when compared to miRNA-9, miRNA-125b and miRNA-155, it is the most inducible and up-regulated miRNA in human neuronal and astroglial cells compared to all other NF-кB-regulated miRNA species so far indentified (Figure 1) [21-23,45]. Interestingly, miRNA- 146a may be the most induced of these up-regulated miRNAs due to the presence of 3 tandem, canonical NF-кB binding sites in the human pre-miRNA-146a promoter [23,45,68].
miRNA-155
MiRNA-155 is a cytokine, NF-кB and cell cycle-regulated miRNA also abundant in the human neocortex and retina, and has approximately 45% sequence homology to miRNA-146a (Figure 2) [25,31,72]. Interestingly, miRNA- 146a and miRNA-155 have partially overlapping binding (recognition) sites in the CFH mRNA 3’- UTR, and miRNA-146a and miRNA-155 together define an overlapping miRNA regulatory control (MiRC) region in the CFH 3’-untranslated region (3’-UTR; 5’-TTTAGTATTAA-3’) wherein either of these miRNAs may interact (Figure 2). As is true for miRNA-146a, inflammatory cytokines increase miRNA-155 expression in human retinal pigment epithelial cells by activation of the JAK/ STAT signaling pathway [72]. Several additional studies indicate that miRNA-146a and miR-155 together play a key role in regulating several critical pathways that orchestrate innate immune responses and chronic inflammatory processes that are conserved across many different human tissue systems [77,81].
Summary and conclusions
The five main conclusions of the data presented in this report are: (a) that normally aging brain neocortex and retinal tissues exhibit overlap in the speciation of their resident miRNAs; (b) that several brain and retinal miRNAs are similarly up-regulated during age-related inflammatory degeneration, and these include miRNA-9 and more significantly miRNA-125b, miRNA-146a and miRNA-155; (c) that miRNA-125b, miRNA- 146a and miRNA-155 all have high affinity binding sites in the CFH mRNA 3’-UTR, supportive of their roles in regulation of CFH and the immune response; (d) that miRNA-146a and miRNA-155 both recognize an overlapping MiRC region in the CFH mRNA 3’-UTR that is conducive to down -regulation in the expression of CFH in both the brain and retina; and (e) that multiple miRNAs are capable of targeting and down-regulating an important repressor of the innate immune and inflammatory response. It is tempting to speculate that a subfamily of human miRNAs that include miRNA-125b, miRNA-146a, miRNA-155, and perhaps miRNA-9 and others, may help coordinate innate immune and inflammatory signals across the entire anteroposterior axis of the retinal-primary visual cortex pathway, and perhaps also in other immune-responsive cells and tissues. Other brain and retinal mRNA 3’- UTR targets, and ensuing down-regulated gene expression are probably affected by this miRNA-mRNA control system. This work in progress presents several testable hypotheses. For example it will extremely interesting to expand our understanding of the role of NF-кB with miRNAs and with other transcription factors, chromatin-remodeling mechanisms, and other epigenetic influences on specific miRNA-mRNA activation pathways to further understand their surprisingly dynamic interactive roles, and their coordinate contribution to the neurogenetic control of brain cell aging in health, aging and degenerative disease. It will be further interesting to see what individual brain and retinal cell types might be contributing to these multiple miRNA and CFH aberrations in AD and AMD [16,22,31,45], and how this knowledge can be profitably manipulated using anti-miRNA and related pharmacological approaches that have not yet been considered in the effective management of these diseases.
Acknowledgements
The studies were presented in part at the 11th annual Alzheimer’s Association International Conference on Alzheimer’s disease (AAICAD 11) conference in Versailles, France, 16-20 July 2011; thanks are extended to Drs. C Chen, Y Zhao, EI Rogaev, E. Head, Chris Hebel, W Poon and Darlene Guillot for provision of human brain and retina cells, tissues and/or extracts and expert technical assistance, unpublished data, recent pre-publications in this research area and helpful interpretative discussions. Some of the human brain and retinal tissues used in this study were provided by the Southern Eye Bank (Metairie, LA), the Institute for Memory Impairments and Neurological Disorders and the University of California at Irvine Alzheimer's Disease Research Center (UCI-ADRC); funding for the UCI-ADRC was provided by NIH/NIA Grant P50 AG16573. Research on the structure and function of NF-кB and miRNA expression in the Lukiw laboratory were supported through Grant Number P20RR016456 from the National Center for Research Resources (NCRR), Translational Research Initiative (TRI) Grants from LSU Health Sciences Center New Orleans, an Alzheimer Association Investigator-Initiated Research Grant IIRG-09-131729 (WJL), and NIH NIA Grants AG18031 and AG038834 (WJL). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Center for Research Resources, or the National Institutes of Health.
References
- 1. http://www.preventblindness.org/resources/visiondata.html; http://www.nei.nih.gov/ health/maculardegen/armdfacts.asp.
- 2.Zipfel PF, Lauer N, Skerka C. The role of complement in AMD. Adv Exp Med Biol. 2010;703:9–24. doi: 10.1007/978-1-4419-5635-4_2. [DOI] [PubMed] [Google Scholar]
- 3.Donoso LA, Vrabec T, Kuivaniemi H. The role of complement Factor H (CFH) in age-related macular degeneration: a review. Surv Ophthalmol. 2010;55:227–246. doi: 10.1016/j.survophthal.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010;128:349–358. doi: 10.1001/archophthalmol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondo N, Bessho H, Honda S, Negi A. Complement factor HY402H variant and risk of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2011;118:339–344. doi: 10.1016/j.ophtha.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Takata K, Kitamura Y. Molecular approaches to the treatment, prophylaxis, and diagnosis of Alzheimer's disease: tangle formation, amyloid- β peptides, and microglia in Alzheimer's disease. J Pharmacol Sci. 2012 doi: 10.1254/jphs.11r10fm. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Cui JG, Hill JM, Zhao Y, Lukiw WJ. Expression of inflammatory genes in the primary visual cortex of late-stage Alzheimer's disease. Neuroreport. 2007;18:115–119. doi: 10.1097/WNR.0b013e32801198bc. [DOI] [PubMed] [Google Scholar]
- 8.Ohno-Matsui K. Parallel findings in age-related macular degeneration and Alzheimer's disease. Prog Retina Eye Res. 2011;30:217–238. doi: 10.1016/j.preteyeres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Alexandrov PN, Pogue AI, Bhattacharjee S, Lukiw WJ. Retinal amyloid-beta and complement factor H expression in transgenic models of Alzheimer’s disease. Neuroreport. 2011;22:623–627. doi: 10.1097/WNR.0b013e3283497334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu K, Chan TF, Wu A, Leung IY, So KF, Chang RC. Neurodegeneration of the retina in mouse models of Alzheimer's disease: what can we learn from the retina? Age (Dordr) 2011 doi: 10.1007/s11357-011-9260-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaarniranta K, Salminen A, Haapasalo A, Soininen H, Hiltunen M. Age-related macular degeneration (AMD): Alzheimer's disease in the eye? J Alzheimers Dis. 2011;24:615–631. doi: 10.3233/JAD-2011-101908. [DOI] [PubMed] [Google Scholar]
- 12.Cui JG, Kuroda H, Chandrasekharan NV, Pelaez RP, Simmons DL, Bazan NG, Lukiw WJ. Cyclooxygenase-3 gene expression in Alzheimer hippocampus and in stressed human neural cells. Neurochem Res. 2004;29:1731–1737. doi: 10.1023/b:nere.0000035809.70905.8a. [DOI] [PubMed] [Google Scholar]
- 13.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YY, Cui JG, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ. Increased expression of miRNA-146a in Alzheimer's disease transgenic mouse models. Neuroscience Letters. 2011;487:94–98. doi: 10.1016/j.neulet.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher EL, Jobling AI, Vessey KA, Luu C, Guymer RH, Baird PN. Animal models of retinal disease. Prog Mol Biol Transl Sci. 2011;100:211–286. doi: 10.1016/B978-0-12-384878-9.00006-6. [DOI] [PubMed] [Google Scholar]
- 16.Li YY, Cui JG, Dua P, Pogue AI, Bhattacharjee S, Lukiw WJ. Differential expression of miRNA- 146a-regulated inflammatory genes in human primary neural, astroglial and microglial cells. Neuroscience Letters. 2011;499:109–113. doi: 10.1016/j.neulet.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutescu RM, Li QX, Crowston J, Masters CL, Baird PN, Culvenor JG. Amyloid precursor protein processing and retinal pathology in mouse models of Alzheimer's disease. Graefes Arch Clin Exp Ophthalmol. 2009;247:1213–1221. doi: 10.1007/s00417-009-1060-3. [DOI] [PubMed] [Google Scholar]
- 18.Ramkumar HL, Zhang J, Chan CC. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD) Prog Retin Eye Res. 2010;29:169–190. doi: 10.1016/j.preteyeres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeiss CJ. Animals as models of age-related macular degeneration: an imperfect measure of the truth. Vet Pathol. 2010;47:396–413. doi: 10.1177/0300985809359598. [DOI] [PubMed] [Google Scholar]
- 20.Telander DG. Inflammation and age-related macular degeneration (AMD) Semin. Ophthalmol. 2011;26:192–197. doi: 10.3109/08820538.2011.570849. [DOI] [PubMed] [Google Scholar]
- 21.Lukiw WJ, Dua P, Pogue AI, Eicken C, Hill JM. Up -regulation of micro RNA-146a (miRNA-146a), a marker for inflammatory neurodegeneration, in sporadic Creutzfeldt-Jakob disease (sCJD) and Gerstmann-Straussler Scheinker (GSS) syndrome. Journal of Toxicology and Environmental Health. 2011;74:1460–1468. doi: 10.1080/15287394.2011.618973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogue AI, Percy ME, Cui JG, Li YY, Bhattacharjee S, Hill JM, Kruck TPA, Zhao Y, Lukiw WJ. Up-regulation of NF-kB-sensitive miRNA-125b and miRNA-146a in metal sulfate-stressed human astroglial (HAG) primary cell cultures. J Inorganic Biochem. 2011;105:1434–1437. doi: 10.1016/j.jinorgbio.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukiw WJ, Zhao Y, Cui JG. An NF-kB-sensitive micro RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human neural cells. J Biol Chem. 2008;283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YY, Alexandrov PN, Pogue AI, Zhao Y, Bhattacharjee S, Lukiw WJ. miRNA-155 up-regulation and complement factor H (CFH) deficits in Down’s Syndrome. Neuroreport. 2012;23:168–173. doi: 10.1097/WNR.0b013e32834f4eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukiw WJ, Alexandrov PN. Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’1(1):s disease brain. Molecular Neurobiology. 2012 doi: 10.1007/s12035-012-8234-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasluosta CF, Dua P, Lukiw WJ. Nearest hyperplane distance neighbor clustering algorithm applied to gene co-expression analysis in Alzheimer's disease, Proceedings of the IEEE Engineering in Medicine and Biology Society. EMBS. 2011;6091344:5559–5562. doi: 10.1109/IEMBS.2011.6091344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M, Forrester JV, Xu H. Synthesis of complement factor H (CFH) by retinal pigment epithelial cells is down-regulated by oxidized photoreceptor outer segments. Exp Eye Res. 2007;84:635–645. doi: 10.1016/j.exer.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Kociok N, Joussen AM. Enhanced expression of the complement factor H (CFH) mRNA in proliferating human RPE cells. Graefes Arch Clin Exp Ophthalmol. 2010;248:1145–1153. doi: 10.1007/s00417-010-1371-4. [DOI] [PubMed] [Google Scholar]
- 29.Ding JD, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, Mace BE, Sullivan P, Jamison JA, Kelly U, Harrabi O, Bollini SS, Dilley J, Kobayashi D, Kuang B, Li W, Pons J, Lin JC, Bowes Rickman C. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci USA. 2011;108:279–287. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prakasam A, Muthuswamy A, Ablonczy Z, Greig NH, Fauq A, Rao KJ, Pappolla MA, Sambamurti K. Differential accumulation of secreted βAPP metabolites in ocular fluids. J Alzheimers Dis. 2010;20:1243–1253. doi: 10.3233/JAD-2010-100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukiw WJ. NF-kB-regulated micro RNAs (miRNAs) in primary human brain and retinal cells. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.11.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barile GR, Schmidt AM. RAGE and its ligands in retinal disease. Curr Mol Med. 2007;7:758–765. doi: 10.2174/156652407783220778. [DOI] [PubMed] [Google Scholar]
- 33.Zetterberg M, Landgren S, Andersson ME, Palmér MS, Gustafson DR, Skoog I, Minthon L, Thelle DS, Wallin A, Bogdanovic N, Andreasen N, Blennow K, Zetterberg H. Association of complement factor H Y402H gene polymorphism with Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147:720–726. doi: 10.1002/ajmg.b.30668. [DOI] [PubMed] [Google Scholar]
- 34.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian miRNAs act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang LL, Huang Y, Wang G, Chen SD. The potential role of microRNA-146 in Alzheimer's disease: Biomarker or therapeutic target? Medical Hypotheses. 2012;78:398–401. doi: 10.1016/j.mehy.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Jiang M, Xiang Y, Wang D, Gao J, Liu D, Liu Y, Liu S, Zheng D. Dysregulated expression of miRNA-146a contributes to age-related dysfunction of macrophages. Aging Cell. 2012;11:29–40. doi: 10.1111/j.1474-9726.2011.00757.x. [DOI] [PubMed] [Google Scholar]
- 37.Xu D, Sharma C, Hemler ME. Tetraspanin 12 regulates ADAM10-dependent cleavage of amyloid precursor protein. FASEB J. 2009;23:3674–3681. doi: 10.1096/fj.09-133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye W. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 39.Chan LW. Modeling equilibrium of microRNA Expression. Front Genet. 2011;2:35. doi: 10.3389/fgene.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burmistrova OA, Goltsov AY, Abramova LI, Kaleda VG, Orlova VA, Rogaev EI. MicroRNA in schizophrenia: genetic and expression analysis of miR-130b (22q11) Biochemistry (Mosc) 2007;72:578–582. doi: 10.1134/s0006297907050161. [DOI] [PubMed] [Google Scholar]
- 41.Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer's disease temporal lobe neocortex. Neurosci Lett. 2009;459:100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 43.Yuva-Aydemir Y, Simkin A, Gascon E, Gao FB. MicroRNA-9: functional evolution of a conserved small regulatory RNA. RNA Biol. 2011;8:557–564. doi: 10.4161/rna.8.4.16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao JL, Yu J, Huang K, Hu J, Diemer T, Ma Z, Dvash T, Yang XJ, Travis GH, Williams DS, Bok D, Fan G. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet. 2010;19:4229–4238. doi: 10.1093/hmg/ddq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ. Differential regulation of interleukin-1 receptor- associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-kB in stressed human astroglial cells. J Biol Chem. 2010;285:38951–38960. doi: 10.1074/jbc.M110.178848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 (NPD1) induces neuronal survival via secretase- and PPARγ- mediated mechanisms in Alzheimer's disease models. PLoS One. 2011;6:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atwood CS, Martins RN, Smith MA, Perry G. Senile plaque composition and post-translational modification of amyloid-beta peptide and associated proteins. Peptides. 2002;23:1343–1350. doi: 10.1016/s0196-9781(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 48.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Li CM, Rudolf M, Belyaeva OV, Chung BH, Messinger JD, Kedishvili NY, Curcio CA. Lipoprotein particles of intraocular origin in human Bruch membrane: an unusual lipid profile. Invest Ophthalmol Vis Sci. 2009;50:870–877. doi: 10.1167/iovs.08-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudolf M, Clark ME, Chimento MF, Li CM, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung T, Catalgol B, Grune T. The proteasomal system. Mol Aspects Med. 2009;30:191–296. doi: 10.1016/j.mam.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Hebert DN, Bernasconi R, Molinari M. ERAD substrates: which way out? Semin Cell Dev Biol. 2010;21:526–532. doi: 10.1016/j.semcdb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Decembrini S, Bressan D, Vignali R, Pitto L, Mariotti S, Rainaldi G, Wang X, Evangelista M, Barsacchi G, Cremisi F. MicroRNAs couple cell fate and developmental timing in retina. Proc Natl Acad Sci USA. 2009;106:21179–21184. doi: 10.1073/pnas.0909167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Hooks JJ, Redmond TM. Inflammatory cytokines regulate microRNA-155 expression in human retinal pigment epithelial cells by activating JAK/STAT pathway. Biochem Biophys Res Commun. 2010;402:390–395. doi: 10.1016/j.bbrc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA. 2009;106:5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibata M, Nakao H, Kiyonari H, Abe T, Aizawa S. MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J Neurosci. 2011;31:3407–3422. doi: 10.1523/JNEUROSCI.5085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kutty RK, Samuel W, Jaworski C, Duncan T, Nagineni CN, Raghavachari N, Wiggert B, Redmond TM. microRNA expression in human retinal pigment epithelial (ARPE-19) cells: increased expression of microRNA-9 by N-(4- hydroxyphenyl)retinamide. Mol Vis. 2010;16:1475–1486. [PMC free article] [PubMed] [Google Scholar]
- 60.Arora A, Guduric-Fuchs J, Harwood L, Dellett M, Cogliati T, Simpson DA. Prediction of microRNAs affecting mRNA expression during retinal development. BMC Dev Biol. 2010;10:1. doi: 10.1186/1471-213X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonev B, Pisco A, Papalopulu N. MicroRNA-9 reveals regional diversity of neural progenitors along the anterior-posterior axis. Dev Cell. 2011;20:19–32. doi: 10.1016/j.devcel.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Z, Chang H, Li Y, Zhang T, Zou J, Zheng X, Wu J. MicroRNAs: potential regulators involved in human anencephaly. Int J Biochem Cell Biol. 2010;42:367–374. doi: 10.1016/j.biocel.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 63.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pogue AI, Cui JG, Li YY, Zhao Y, Culicchia F, Lukiw WJ. miRNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci Lett. 2010;476:18–22. doi: 10.1016/j.neulet.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 65.Feng J, Kim ST, Liu W, Kim JW, Zhang Z, Zhu Y, Berens M, Sun J, Xu J. An integrated analysis of germline and somatic, genetic and epigenetic alterations at 9p21.3 in glioblastoma. Cancer. 2011;118:232–240. doi: 10.1002/cncr.26250. [DOI] [PubMed] [Google Scholar]
- 66.Hennig R, Kehl T, Noor S, Ding XZ, Rao SM, Bergmann F, Fürstenberger G, Büchler MW, Friess H, Krieg P, Adrian TE. 15-lipoxygenase-1 production is lost in pancreatic cancer and over -expression of the gene inhibits tumor cell growth. Neoplasia. 2007;9:917–926. doi: 10.1593/neo.07565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao PJ, Zhu M, Pyun EI, Brooks AI, Therianos S, Meyers VE, Coleman PD. Defects in expression of genes related to synaptic vesicle trafficking in frontal cortex of Alzheimer's disease. Neurobiol Dis. 2003;12:97–109. doi: 10.1016/s0969-9961(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 68.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-кB-dependent induction of miRNA -146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saba R, Gushue S, Huzarewich RL, Manguiat K, Medina S, Robertson C, Booth SA. MicroRNA 146a (miR-146a) is over-expressed during prion disease and modulates the innate immune response and the microglial activation state. PLoS One. 2012;7:e30832. doi: 10.1371/journal.pone.0030832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aronica E, Fluiter K, Iyer A, Zurolo E, Vreijling J, van Vliet EA, Baayen JC, Gorter JA. Expression pattern of miRNA-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur J Neurosci. 2010;31:1100–1107. doi: 10.1111/j.1460-9568.2010.07122.x. [DOI] [PubMed] [Google Scholar]
- 71.Feng B, Chen S, McArthur K, Wu Y, Sen S, Ding Q, Feldman RD, Chakrabarti S. miR-146a- Mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60:2975–2984. doi: 10.2337/db11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Hooks JJ, Redmond TM. Inflammatory cytokines regulate microRNA-155 expression in human retinal pigment epithelial cells by activating JAK/STAT pathway. Biochem Biophys Res Commun. 2010;402:390–395. doi: 10.1016/j.bbrc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, Thompson HW, Bazan NG. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest Ophthalmol Vis Sci. 2003;44:4163–4170. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- 74.Kovacs B, Lumayag S, Cowan C, Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin- induced diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:4402–4409. doi: 10.1167/iovs.10-6879. [DOI] [PubMed] [Google Scholar]
- 75.Lukiw WJ, Mukherjee PK, Cui JG, Bazan NG. A2E selectively induces COX-2 in ARPE-19 and human neural cells. Curr Eye Res. 2006;31:259–263. doi: 10.1080/02713680600556974. [DOI] [PubMed] [Google Scholar]
- 76.Hébert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends in Neuroscience. 2009;32:199–206. doi: 10.1016/j.tins.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Tsitsiou E, Lindsay MA. microRNAs and the immune response. Curr Opin Pharmacol. 2009;9:514–520. doi: 10.1016/j.coph.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maiorano NA, Hindges R. Non-Coding RNAs in Retinal Development. Int J Mol Sci. 2012;13:558–578. doi: 10.3390/ijms13010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu S. microRNA expression in the eyes and their significance in relation to functions. Prog Retin Eye Res. 2009;28:87–116. doi: 10.1016/j.preteyeres.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Karali M, Peluso I, Gennarino VA, Bilio M, Verde R, Lago G, Dollé P, Banfi S. miRNeye: a microRNA expression atlas of the mouse eye. BMC Genomics. 2010;11:715. doi: 10.1186/1471-2164-11-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roy S, Sen CK. MiRNA in innate immune responses: novel players in inflammation. Physiol Genomics. 2011;43:557–565. doi: 10.1152/physiolgenomics.00160.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]