Abstract
Hedgehog (Hh) signaling is frequently activated in human cancer, including esophageal cancer. Most esophageal cancers are diagnosed in the advanced stages, therefore, identifying the very alterations that drive esophageal carcinogenesis may help designing novel strategies to diagnose and treat the disease. Analysis of Hh signaling in precancerous lesions is a critical first step in determining the significance of this pathway for carcinogenesis. Here we report our data on Hh target gene expression in 174 human esophageal specimens [28 esophageal adenocarcinomas (EAC), 19 Barrett’s esophagus, 103 cases of esophageal squamous cell carcinoma (ESCC), and 24 of squamous dysplastic lesions], and in two rat models of esophageal cancer. We found that 96% of human EAC express Hh target genes. We showed that PTCH1 expression is the most reliable biomarker. In contrast to EAC, only 38% of ESCC express Hh target genes. We found activation of Hh signaling in precancerous lesions of ESCCs and EACs in different degrees (21% and 58% respectively). Expression of Hh target genes is frequently detected in severe squamous dysplasia/ carcinoma in situ (p=0.04) and Barrett’s esophagus (p=0.01). Unlike EAC, sonic hedgehog (Shh) expression was rare in ESCCs. Consistent with the human specimen data, we found a high percentage of Hh signaling activation in precancerous lesions in rat models. These data indicate that Hh signaling activation is an early molecular event in the development of esophageal cancer, particularly EAC.
Keywords: Esophageal adenocarcinoma (EAC), esophageal squamous cell carcinoma (ESCC), hedgehog (Hh), patched-1 (PTCH1 for humans and Ptch1 for animals), Gli2, sFRP-1, human homologue of hedgehog-interaction protein (HHIP), rat model, Barrett’s esophagus (BE)
Introduction
Esophageal cancer is the 6th cause of cancer-related death worldwide and the 7th cause of cancer-related death in American men [1]. The two types of esophageal cancer, squamous cell carcinoma (ESCC) and adenocarcinoma (EAC), have different incidences in different geographic regions: squamous cell carcinoma of esophagus is a predominant type worldwide whereas in the United States the incidence of adenocarcinomas approaches the incidence of ESCC in Caucasians [2]. China is one of the countries with the highest incidence of esophageal cancer, mostly ESCC [3]. It is known that the etiology of esophageal adenocarcinoma includes long standing acid/bile reflux esophagitis and development of Barrett’s esophagus, an intestinal type metaplasia of the normal squamous epithelium. Similarly, ESCC is considered to arise from multiple steps through the progression of precancerous dysplastic lesions to invasive ESCC [4]. Most esophageal cancers are diagnosed in the advanced stages. Thus, identifying gene alterations that drive the carcinogenesis process of esophageal cancer may help design novel strategies to diagnose and treat the disease.
The Hh signaling pathway plays an important role in embryonic development, cell proliferation, tissue polarity and carcinogenesis [5-8]. Hh protein binds to its receptor human patched 1 homologue (PTCH1), and relieves PTCH1’s inhibition on smoothened (SMO), allowing SMO to signal downstream to GLI transcriptional factors, which activates the target genes via specific genomic DNA sequences (TGGGTGGTC) [9,10].
Activation of Hh signaling has been reported in many cancer types [11]. Expression of Hh pathway components and their target genes is found in gastrointestinal cancer [4,12-15], prostate cancer [16-18], pancreatic cancer, and other cancer types [14,15]. Previously, we and others found activation of Hh signaling in esophageal cancer [14,19,20]. Elevated expression of sonic hedgehog (Shh) and its target genes was observed in several esophageal cancer cell lines and cancer specimens [4,15,21]. Recent studies indicate that Shh is induced in Barrett’s esophagus to mediate paracrine Hh signaling [22]. Studies also suggest that activation of Hh signaling is associated with poor prognosis [21] of esophageal squamous cell carcinomas. While most of the studies have been focused on Barrett’s esophagus and EAC, only limited studies on hedgehog signaling activation in ESCC and its precursors have been reported. At present, N -nitrosomethylbenzylamine (NMBA)-induced carcinogenesis in rat esophagus is the most physiologically relevant animal model of ESCC while the esophagogastroduodenal anastomosis (EGDA) procedure in rats is the reliable animal model for EAC. Thus far, it is not known if Hh signaling is activated in the physiologically relevant animal models of esophageal cancers. To understand the role of Hh signaling activation in the development of esophageal cancer, we examined expression of Hh target genes in pre-cancerous lesions as well as in esophageal tumors of humans and rats.
Materials and methods
Patient specimens
Specimen from 19 cases of Barrett’s esophagus (BE) and 28 cases of esophageal adenocarcinoma arising in association with BE were obtained from the Pathology Departments of University of Texas Medical Branch Hospitals and Creighton University Medical Center. The human related studies have been approved by the Institutional Review Board in these two institutions. Twenty four pre-cancerous lesions and 103 cases of squamous cell carcinoma (in tissue array) were obtained from the Cancer Hospital in Beijing, China. Table 1 shows the patient demographic information. According to the common clinical procedures in all research institutions involved in this study, all specimens were fixed in formalin for 24 hours before proceeding to paraffin tissue embedding, and all the tissue blocks and slides were stored at room temperature. The diagnosis of the tumor specimens was performed in the pathology department in which the tissues were collected. The TNM staging system of the American Joint Committee on Cancer and the International Union Against Cancer for esophageal cancer is used universally. Additional confirmation of the tissue sections used in this study were carried out by two pathologists (Z.G. and S.Q.).
Table 1.
Patient and Tumor Characteristics
| Adenocarcinoma (n=28) | ESCC (n= 103) | |
|---|---|---|
| Age | ||
| Mean (Median) | 59.9 (59) | 59.9 (60) |
| Range | 48 - 81 | 36 - 79 |
| Gender | ||
| M | 23 (82.1%) | 86 (83.5%) |
| F | 5 (17.9%) | 17 (16.5%) |
| Differentiation | ||
| Well | 5 (17.9%) | 9 (8.7%) |
| Well-moderate | 1 (3.6%) | 42 (40.8%) |
| Moderate | 13 (46.4%) | 28 (27.2%) |
| Moderate-Poor | 0 (0) | 15 (14.6%) |
| Poor | 9 (32.1%) | 9 (8.7%) |
| Stage | ||
| I | 2 (8.7%) | 1 (1%) |
| II | 9 (39.1%) | 73 (71%) |
| III | 7 (30.4%) | 28 (27%) |
| IV | 5 (21.7%) | 0 (0) |
| Missing | 5 (--) | 1 (1%) |
| Lymph node Metastasis | ||
| Positive | 15 (62%) | 34 (33%) |
| Negative | 9 (38%) | 69 (67%) |
| Missing | 4 (--) | |
| Smoking | ||
| Yes | -- | 50 (48.5%) |
| No | -- | 53 (51.5%) |
| Drinking | ||
| + | -- | 14 (13.6%) |
| ++ | -- | 28 (27.2%) |
| - | -- | 61 (59.2%) |
Animal
Male F344 rats, 4-6 weeks old, were obtained from Harlan Sprague-Dawley (Indianapolis, IN). The animals were housed and maintained according to the recommendations of the American Association of Laboratory Animal Care (AALAC), as reported previously [23,24]. Animal studies have been approved by The Institutional Animal Care and Use Committee (IACUC) of the relevant institutions (EGDA model by North Carolina Central University and chemical carcinogenesis models by Medical College of Wisconsin).
Two weeks after arrival in the animal facility, the rats were randomly assigned into 2 groups of 15 animals each. One group of rats was injected s.c. with N-nitrosomethylbenzylamine (NMBA) (0.3 mg/kg b.w., 3x/wk for 5 wks) and control rats were injected s.c. with a solution of DMSO/water (20:80), the vehicle for NMBA [24]. At 9, 15 and 35 wks, 5 rats from each group were killed by CO2 asphyxiation, the esophagus of each animal was opened longitudinally, and the surface tumors (only found at wk 35) were mapped, counted, and sized. Each esophagus and the individual tumors were fixed in 10% neutral buffered formalin for subsequent histopathologic evaluation and immunohistochemical staining. Five mice in each group were used in this study.
For esophagogastroduodenal anastomosis (EGDA), six-week-old male Sprague-Dawley rats were allowed to acclimatize for 2 weeks prior to surgery. Solid food was withdrawn 1 day before and for 1 day after the surgery [23]. EGDA was performed under general anesthesia (80 mg ketamine and 12 mg xylazine per kg body wt, i.p.), through an upper midline incision as previously reported [23]. This procedure was approved by the Animal Care and Facilities Committee at the North Carolina Central University. Food intake was measured three times during the experiment (4, 16 and 30 weeks after surgery) with each time for 3 consecutive days, and the average values of food intake (g diet/day) for the 3 days were calculated. All animals were kept for 40 weeks after surgery. 10 mice were used for the study, with the same results.
Immunohistochemistry
A standard avidin-biotin immunostaining technique was performed using a kit from Vector laboratories with specific antibodies to SHH (Santa Cruz Biotechnology Cat# 9024), PTCH1 (Santa Cruz Biotechnology Cat# 6149), human hedgehog-interacting protein (HHIP) (R&D systems Cat# AF1568), Gli2 (ABCam Cat# 26056) and secreted frizzled related protein 1 (sFRP1) (R&D systems Cat# 1384). Positive staining was in red (Noval red substrate) or brown (DAB substrate). Specificity of the antibodies was tested as described previously [16]. Hematoxylin was used for counterstaining (in blue). Tissue pathology slides were reviewed and confirmed by two pathologists (Z.G. and S.Q.). The overall IHC score (0, 0.5, 1, 1.5, 2, 3) was calculated by multiplying intensity (0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining) by proportion positively stained cells (0, <10%; 0.5, 10-30%; 1, >30%). Due to a small number of specimens in each IHC score, expression of SHH and PTCH1 in this study was categorized as negative (IHC score = 0) or positive expression (IHC score>0) for further analyses (Supplementary Tables 1-3).
Statistical methods
Patient and tumor characteristics were summarized for the adenocarcinomas (ACE) (n=28) and squamous cell carcinoma (ESCC) (n=103) samples. Because Gli2 and PTCH1 had the same pattern of expression, all statistical analyses were performed using the data from PTCH1. Comparison of the frequency of PTCH1 positivity between the two subtypes of esophageal cancers was carried out using the Chi-square test. The Chi-square or Fisher’s exact test was used to assess modulation of Hh targets and SHH levels between pre-cancerous tissues (Barrett’s esophagus or pre-ESCC dysplasia samples) versus the ACE or ESCC tissues. Paired test using the McNemar’s Chi-square test was employed to compare PTCH1 positivity rates between paired normal and cancer specimens from ESCC patients. The PTCH1 positivity rate was compared for age, gender, clinical tumor stages, lymph node metastases and tumor differentiation, as well as smoking and drinking status using the Chi-square or Fisher’s exact test. The logistic regression model was employed to perform comparison of PTCH1 level between ACE vs. ESCC with adjustment for tumor stage, differentiation, and lymph node status.
Results
Expression of hedgehog target genes in the development of human ESCC and the dysplastic lesions
To examine whether Hh signaling is important for development of esophageal cancer, a specific PTCH1 antibody [16] was used to detect Hh signaling activation. To confirm the sensitivity of the antibody, we detected expression of Hh target genes PTCH1 and sFRP1 in ESCCs and EACs with known activation of Hh signaling (as shown in Figure S1). Immunohistochemistry results showed better staining from PTCH1 antibodies. Our previous studies also indicated that detection of PTCH1 protein confirmed the data from in-situ hybridization (as summarized in [19,25]). We further confirmed our data using expression of Gli2 (Figure S1), a downstream transcriptional factor of the Hh pathway with elevated expression in Hh signaling activated tumors [26,27].
First, we examined expression of PTCH1 in squamous dysplasia, the precancerous lesion for ESCC. ESCCs are thought to be derived from lesions that are moderately to severely dysplastic [28]. PTCH1 expression was found in 21% of dysplastic lesions (Figure 1 and Table 2). Further analysis showed that PTCH1 expression was detected only in tissues with severe dysplasia or carcinoma in situ but not in those with mild/moderate dysplasia (p = 0.04, see Table 2). We confirmed the results with Gli2 expression (Figure 1), suggesting that Hh signaling activation may promote formation of carcinoma in situ. Most staining of PTCH1 and Gli2 was detected in the epithelial cells of the lesion (Figure 1, as indicated by arrows) with little observed in the stroma (as indicated by arrowheads).
Figure 1.
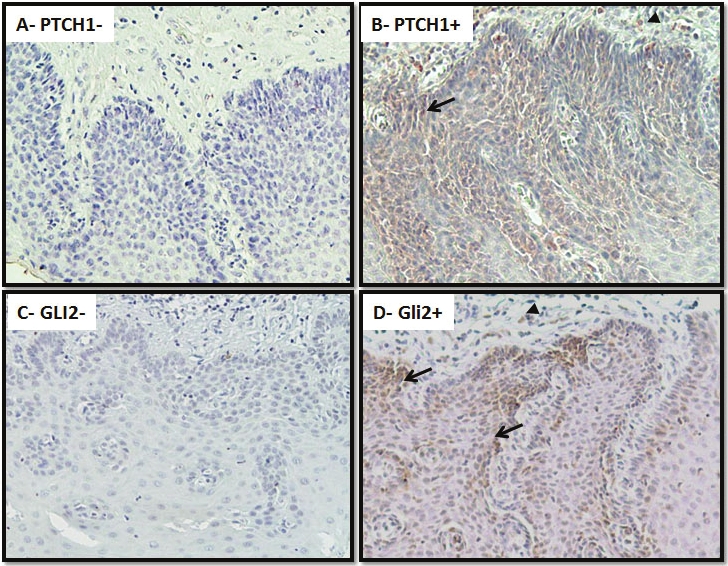
Expression of PTCH1/GLI2 in squamous dysplastic lesions. Expression of PTCH1/GLI2 was examined in 21 cases with squamous dysplastic lesions and 103 cases of ESCCs using standard immunohistochemistry. Summary of our data is shown in Tables S1 and 3. This figure shows representative results. Positives (B for PTCH1 and D for GLI2) and negatives (A for PTCH1 and C for GLI2) staining of severe squamous dysplasia are shown in brown. Please note that expression of PTCH1/GLI2 is mostly in the epithelial lesions (arrows) with some staining in the stroma (arrow heads). Specificities of these antibodies have been shown in our previous publication (see [16] for PTCH1, HHIP; [43] for sFRP1), or by the vendor for GLI2 (Abcam Inc.)
Table 2.
Comparison of PTCH1 between two forms of Esophageal Cancer and precancerous lesions.
| PTCH1 Positive | PTCH1 Negative | p-value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Adenocarcinoma | 27(96%) | 1 (4%) | |
| ESCC (Cancer tissue samples) | 39(38%) | 64(62%) | <0.0001a |
| ESCC (Normal tissue samples) | 0 (0%) | 103 (100%) | <0.0001a |
| PTCH1 Positive | PTCH1 Negative | p-value | |
| N (%) | N (%) | ||
| Squamous dysplasia | 5 (21%) | 19(79%) | 0.01b |
| Barrett’s Esophagus | 11(58%) | 8 (42%) | |
| PTCH1 Positive | PTCH1 Negative | p-value | |
| N (%) | N (%) | ||
| Squamous dysplasia | |||
| Mild/ moderate | 0 (0) | 12(100%) | |
| Severe/ carcinoma in situ | 5 (42%) | 7 (58%) | 0.04c |
| ESCC | 39(38%) | 64(62%) | 0.008c |
p-value based on the chi-square or Fisher’s exact test in comparison with adenocarcinoma;
p-value based on the chi-square or Fisher’s exact test;
p-value in comparison with Pre-ESCC mild/moderate based on Fisher’s exact test.
Expression of PTCH1 and Gli2 was also examined in ESCCs. Using PTCH1 data, a paired comparison between matched tumors vs. normal tissues from ESCC patients showed PTCH1 expression only in tumor tissue (38% of tumors) and not in normal tissue (0%) (p<0.001, Table 2). We found an increasing trend of PTCH1 and Gli2 expression in squamous dysplasia (21%) versus ESCC (38%) (Table 2 for PTCH1 analysis, and Figure S1 for the picture). Additional association analysis revealed that female ESCC patients had a higher rate of PTCH1 positive staining (59%) in tumors compared to male patients (34%, p=0.05). Shh expression was not detected in any of the ESCC specimens, indicating that Shh expression by itself is not responsible for pathway activation in this subset of ESCCs.
Expression of hedgehog target genes in a rat model of ESCC
To substantiate our data on PTCH1/Gli2 protein expression in human esophageal specimens, we examined their expression in chemically-induced squamous dysplasia in the rat esophagus [24]. Fischer-344 rats were treated with N-nitrosomethylbenzylamine (NMBA) for 5 wks and then sacrificed at 9, 15 and 35 wks of the bioassay. Esophageal tissues and papillomas (only found at wk 35) were examined for expression of Ptch1 and Gli2. Expression of Ptch1 and Gli2 was detected in 8 of 15 (53%) of esophageal tissue specimens (Table S3) and only in moderately to severely dysplastic lesions (Figure 2) collected at 15 and 35 weeks of the bioassay (Table S3). We had little opportunity to examine Ptch1/Gli2 expression in esophageal cancer because, in this model, the rats succumb to the occlusive effects of large papillomas before carcinomas can develop. The high level of Ptch1/ Gli2 expression in precancerous lesions suggests that Hh signaling activation is an important molecular event in the development of ESCC in rats. It appears that the rat model resembles only some phenotypes in human esophageal dysplasia because only 21% of the dysplastic lesions in human esophagus (all carcinomas in situ or severe squamous dysplasia) expressed PTCH1/GLI2. Nevertheless, the existence of Hh signaling in this rat model will support additional functional studies for the role of Hh signaling in the development of ESCCs.
Figure 2.
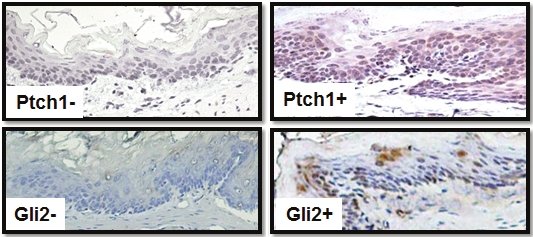
Expression of Ptch1/Gli2 in squamous dysplasia of rat esophagus Expression of Ptch1/Gli2 was detected by immunohistochemistry using specific antibodies to Ptch1 and Gli2 (see methods). Please note that the staining (Brown) is mainly in the cytoplasm of the epithelium with some in the nucleus, which was also reported previously for interactions with cyclin B1 [44]. Gli2 was mostly detected in the nucleus.
Activation of Hh signaling in human EAC and Barrett’s esophagus
Unlike ESCC, the incidence of esophageal adenocarcinoma (EAC) continues to rise faster than that of any other malignancy in the United States [1]. Barrett's esophagus (BE) is the most significant risk factor for the development of EAC [2,3]. Progression from Barrett’s esophagus to esophageal adenocarcinomas is believed to occur in an orderly fashion from no dysplasia to low grade dysplasia (LGD) to high grade dysplasia (HGD), and ultimately to adenocarcinoma (EAC) [29,30]. In order to understand the role of hedgehog signaling in adenocarcinoma carcinogenesis, we examined the expression of PTCH1 in Barrett’s esophagus, and detected PTCH1 expression in 58% of these lesions (Table 2). BE tissues with dysplasia had a similar frequency of PTCH1 expression as those without dysplasia (Table S2). The high frequency of PTCH1 expression in BE without dysplasia suggests that activation of Hh signaling may be among the earliest events in the development of adenocarcinomas arising from BE (Figure S2). We further confirmed Hh signaling activation by detection of GLI2 protein expression in the tumor (Figure 3). Unlike EAC, the expression of PTCH1/GLI2 in BE was focal or scattered. Most protein expression was detected in the epithelium. By comparison, PTCH1 expression (same is true for GLI2) was high in BE (58%) and was even higher in adenocarcinoma (96%, p=0.002) (see Table 2 and Figure 3). Expression of Shh was detected principally in the epithelium (Figure S3), and was further increased in EAC (see Table 3 for statistical analysis, with p value= 0.06). These results indicate that activation of Hh signaling is quite common during development of EAC, possibly through elevated expression of ligand Shh.
Figure 3.
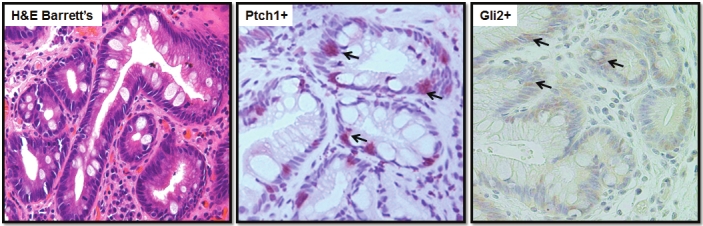
Expression of PTCH1/GLI2 in Barrett’s esophagus (BE) PTCH1/ GLI2 expression was detected by IHC. A shows H&E staining of a tissue with BE, while B (for PTCH1) and C (GLI2) show IHC staining. Please note that PTCH1/ GLI2 staining is focal or scattered (indicated by an arrow, red) in the epithelium.
Table 3.
Comparison of PTCH1 and SHH expression between normal (or non-cancer samples) vs. adenocarcinoma and ESCC.
| PTCH1 Positive | PTCH1 Negative | p-value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Barrett’s | 11(58%) | 8 (42%) | |
| Adenocarcinoma | 27 (96%) | 1 (4%) | 0.002a |
| SHH Positive | SHH Negative | p-value | |
| N (%) | N (%) | ||
| Barrett’s | 8 (42%) | 11 (58%) | |
| Adenocarcinoma | 17 (71%) | 7 (29%) | 0.06a |
| PTCH1 Positive | PTCH1 Negative | p-value | |
| N (%) | N (%) | ||
| Squamous dysplasia | 5 (21%) | 19 (79%) | |
| ESCC (Normal tissue samples) | 0 (0%) | 103 (100%) | 0.003b |
| ESCC (Cancer tissue samples) | 39 (38%) | 64 (62%) | 0.11b |
| ESCC Normal tissue samples | |||
| PTCH1 Positive | PTCH1 Negative | p-value | |
| ESCC Cancer tissue samples | <0.0001c | ||
| PTCH1 positive | 0 (0%) | 39 (38%) | |
| PTCH1 negative | 0 (0%) | 64(62%) | |
Based on chi-square or Fisher’s exact test in comparison with Barrett’s esophagus;
Based on the chi-square or Fisher’s exact test in comparison with Pre-ESCC;
Based on the McNemar’s test for paired comparison of normal vs. matched cancer tissue samples.
Expression of hedgehog target genes in a rat model of EAC
Currently, the esophagogastroduodenal anastomosis (EGDA) model in rats most closely represents Barrett’s esophagus and EAC seen in humans. In this model, esophagitis begins to develop within one week after surgery. Forty weeks after surgery, both columnar-lined esophagi (CLE), CLE with dysplasia as well as well-differentiated adenocarcinomas appear near the site of EGDA [31]. CLE is morphologically similar to Barrett’s esophagus. To examine if hedgehog signaling is activated in this model, we examined the expression of Hh target gene Ptch1 and the downstream transcription factor Gli2. As shown in Figure 4, Ptch1/Gli2 was expressed in CLE, a BE-like precancerous lesion. Unlike the ESCC model, we noticed Ptch1/Gli2 expression mainly in the stroma (as indicated by arrowheads). Our data indicate that this animal model can be used to examine the effect of Hh signaling in the development of Barrett’s esophagus and EAC.
Figure 4.
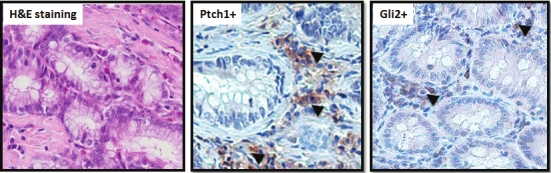
Expression of Ptch1 in a rat model of EAC Thirty weeks following EGDA, rat esophagi were examined for the presence of CLE, an esophageal metaplasia similar to Barrett’s esophagus in humans (see A). Expression of Ptch1/ Gli2 is shown in B and C (indicated by arrow heads). Please note Ptch1/Gli2 was detectable mainly in the stroma.
Differences in Hh signaling activation between human ESCC and EAC
Previous genetic analysis indicates that these two histological subtypes of esophageal cancer in humans share some biological features but differ in others [32-34]. Our results showed that the expression of PTCH1/GLI2 is significantly higher (p<0.0001) in tumor samples from EAC patients (96%) than from ESCC patients (38%). Normal esophagus samples from both EAC and ESCC patients had no PTCH1 expression (Table 2). After we adjusted for differentiation stage, tumor stage and lymph node metastasis by logistic regression, there were still significant (p=0.0006) differences in PTCH1 expression between EAC vs. ESCC, indicating that PTCH1 was an independent predictor for the two diseases (Figure 5). Furthermore, as indicated above our analysis suggests that female patients with ESCC have a high rate of PTCH1 expression. Nevertheless, the molecular basis underlying this finding is yet to be established. Our studies suggest that ESCC may represent a heterogeneous disease in regarding to Hh signaling activity. Consistent with this hypothesis is the fact that most reports have found that molecular alterations in human ESCC occur in less than 50% of the cases [29,35,36]. Additional work is needed to further classify ESCC at the molecular level, which will require assessment of multiple molecular alterations in the same tumor in a large cohort study.
Figure 5.
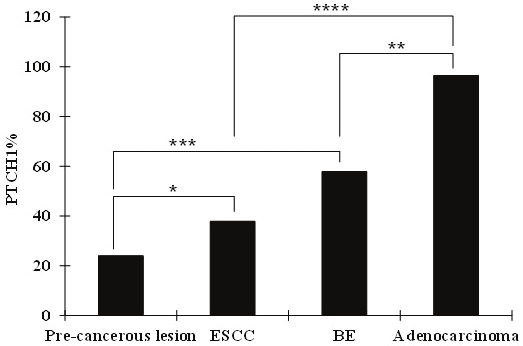
Statistical analysis of PTCH1 expression in esophageal specimens The frequency of PTCH1 expression in different types of specimens was compared. Through statistical analyses, it was shown that the difference in PTCH1 expression between squamous dysplasia (pre-cancerous lesion) and ESCC was significant (p < 0.05, indicated by *). Similarly, BE and EAC (adenocarcinomas) are significantly different in PTCH1 expression (P < 0.05 and indicated by **). Further analyses also indicate statistically significant differences of PTCH1 expression between ESCC and EAC (indicated by ****), and between Squamous dysplasia and BE (indicated by ***). The analysis was performed with the data from PTCH1 because Gli2 had the same staining pattern.
Precancerous lesions for ESCC also differ molecularly from those for EAC. First, Hh signaling was more frequently activated in Barrett’s esophagus (58%) than in squamous dysplasia (21%) (Table 2), Second, most squamous dysplasia specimens with Hh signaling activation were found to be either severely dysplastic or carcinoma in situ. In contrast, both metaplastic and dysplastic BE lesions had a similar frequency of Hh signaling activation.
In summary, we found that Hh signaling is frequently activated in precancerous lesions of esophageal ESCC and EAC. In comparison with the tumors, Hh target gene expression was less frequent in precancerous lesions. ESCC and EAC were found to differ in their frequency of Hh signaling activation.
Discussion
The role of Hh signaling in precancerous lesions of esophageal cancer
Human ESCC typically evolves through a sequence of defined histopathologic lesions, including mild to severe squamous dysplasia or squamous carcinoma in situ, and finally, invasive carcinoma [28]. Barrett’s esophagus (intestinal metaplasia) is the major risk factor and a precursor for EAC [36,37]. It also progresses through increasing degrees of dysplasia and carcinoma in situ. In our study, PTCH1/GLI2 expression was observed in Barrett’s esophagus and in severe squamous dysplasia /carcinoma in situ, indicating that detection of Hh signaling activation may be useful for early diagnosis of esophageal cancer. Our data on Hh signaling activation from both types of precancerous lesions (BE 58%, squamous dysplasia 21%, p=0.01) indicate that activation of Hh signaling is an early event in the development of esophageal cancer. Our findings of Ptch1/Gli2 expression in two rat esophageal cancer models further confirms that Hh signaling is involved in tumorigenesis of esophageal cancer. Although not in the scope of this study, further studies using Hh signaling inhibitors in these models will provide evidence for preventative effects of Hh signaling inhibitors for esophageal cancer development and progression.
Clinical implications of active Hh signaling in precancerous lesions of esophageal cancer
Diagnosis of cancer at later stages is a major factor for the mortality of esophageal cancer. Frequently, precancerous lesions do not have symptoms in patients. Our findings that activation of Hh signaling occurs in precancerous lesions (Barrett’s esophagus or squamous dysplastic lesions) could help design new strategy for early diagnosis of esophageal cancer. Using commercially available small molecules targeting specifically to the Hh pathway [38], one could design a probe to detect pre-cancerous lesions using imaging system. Another possibility is to use clinical specimens of gastroesophageal reflux disease (GERD) patients (e.g. fine-needle aspiration specimens) to detect expression of PTCH1 and GLI2. Positive patients may be eligible for treatment with Hh signaling inhibitors which are in phase II clinical trials (reviewed in [38]), and may be in clinic soon [39].
In addition, our findings of the link of Hh signaling activation in female ESCC patients may help focus on early diagnosis in a subset of population. The heterogeneity nature of esophageal cancer [29,36] makes it difficult to identify biomarkers. The fact that activation of Hh signaling was detected in 58% of Barrett’s esophagus and 96% of EAC indicates a high possibility that early detection of Hh signaling activation may be effective in detection of EAC.
Molecular basis of Hh signaling activation in esophageal cancer
Previous studies indicated that Hh signaling activation is frequently associated with elevated expression of Shh [15,22]. In this study, we assessed expression of Shh in 19 BE, 24 EAC and 103 ESCC specimens. We found that 42 % (8 of 19) of BE and 71% (17 of 24) of EAC showed positive staining (p=0.06, see Table 3). In BE, 6 of 19 (32%) specimens co-expressed Shh and PTCH1 (p=0.20), whereas in EAC, 16 of 23 (70%) specimens showed Shh and PTCH1 co -expression (p=0.51). No expression of Shh was detected in the 103 ESCC specimens. Our data suggest that Shh expression may not be responsible for Hh signaling activation in ESCC. Other alterations in the Hh pathway that may be responsible for Hh signaling activation include elevated expression of Indian hedgehog, hypermethylation of negative regulators of the pathway [i.e. HHIP, SU(FU)] and gene amplification downstream of the pathway (GLI transcriptional factors) [40]. Low expression of HHIP in gastric, esophageal and colon cancers has been associated with hypermethylation of the HHIP promoter [41]. We have previously shown that loss of negative regulator SU(FU) through methylation in lung cancer cells [42]. Although the exact mechanism for Hh signaling activation in each specimen from both precancerous lesions and tumors in ESCC and EAC will require additional investigation, it is clear that elevated expression of Shh is not the only mechanism responsible for pathway activation.
Activation of Hh signaling in tumor vs stroma of cancerous lesions
A recent study by Dr. Wang et al [22] showed that high expression of Shh in Barrett’s esophagus is correlated with stromal expression of PTCH1, and this paracrine signaling is a feature of Barrett’s esophagus. Our data from Barrett’s esophagus showed a more heterogeneous picture in Shh, PTCH1 and GLI2 expression. First, we found that not all BE tissues express these proteins. Second, we found that Shh, GLI2 and PTCH1 are expressed focally within the epithelium. Our data are consistent with the study of Wang et al in that Shh expression is focally expressed in the epithelium of Barrett’s esophagus. However, we found that stromal expression of PTCH1 is rather weak in all BE samples and in the rat model. Since most of their staining was done using immunofluorescence, the results are difficult to compare directly with ours. Further studies using a large cohort of specimens may be necessary to explain the difference.
In summary, we found Hh target gene expression in precancerous lesions of ESCC and EAC, suggesting a role of this pathway in the development of esophageal cancer. In two rat models of esophageal cancer, we also detected Ptch1 expression in precancerous lesions. These data suggest that Hh signaling plays a significant role in the development of esophageal cancer as indicated in Figure S2.
Acknowledgements
This research was supported by a grant from the NIH (R01-CA94160) and Wells Center for Pediatric Research of Indiana University.
Supporting Information
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He YT, Hou J, Chen ZF, Qiao CY, Song GH, Meng FS, Jin HX, Chen C. Decrease in the esophageal cancer incidence rate in mountainous but not level parts of Cixian County, China, over 29 years. Asian Pac J Cancer Prev. 2005;6:510–514. [PubMed] [Google Scholar]
- 4.Ma X, Chen K, Huang S, Zhang X, Adegboyega PA, Evers BM, Zhang H, Xie J. Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas. Carcinogenesis. 2005;26:1698–1705. doi: 10.1093/carcin/bgi130. [DOI] [PubMed] [Google Scholar]
- 5.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 6.Toftgard R. Hedgehog signalling in cancer. Cell Mol Life Sci. 2000;57:1720–1731. doi: 10.1007/PL00000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 8.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 9.Kinzler KW, Vogelstein B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol. 1990;10:634–642. doi: 10.1128/mcb.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 11.Xie J. Hedgehog signaling pathway: development of antagonists for cancer therapy. Curr Oncol Rep. 2008;10:107–113. doi: 10.1007/s11912-008-0018-7. [DOI] [PubMed] [Google Scholar]
- 12.Fukaya M, Isohata N, Ohta H, Aoyagi K, Ochiya T, Saeki N, Yanagihara K, Nakanishi Y, Taniguchi H, Sakamoto H, Shimoda T, Nimura Y, Yoshida T, Sasaki H. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology. 2006;131:14–29. doi: 10.1053/j.gastro.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Han HS, Lee KY, Hwang TS, Kim JH, Sung IK, Park HS, Jin CJ, Choi KW. Sonic hedgehog expression in gastric cancer and gastric adenoma. Oncol Rep. 2007;17:1051–1055. [PubMed] [Google Scholar]
- 14.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 16.Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 19.Ma X, Sheng T, Zhang Y, Zhang X, He J, Huang S, Chen K, Sultz J, Adegboyega PA, Zhang H, Xie J. Hedgehog signaling is activated in subsets of esophageal cancers. Int J Cancer. 2006;118:139–148. doi: 10.1002/ijc.21295. [DOI] [PubMed] [Google Scholar]
- 20.Xie K, Abbruzzese JL. Developmental biology informs cancer: the emerging role of the hedgehog signaling pathway in upper gastrointestinal cancers. Cancer Cell. 2003;4:245–247. doi: 10.1016/s1535-6108(03)00246-0. [DOI] [PubMed] [Google Scholar]
- 21.Mori Y, Okumura T, Tsunoda S, Sakai Y, Shimada Y. Gli-1 expression is associated with lymph node metastasis and tumor progression in esophageal squamous cell carcinoma. Oncology. 2006;70:378–389. doi: 10.1159/000098111. [DOI] [PubMed] [Google Scholar]
- 22.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, Corcoran-Schwartz IM, Wilburn DL, Montgomery EA, Wang JS, Jenkins NA, Copeland NA, Harmon JW, Phillips WA, Watkins DN. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett's metaplasia. Gastroenterology. 2010;138:1810–1822. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Yang G, Ding WY, Bondoc F, Curtis SK, Yang CS. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis. 1999;20:1801–1808. doi: 10.1093/carcin/20.9.1801. [DOI] [PubMed] [Google Scholar]
- 24.Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, McIntyre C, Rocha C, Lechner JF, Stoner GD. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res (Phila) 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Bian Y, Huang S, Ma X, Zhang C, Su X, Chen ZJ, Xie J, Zhang H. Identification of signature genes for detecting hedgehog pathway activation in esophageal cancer. Pathol Oncol Res. 2011;17:387–391. doi: 10.1007/s12253-010-9337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA, Rojkind M, Diehl AM. Hedgehog pathway activation and epithelial-to -mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexaki VI, Javelaud D, Van Kempen LC, Mohammad KS, Dennler S, Luciani F, Hoek KS, Juarez P, Goydos JS, Fournier PJ, Sibon C, Bertolotto C, Verrecchia F, Saule S, Delmas V, Ballotti R, Larue L, Saiag P, Guise TA, Mauviel A. GLI2-mediated melanoma invasion and metastasis. J Natl Cancer Inst. 2010;102:1148–1159. doi: 10.1093/jnci/djq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 29.McManus DT, Olaru A, Meltzer SJ. Biomarkers of esophageal adenocarcinoma and Barrett's esophagus. Cancer Res. 2004;64:1561–1569. doi: 10.1158/0008-5472.can-03-2438. [DOI] [PubMed] [Google Scholar]
- 30.Wang DH, Souza RF. Biology of Barrett's esophagus and esophageal adenocarcinoma. Gastrointest Endosc Clin N Am. 2011;21:25–38. doi: 10.1016/j.giec.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Li N, Wang S, Hong J, Fang M, Yousselfson J, Yang P, Newman RA, Lubet RA, Yang CS. Aberrant arachidonic acid metabolism in esophageal adenocarcinogenesis, and the effects of sulindac, nordihydroguaiaretic acid, and alpha-difluoromethylornithine on tumorigenesis in a rat surgical model. Carcinogenesis. 2002;23:2095–2102. doi: 10.1093/carcin/23.12.2095. [DOI] [PubMed] [Google Scholar]
- 32.Lin J, Beerm DG. Molecular biology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:476–486. doi: 10.1053/j.seminoncol.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Okano J, Snyder L, Rustgi AK. Genetic alterations in esophageal cancer. Methods Mol Biol. 2003;222:131–145. doi: 10.1385/1-59259-328-3:131. [DOI] [PubMed] [Google Scholar]
- 34.McManus DT, Olaru A, Meltzer SJ. Biomarkers of esophageal adenocarcinoma and Barrett's esophagus. Cancer Research. 2004;64:1561–1569. doi: 10.1158/0008-5472.can-03-2438. [DOI] [PubMed] [Google Scholar]
- 35.Mandard AM, Hainaut P, Hollstein M. Genetic steps in the development of squamous cell carcinoma of the esophagus. Mutat Res. 2000;462:335–342. doi: 10.1016/s1383-5742(00)00019-3. [DOI] [PubMed] [Google Scholar]
- 36.Moyes LH, Going JJ. Still waiting for predictive biomarkers in Barrett's oesophagus. J Clin Pathol. 2011;64:742–750. doi: 10.1136/jclinpath-2011-200084. [DOI] [PubMed] [Google Scholar]
- 37.Hormi-Carver K, Souza RF. Molecular markers and genetics in cancer development. Surg Oncol Clin N Am. 2009;18:453–467. doi: 10.1016/j.soc.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 39.Carey K. Hedgehog inhibitor single arm. Nat Biotechnol. 2011;29:957. [Google Scholar]
- 40.Snijders AM, Huey B, Connelly ST, Roy R, Jordan RC, Schmidt BL, Albertson DG. Stromal control of oncogenic traits expressed in response to the overexpression of GLI2, a pleiotropic oncogene. Oncogene. 2009;28:625–637. doi: 10.1038/onc.2008.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniguchi H, Yamamoto H, Akutsu N, Nosho K, Adachi Y, Imai K, Shinomura Y. Transcriptional silencing of hedgehog-interacting protein by CpG hypermethylation and chromatic structure in human gastrointestinal cancer. J Pathol. 2007;213:131–139. doi: 10.1002/path.2216. [DOI] [PubMed] [Google Scholar]
- 42.Chi S, Huang S, Li C, Zhang X, He N, Bhutani MS, Jones D, Castro CY, Logrono R, Haque A, Zwischenberger J, Tyring SK, Zhang H, Xie J. Activation of the hedgehog pathway in a subset of lung cancers. Cancer Lett. 2006;244:53–60. doi: 10.1016/j.canlet.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 43.He J, Sheng T, Stelter AA, Li C, Zhang X, Sinha M, Luxon BA, Xie J. Suppressing Wnt signaling by the hedgehog pathway through sFRP -1. J Biol Chem. 2006;281:35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- 44.Adolphe C, Hetherington R, Ellis T, Wainwright B. Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res. 2006;66:2081–2088. doi: 10.1158/0008-5472.CAN-05-2146. [DOI] [PubMed] [Google Scholar]


