Abstract
DNA double strand breaks (DSB) are among the most lethal forms of DNA damage and, in humans, are repaired predominantly by the non-homologous end joining (NHEJ) pathway. NHEJ is initiated by the Ku70/80 heterodimer binding free DNA termini and then recruiting the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) to form the catalytically active DNA-PK holoenzyme. The extreme C-terminus of Ku80 (Ku80CTD) has been shown to be important for in vitro stimulation of DNA-PK activity and NHEJ in vivo. To better define the mechanism by which the Ku80CTD elicits these activities, we assessed its functional and physical interactions with DNA-PKcs and Ku70/80. The results demonstrate that DNA-PKcs activity could not be complemented by addition of a Ku80CTD suggesting that the physical connection of the C-terminus to the DNA binding domain of Ku70/80 is required for DNA -PKcs activation. Analysis of protein-protein interactions revealed a low but measurable binding of the Ku80CTD for Ku70/80ΔC and for DNA-PKcs while dimer formation and the formation of higher ordered structures of the Ku80CTD was readily apparent. Ku has been shown to tether DNA termini possibly due to protein/protein interactions. Results demonstrate that the presence of the Ku80CTD stimulates this activity possibly through Ku80CTD/Ku80CTD interactions.
Keywords: NHEJ, Ku, DNA-PK, chemical crosslinking, dimerization, DNA repair
Introduction
DNA double strand breaks (DSB) can arise from both endogenous and exogenous sources including reactive oxygen species (ROS), ionizing radiation (IR), and radiomimetic drugs. If not repaired, DSBs can result in chromosomal translocations, genetic instability, and cell death. The two main pathways to repair DSBs are homology directed repair (HDR), which employs a subset of the HR protein machinery and non-homologous end joining (NHEJ) [1]. HDR is highly accurate and proceeds with minimal loss of genetic material while NHEJ is considerably more error-prone [2]. The NHEJ pathway is initiated by Ku, a heterodimeric protein comprised of Ku70 and Ku80 subunits which binds DNA termini generated from DSB. Upon binding to DNA a conformational change in Ku occurs that facilitates the binding of the DNA dependent protein kinase catalytic subunit (DNA-PKcs) to the site of a DSB through an interaction with both Ku and the DNA termini, thus generating the DNA-PK holoenzyme [3]. It has also been suggested that this complex plays a role in stabilizing the two DNA termini at the site of the break [4]. DNA-PK, a serine/threonine protein kinase, is active once bound to the DNA termini and catalyzes autophosphorylation and phosphorylation of other downstream NHEJ proteins including processing proteins like the nuclease Artemis [5]. Following processing of the DNA termini, the Ligase IV/XRCC4/XLF complex is recruited to the termini and catalyzes ligation of the DNA DSB [6].
The crystal structure of Ku revealed a bridge and pillar region comprised of both Ku70 and Ku80 subunits that form a ring around DNA [7]. These studies revealed that the ring shape exists in the presence and absence of DNA and a high degree of structure homology exists between the Ku70 and 80 subunits, despite the fact that they share minimal sequence homology. Studies have shown that Ku binds DNA in a sequence independent fashion by way of several hydrophobic residues that make contact with the major groove of DNA and several basic residues that interact with the phosphate back bone. Photocrosslinking and crystal structure studies have further shown that when bound to DSB DNA, the Ku70 subunit is proximal to the DSB and Ku80 is distal to the DSB [8]. The structure of Ku enables the protein to slide or translocate inward along the length of a DNA molecule in an ATP-independent manner and this sliding movement is thought to coincide with recruitment of DNA-PKcs to the DNA termini. This translocation has been suggested to be important for generating space at the DNA termini for other NHEJ proteins, like Ligase IV, to bind to the DNA.
Previous studies have shown that the C-terminus of Ku80 is essential for efficient activation of DNA-PKcs [9]. Upon removal of the C-terminus of Ku80 the kinase activity of DNA-PK is drastically decreased [10]. These studies show that the final 12 amino acids of the Ku80 subunit are sufficient to bind DNA-PKcs, and their removal resulted in decreased kinase activity of DNA-PK [11]. More recent structural research using small angle X-ray scattering (SAXS) revealed that the C-terminus of Ku80 is attached to the core by a highly flexible, disordered region. This region is capable of extending from the Ku molecule to a distance suitable for an interaction with a DNA-PKcs molecule that is bound to the same DSB end, as well as a DNA-PKcs molecule on an adjacent double strand break [12]. The flexibility of this region also suggests that protein-protein interactions could occur between the CTD region and other parts of the Ku molecule Despite the fact that the crystal structures of Ku and Ku80CTD/DNA-PKcs have been solved, resolution of the C-terminus of Ku80 has been poor due to its highly flexible nature, thus little is known about its interaction with the rest of the Ku molecule or DNA-PKcs.
To further understand how the Ku80CTD participates in DNA-PKcs activation and in protein/ protein interactions we undertook a chemical crosslinking approach to discern the functional and physical interactions. The results unexpectedly reveal the presence of a potential novel dimerization domain in the C-term of Ku80.
Materials and methods
Cloning
The Ku80ΔC construct was prepared via PCR cloning using an anti-sense primer inserting a stop codon after amino acid 548. The PCR product was subcloned directly into pBacPAK 8 and used to generate a recombinant baculovirus as described by the manufacturer (Clonetech). Plaque purified virus was amplified, titered and used to infect Sf9 cells for protein production. Production of the Ku70/80ΔC and wtKu was achieved by co-infection with wild type [His]6 Ku70 virus as previously described [13].
The Ku80 C-terminal domain (Ku80CTD) construct consists of amino acids 599-732. The final 432 bases of the Ku80 gene were synthesized by GenScript Corporation into pUC57 with an Nde1 restriction enzyme cut site at the 5’ end and BamH1 restriction enzyme cut site at the 3’ end. This was then subcloned into pET15b placing a [His]6 tag on the N-terminus of the protein that can be removed via thrombin cleavage. The Ku80CTD mutant construct containing amino acids 599-720 was generated by insertion of stop codons into the pET15b plasmid via site directly mutagenesis.
Protein purification
Human Ku was purified from Sf9 cells infected with baculovirus containing Ku70 and either wtKu80 or Ku80ΔC with an M.O.I. of 5 and 10 respectively. Wild type and Ku70/80ΔC mutants were purified by sequential Ni-NTA and Q-Sepharose column chromatography as previously described. Fractions containing Ku were identified based on SDS-PAGE and visualized by coomassie blue staining. Peak fractions were pooled and dialyzed overnight in either Buffer A or HEPES buffer (buffer A: 25 mM Tris pH 8.0, 75 mM potassium chloride, 10% glycerol, 0.0025% triton X-100 and 2 mM DTT; HEPES buffer: 20 mM HEPES pH 6.0, 75 mM potassium chloride, 10% glycerol, 0.005% triton X- 100, 2 mM DTT) and stored at -80°C. SDS-PAGE analysis of the purified Ku70/80ΔC and wtKu is presented in Figure 1B and C respectively.
Figure 1.
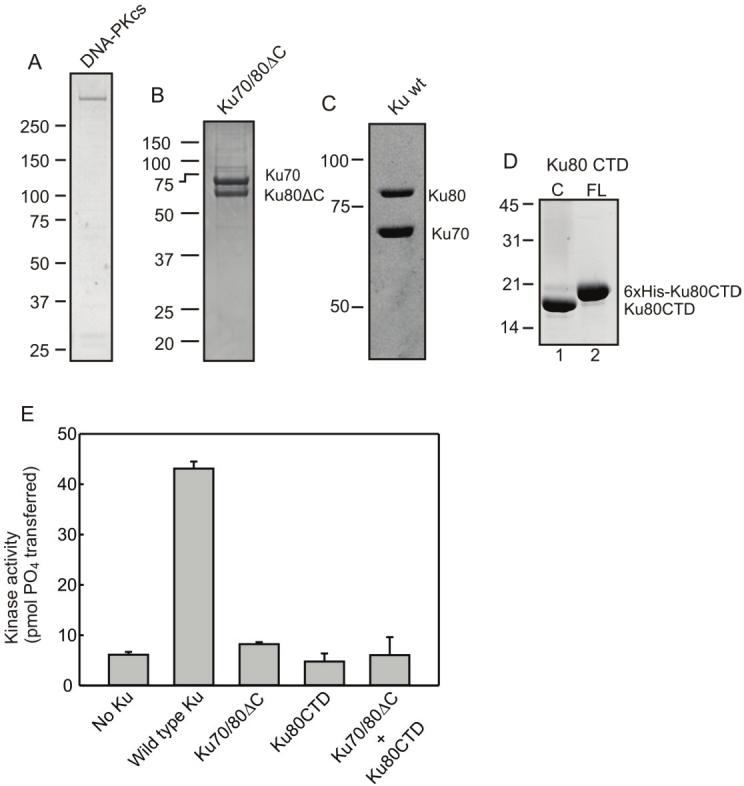
Ku constructs purity, stoichiometry, and DNA-PKcs activation. A-D) Purified proteins used in biochemical assays were subjected to SDS-PAGE and visualized with Coomassie staining. In all cases Ku70 constructs contained a [His]6-tag. Ku80ΔC construct contains amino acids 1-554 while Ku80CTD constructs contain amino acids 590-732 unless otherwise indicated. Ku80CTD construct contains a cleavable [His]6-tag as shown in D. E) Effect of Ku80 C-terminus on DNA-PK activation. DNA-PK kinase assays were performed as described in materials and methods in the presence of wtKu (1 pmol), Ku70/80ΔC (1 pmol) or Ku80CTR (10 pmol) as indicated. Results are presented as picomoles of 32P transferred to a synthetic peptide in a 15 minute reaction. Data presented is the average and standard deviation of three analyses.
Both Ku80CTD constructs were purified from BL21 E.coli cells. Briefly, pET15b-Ku80CTD was transformed into BL21 E.coli cells and induced with 0.4 mM Isopropyl β - D - 1 - thiogalactopyranoside for one hour. Cells were then harvested via centrifugation and lysed with a buffer containing 50 mM sodium phosphate pH 8.0, 1 M potassium chloride, 10% glycerol, 0.25% triton X-100, and 7 mM 2- mercaptoethanol. Cell free extract was then supplemented with 20 mM imidazole and applied to a 2 ml Ni-NTA column. Protein was eluted with lysis buffer containing 350 mM imidazole. Fractions containing Ku80CTD were identified based on SDS-PAGE and visualized by coomassie blue staining. Peak fractions were pooled and dialyzed overnight in either Buffer A or HEPES buffer and stored at -80°C.
The Ku80CTD [His]6 tag was removed via thrombin cleavage. Cleavage reactions were carried out in cleavage buffer containing 20 mM Tris- HCl, 150 mM NaCl, and 2.5 mM CaCl2, pH 8.4. 400 μg Ku80CTD and 0.05 units of thrombin (Novagen) diluted in 50 mM sodium citrate, 200 mM NaCl, 0.1% PEG-8000, and 50% glycerol pH 6.5 in a final reaction volume of 500 μl. Reactions were incubated at room temperature for 2 hours. Imidazole was then added to a final concentration of 20 mM and reactions were applied to a Ni-NTA spin column that had been equilibrated with cleavage buffer supplemented with 20 mM imidazole. Columns were centrifuged at 270 x g for 5 minutes and flow through containing cleaved Ku80CTD was collected and dialyzed overnight against either Buffer A or HEPES buffer. SDS-PAGE of the purified cleaved and un -cleaved Ku80-CTD is presented in Figure 1D.
DNA-PKcs was purified from cell-free extracts prepared from 4L of HeLa cells as previously described [14]. Pooled fractions were dialyzed in HEPES buffer and stored at -80°C. SDS-PAGE of the final purified protein is presented in Figure 1A.
SDS-PAGE and western blot
Proteins were separated via SDS-PAGE. Samples were denatured with 6X loading dye, heated to 95°C for 5 minutes, and separated via SDS PAGE according to manufacturers specifications (Invitrogen). Gels were either stained with coomassie blue or transferred to PVDF membrane for Western blot analysis according to manufacturer’s specifications. Membranes were blocked with 2% non-fat dry milk in TBS-Tween and probed with the primary antibodies indicated in the figure legends. Bound antibodies were detected with a horse radish peroxidase (HRP) conjugated goat anti-mouse IgG and visualized via chemiluminescence detection and images captured on a Fujifilm LAS-3000 CCD system.
DNA-PK assay
Kinase assays were performed at 37°C in a final volume of 20 μl containing 20 mM HEPES, pH 7.5, 8 mM MgCl2, 1 mM DTT, 5% glycerol, 125 μM ATP, [γ-32P] ATP (0.5 μCi), 2 pmol of a 30-bp double strand DNA, 500 μM p53 synthetic peptide, and 80 fmol DNA-PKcs was incubated with 1 pmol wtKu, 1 pmol Ku70/80ΔC, or 10 pmol Ku80CTD as indicated. The sequences for the DNA substrates are as follows: CCC TAT CCT TTC CGC GTC CTT ACT TCC CC and GGG GAA GTA AGG ACG CGG AAA GGA TAG GGG. Reactions were incubated at 37°C for 15 minutes and stopped with 30% acetic acid. Reaction products were spotted on P81 phosphocellulose filter paper that was then washed 5 times for 5 minutes each in 15% acetic acid, once in 100% methanol and allowed to dry. Samples were exposed to phosphoimager and analyzed using ImageQuant software (Molecular Dynamics).
PICUP
Photo-induced crosslinking of unmodified proteins (PICUP) was performed to analyze protein-protein interactions. Reactions were carried out in buffer containing 15 mM NaPi pH 7.5, 150 mM NaCl, 2.5 mM APS and 0.125 mM Ruthenium as indicated. 900 nM Ku70/80ΔC and varying concentrations of Ku80CTD as indicated was exposed to intense white light for 20 seconds or as indicated. Reactions were placed 6 inches from the intense white light source shining through a 1% Copper Sulfate solution to dissipate heat. Reactions were stopped with either the addition of DTT or 6X SDS loading dye. Reactions were then separated on SDS-PAGE gels and transferred to PVDF membrane for western blot analysis.
EDC coupling
Additional protein crosslinking experiments were performed with 1-ethyl-3-[3- dimethylaminopropyl]carbodiimide hydrochloride (EDC) in the presence of reaction stabilizing reagent N-hydroxysulfosuccinimide (NHS). Proteins involved in EDC coupling were dialyzed in HEPES buffer (20 mM HEPES pH 6.0, 75 mM KCl, 10% glycerol, 0.005% triton X-100 and 2 mM DTT). Reaction buffer contained 100 mM MES pH 6.0 and 500 mM NaCl. Optimal one step EDC coupling conditions were determined by titration of EDC, titration of Ku80CTD, and time course studies. From these studies it was determined that the optimal conditions were 1 mM EDC, 1.4 mM NHS, 900 nM Ku70/80ΔC and varied concentrations of Ku80CTD. For the Ku80CTD dimer experiments, equal concentration of 2 mM cleaved and uncleaved Ku80CTD were incubated in reaction conditions stated above. Ku80CTD dimer experiments using the 599-720 construct were performed identically to the Ku80CTD 599-732 experiments. Reactions were incubated for 30 minutes at room temperature and stopped by the addition of 6X SDS loading dye. Samples were then loaded onto SDS-PAGE gels followed by transfer to PVDF membrane for western blot analysis.
DNA-PKcs- EDC was titrated at 1, 2, 5, and 10 mM concentrations in reactions containing 1.3 μM Ku80CTD, 333 nM 30-bp double strand DNA, 14 mM NHS and in the presence or absence of 55.6 nM DNA-PKcs as indicated. Coupling reactions were preformed as above but were stopped with the addition of 2- mercaptoethanol to a final concentration of 20 mM. Samples were loaded onto an 8% Tris- Glycine gel after the addition of 6X SDS loading dye and transferred to PVDF membrane for western blot analysis. DNA-PKcs was titrated at 0, 18.3, 36.7, and 73.3 nM concentrations in reactions with fixed concentrations of 333 nM 30-bp double strand DNA, 667 nM Ku80CTD, 10 mM EDC and 14 mM NHS. Reactions were preformed as above and were stopped by the addition of 2-mercaptoethanl to a final concentration of 5 mM. Samples were loaded onto an 8% Tris-Glycine gel after the addition of 6X SDS loading dye and transferred to PVDF membrane for western blot analysis.
EMSA assay
Electrophoretic mobility shift assays (EMSA) were used to investigate the influence of the Ku80CTD on DNA binding and tethering. wtKu and Ku70/80ΔCTD was titrated at concentrations 0.1, 0.5, 1, 5, and 10 μM using a fixed concentration of 3.4 pM for the 21/34 duplex DNA substrate. The 21 base pair oligomer was 32P-labeled and annealed to the 34 base pair oligomer in a 1:2 ratio. This substrate has previously been used in structural studies and has been shown to accommodate the binding of a single Ku heterodimer as the 34 bp substrate forms a hairpin loop [15]. The sequences for the substrates are as follows: GTT TTT AGT TTA TTG GGC GCG and CGC GCC CAG CTT TCC CAG CTA ATA AAC TAA AAA C. In order to maintain the hairpin structure, following annealing the substrate was kept at 4°C and binding assays were done on ice. Reactions were loaded onto 6% native polyacrylamide gels and run with 1X TBE buffer. Gels were exposed to a PhosphorImager screen and analyzed using ImageQuant software (GE Healthcare Life Sciences, NJ).
Results
The Ku80CTD is necessary for DNA-PK activation
We and others have previously demonstrated that the C-terminal domain of Ku80 (CTD) undergoes a conformational change upon Ku binding to DNA. We then asked whether the CTD was physically interacting with the components of the DNA-PK protein complex. A C-terminal truncation of Ku80, Ku80Δ555-732, was purified with 6xHis tagged Ku70 from SF9 cells (Figure 1B). The Ku80CTD (amino acids 599- 732) was purified with a cleavable 6xHis tag (Figure 1D). wtKu80 was purified with 6xHis tagged Ku70 from SF9 cells while DNA-PKcs was purified free from endogenous Ku from HeLa cells. The ability of each of these constructs in DNA-PKcs activation was tested in vitro. The results demonstrate that wtKu was able to stimulate DNA-PKcs kinase activity by a factor of 30, indicating that the DNA-PKcs preparation was purified away from the majority of Ku and verified reconstitution of an active kinase. Inclusion of the Ku70/80ΔC construct into a reaction with DNA-PKcs resulted in kinase activity only slightly above background levels. This result is consistent with other in vitro data that demonstrate the essential nature of the Ku80 CTD for kinase activation. The addition of the Ku80CTD599-732 construct was not able to stimulate kinase activity above background levels independent of the inclusion of Ku70/80ΔC. These results demonstrate that the extreme C-terminus of Ku80 is essential for in vitro stimulation of DNA-PK activity and equally interesting is that the lack of recovery of kinase activity when the Ku80CTD599-732 was added to reactions containing Ku70/80ΔC suggests that the CTD must be physically tethered to the main Ku70/80 DNA binding domain to exert this stimulatory activity.
The Ku80CTD599-732 interacts with the Ku70/80 -DNA binding domain
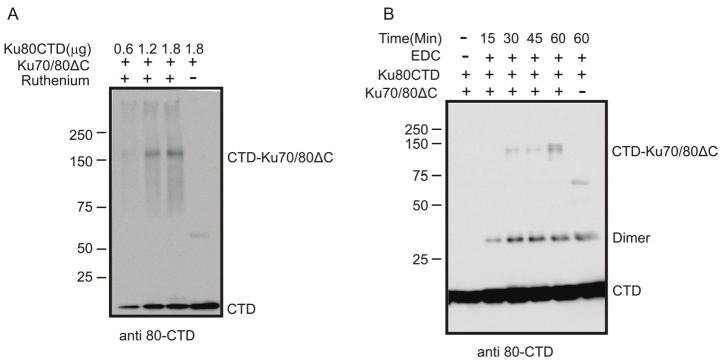
As the ability to stimulate DNA-PK activity is likely a result of specific protein-protein interactions we sought to determine the extent of CTD interactions with Ku and DNA-PKcs. Because the CTD of Ku80 undergoes a conformational change upon Ku binding to DNA and becomes extended form the core, DNA binding domain of Ku, we asked if the Ku80CTD599-732 interacts with the central region of Ku in the absence of DNA. We initially undertook a chemical crosslinking approach as this method allows for the sensitive detection of low affinity protein-protein interactions. Two independent crosslinking methods were used each utilizing unique chemical mechanisms. Both involve a zero distance cross-linker that covalently binds molecules in close proximity which is indicative of protein-protein interactions. PICUP relies on the formation of free radicals on Tryosines which is stimulated by the presence of ruthenium and light [16]. Alternatively, EDC catalyzes covalent crosslinking between carboxyl groups and primary amines resulting in amide bonds [17]. Ku70/80ΔC and Ku80CTD599-732 constructs were subjected to crosslinking conditions and analyzed via western blot resulting in species that migrate at approximately 150kDa and are recognized by an antibody to the Ku80CTD (Figure 2A and 2B). The molecular weight of these species is expected of a Ku70/80ΔC/ Ku80CTD species suggesting the Ku80CTD599-732 interacts with the core, DNA binding domain of Ku. Additional analysis of these cross-linking reactions using an antibody for Ku70 confirms that 150kDa species contain Ku70 (data not shown). Bands were also observed at 55kDa and 65kDa but these are thought to be nonspecific (Figure 2A lane 4 and 2B lane 6). Unexpectedly we also observed species migrating at 33kDa (Figure 2B). We interpreted these species to be dimers of the 16.5kDa Ku80CTD599-732.
Figure 2.
The Ku80CTD cross-links to Ku70/80ΔC. A) PIC-UP Reactions were prepared in the presence of Ku70/80ΔC (0.9μM) and increasing concentrations of the Ku80CTD (1.17μM, 2.35μM, and 3.5μM respectively). Ruthenium was added as indicated to stimulate free radical formation and thus facilitate cross-linking. B) EDC cross-linking reactions were prepared in the presence of EDC (0.5mM), Ku80CTD (2μM), and Ku70/80ΔC (0.58μM) as indicated. Reactions were incubated for 0, 5, 10, 15 and 30 minutes and were stopped with the addition of 6xSDS loading dye. Products were analyzed as in A. Under both conditions non cross-linked Ku80CTD ran at 16.5kDa as expected. Ku80CTD cross -linked to the Ku70/80ΔC heterodimer ran at approximately 150kDa. Ku80CTD-Ku80CTD dimers were observed under EDC cross-linking conditions at approximately 33kDa. Non-specific bands are observed in both experiments at approximately 60kDa. Products were analyzed via western blot using monoclonal Ku80CTD anti-body from NeoMarkers (MS-285).
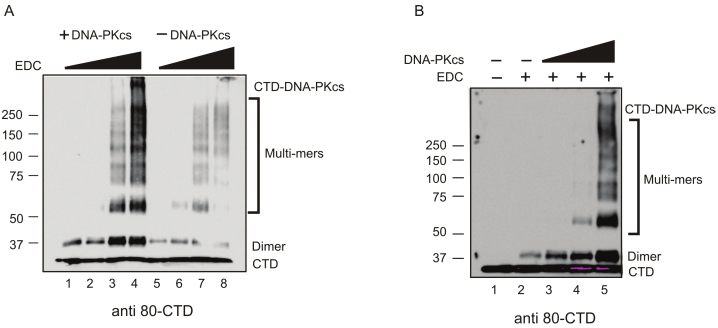
The Ku80CTD599-732 interacts with DNA-PKcs
Because the Ku80CTD has been implicated in DNA-PK activation, we asked if the Ku80CTD directly interacts with DNA-PKcs. Using similar methodology as Figure 2 we titrated EDC and DNA-PKcs into reactions with the Ku80CTD599-732 and analyzed products via western blot using a Ku80CTD antibody. We observed cross-linking species that are recognized by the CTD antibody which migrate well above 250kDa that only occur in the presence of DNA-PKcs (Figure 3A and 3B). DNA-PKcs is approximately 469kDa and thus we interpret the high molecular weight bands in Figure 3A lane 4 and Figure 3B lane 5 to be Ku80CTD599-732 -DNA-PKcs cross-linked species. These data are consistent with previous reports and suggests an interaction between the Ku80 C-terminus and DNA-PKcs. As in Figure 2, we observed cross-linked products at16.5kDa sized intervals which we believe represent Ku80CTD599-732 multimers (Figure 3A and 3B). Interestingly, the concentration of these products as determined by band intensity increases in the presence of DNA-PKcs. This difference in product formation is not due to differences in CTD concentration as an equal amount was used in all reactions. This fact is not obvious because the non-cross-linked Ku80CTD599-732 monomer ran with the dye front on these low percentage gels making their detection difficult. This may mean that DNA-PKcs facilitates a CTD/ CTD interaction thus increasing cross-linking efficiency.
Figure 3.
The Ku80CTD Cross-links to DNA-PKcs. A. Titration of EDC: Reactions were performed in 1, 2, 5, or 10mM EDC in the presence or absence of DNA-PKcs as indicated. B. Titration of DNA-PKcs: Reactions were performed with 0, 18.3, 36.7, or 73.3nM DNA-PKcs in the presence or absence of 10mM EDC. Cross-linked products above 250kDa represent CTD-DNA-PKcs species while the presence of CTD dimers and multimers were observed in all cross-linking conditions. Products were analyzed via western blot as in Figure 2.
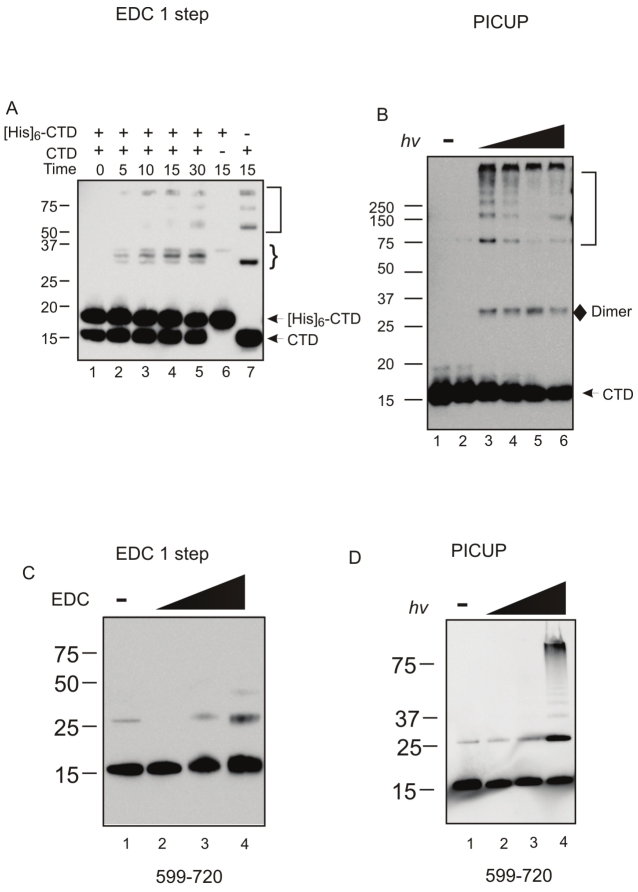
The Ku80CTD forms dimers suggesting the formation of a Ku dimer of dimers
The potential for CTD-CTD interactions is intriguing in the context of the role of Ku in DNA DSB repair. Previously Ku was shown to tether DNA ends in the absence and presence of DNA-PKcs, possibly through a protein/protein interaction [18,19]. One could envision a model where by this activity could be facilitated through the dimerization of Ku dimers bound to separate DNA termini through Ku80CTD-Ku80CTD interactions. To determine if this was a true Ku80CTD dimer we employed two constructs of the Ku80CTD599-732; one which retained the 6xHis tag and one whose tag was removed via thrombin cleavage. The different constructs are separable via SDS page migrating at 13 and 16.5kDa (Figure 1D). In cross-linking reactions with either protein alone, the expected ladder of products was observed. When combined in a single reaction a triplet of dimer products was observed consistent with His-CTD/His-CTD, CTD/His-CTD, and CTD/CTD complexes (Figure 4A see). Dimer formation was further confirmed independently with PIC-UP cross-linking methods (Figure 4B). The final 12 amino acids of the Ku80 C-terminus (721-732) have previously been shown to be involved in protein/protein interactions. We asked if this region is necessary in the CTD/CTD interactions we identified. Using EDC and PIC-UP cross-linking methods and a construct containing amino acids 590- 720 (Ku80CTD590-720) we determined that the 721-732 region is dispensable for dimer formation. A non-specific band was observed under non cross-linking conditions. This aberrant band is clearly different than the CTD dimer as the CTD dimer’s formation is titratible under increased cross-linking conditions (Figure 4C and 4D). To test the role of this Ku80CTD/Ku80CTD interaction in the tethering of DNA termini, electrophoretic mobility shift assays were employed using a hairpin DNA substrate that has previously been shown to accommodate the binding of a single Ku dimer. Clear gel shifts were observed when both wtKu and Ku70/80ΔC were incubated with the DNA, indicating that the deletion of the C-terminus does not affect DNA binding (Figure 5). An additional, higher molecular weight band was also observed at increased concentrations of Ku. Because this DNA substrate has been shown to accommodate a single Ku molecule, this higher band must represent either a Ku/Ku/DNA or a Ku/DNA/Ku/DNA complex formation. Interestingly, the complex formation occurs at lower Ku concentrations in the wtKu than in the Ku70/80ΔC binding reactions (Figure 5 Compare lanes 5 and 11). Therefore the presence of the Ku80CTD stimulates complex formation possibly through the Ku80CTD/Ku80CTD interaction.
Figure 4.
The Ku80CTD Forms Homodimers and Is Not Dependent on Amino Acids 721-732. A. EDC cross-linking of CTD and [His]6-CTD. Reactions were incubated between 0 and 30 minutes as indicated. B. PIC-UP reactions were exposed to white light for 0, 5, 10, 17, and 25 seconds as indicated. C. EDC cross-linking of Ku80CTD amino acids 590-720. Reactions contained 0, 1, 5, and 10mM EDC as indicated. Products were analyzed via western blot using 6x-His tag antibody (Roche: 04 905 318 001). D. PIC-UP reactions were exposed to white light for 0, 15, 30 and 60 seconds as indicated. Products were analyzed via western blot as in C.
Figure 5.
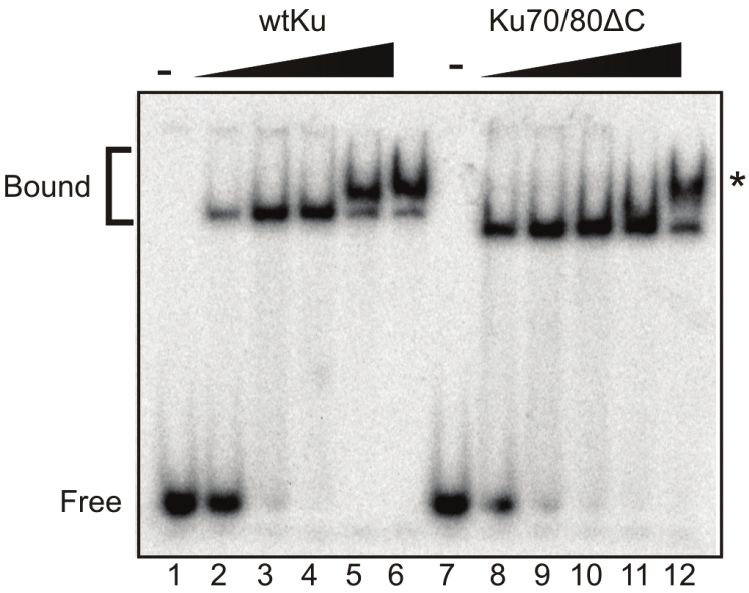
The Presence of the Ku80CTD Stimulates DNA Tethering. 34/21 bp duplex DNA was used in electro mobility shift assays (EMSAs). wtKu and Ku70/80ΔCTD was titrated at concentrations 0.1, 0.5, 1, 5, and 10μM using a fixed concentration of 3.4pM for the 21/34 duplex DNA substrate. Reactions were loaded onto 6% native polyacrylamide gels and run with 1X TBE buffer. Gels were exposed to a PhosphorImager screen.
However the fact that the Ku70/80ΔC protein can form this complex suggests that any Ku80CTD/Ku80CTD interactions that exist are not required for complex formation.
Discussion
The Ku heterodimer is critical for efficient NHEJ and functions as a scaffold protein to which other proteins involved in DNA repair can bind. Among these proteins, DNA-PKcs is recruited to the site of a DSB and is activated via interactions with Ku and DNA [20]. Our results reveal that deleting the carboxy-terminal domain of Ku80 abolishes kinase activity of DNA-PK and this cannot be overcome by the addition of the Ku80CTD construct (Figure 1E). This is interesting in light of our data showing that Ku80CTD interacts directly with DNA-PKcs (Figure 3A and 3B). Together these results demonstrate that the Ku80CTD/DNA-PKcs protein/protein interaction is not sufficient to activate the kinase. Instead the Ku80CTD must be tethered to the Ku core DNA bniding domain to support its role in DNA-PKcs activation.
Previously we showed that the Ku80CTD undergoes a conformational change upon binding to DNA. Recently SAXS confirmed our finding demonstrating that the Ku80CTD becomes extended from the dimerization/DNA binding core domain [12]. Accordingly, we confirmed that the Ku80CTD interacts with the core domain of Ku in the absence of DNA (Figure 2). Unexpectedly we also observed Ku80CTD dimer and multi-mer formation, which was confirmed by two distinct cross-linking methods (Figures 2- 4). Interestingly, the Ku80CTD/ Ku80CTD interactions formed more readily than the Ku80CTD/ DNA-PKcs interactions which have previously been reported (Figure 3). It is thought that during NHEJ, the two DNA termini at the site of the break are brought into close proximity of each other through formation of a synaptic complex. It has been suggested that this synaptic complex is necessary to maintain the DNA ends in proximity of each other for ligation as well as to form a platform for additional NHEJ machinery [21]. Structural and biochemical studies have suggested that the DNA-PK heterotrimeric complex plays a role in stabilizing the two DNA termini at the DSB, and this is further supported by studies reporting that Ku has the ability to tether DNA termini resulting in end-to-end alignment [22]. Our results showing formation of a Ku80CTD dimer further support Ku playing a role in tethering of the two DNA ends through a protein-protein interaction across the DNA break (Figure 5). This model is further supported by structural data that show that upon extension, Ku80CTD is capable of interacting with proteins across the dsDNA synapse.
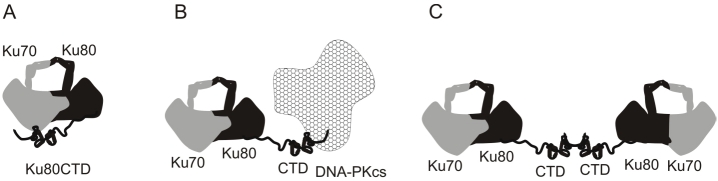
Our data show that the Ku80CTD is not essential for DNA end-to-end alignment, but the presence of the Ku80CTD does stimulate the tethering (Figure 5). Detection of the CTD/CTD interactions is similar to detection of the alignment of DNA termini when bound by Ku. Both events are only observed in assays that take a “snap shot” of molecular events at an instant in time. The alignment of DNA termini bound by Ku is observed following atomic force microscopy and transmission electron microscopy while CTD/ CTD interactions are observed following covalent cross-linking. We believe that this is due to the fact that these events are transient. Determining a role for CTD/CTD interactions in NHEJ is currently under investigation. A summary of the protein-protein interactions revealed in this study is shown in Figure 6. In conclusion our data show that the CTD of Ku80 interacts with the core domain of Ku70/80, DNA-PKcs, and forms homodimers.
Figure 6.
Summary of Protein-Protein Interactions Involving the Ku80CTD. A. The Ku80CTD interacts with the core Ku70/80 dimerization domain. B. The Ku80CTD interacts with DNA-PKcs. C. The Ku80CTD forms homodimers.
Abbreviations
- NHEJ
non-homologous end joining
- DSB
DNA double strand break
- IR
ionizing radiation
- HDR
homology directed repair
References
- 1.Delacote F, Guirouilh-Barbat J, Lambert S, Lopez BS. Homologous recombination, nonhomologous end-joining and cell cycle: Genome's angels. Curr. Genomics. 2004;5:49–58. [Google Scholar]
- 2.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:1–31. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo S, Dynan WS. Geometry of a complex formed by double strand break repair proteins at a single DNA end: recruitment of DNA-PKcs induces inward translocation of Ku protein. Nucleic Acids Res. 1999;27:4679–4686. doi: 10.1093/nar/27.24.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeFazio LG, Stansel RM, Griffith JD, Chu G. Synapsis of DNA ends by DNA-dependent protein kinase. EMBO J. 2002;21:3192–3200. doi: 10.1093/emboj/cdf299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma YM, Pannicke U, Schwarz K, Lieber MR. Hairpin opening and overhang processing by an Artemis/DNA- dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell. 2002;108:781–794. doi: 10.1016/s0092-8674(02)00671-2. [DOI] [PubMed] [Google Scholar]
- 6.Pawelczak KS, Bennett SM, Turchi JJ. Coordination of DNA-PK activation and nuclease processing of DNA termini in NHEJ. Antioxid Redox Signal. 2011;14:2531–2543. doi: 10.1089/ars.2010.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 8.Yoo S, Kimzey A, Dynan WS. Photocrosslinking of an oriented DNA repair complex - Ku bound at a single DNA end. J Biol Chem. 1999;274:20034–20039. doi: 10.1074/jbc.274.28.20034. [DOI] [PubMed] [Google Scholar]
- 9.Singleton BK, Torres-Arzayus MI, Rottinghaus ST, Taccioli GE, Jeggo PA. The C terminus of Ku80 activates the DNA-dependent protein kinase catalytic subunit. Mol Cell Biol. 1999;19:3267–3277. doi: 10.1128/mcb.19.5.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weterings E, Verkaik NS, Keijzers G, Florea BI, Wang SY, Ortega LG, Uematsu N, Chen DJ, van Gent R. The Ku80 carboxy terminus stimulates joining and artemis-mediated processing of DNA ends. Mol Cell Biol. 2009;29:1134–1142. doi: 10.1128/MCB.00971-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gell D, Jackson SP. Mapping of protein-protein interactions within the DNA-dependent protein kinase complex. Nucleic Acids Res. 1999;27:3494–3502. doi: 10.1093/nar/27.17.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammel M, Yu Y, Mahaney BL, Cai B, Ye R, Phipps BM, Rambo RP, Hura GL, Pelikan M, So S, Abolfath RM, Chen DJ, Lees-Miller SP, Tainer JA. Ku and DNA-dependent protein kinase dynamic conformations and assembly regulate DNA binding and the initial nonhomologous end joining complex. J Biol Chem. 2010;285:1414–1423. doi: 10.1074/jbc.M109.065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett SM, Neher TM, Shatilla A, Turchi JJ. Molecular analysis of Ku redox regulation. BMC Mol Biol. 2009;10:86–92. doi: 10.1186/1471-2199-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawelczak KS, Andrews BJ, Turchi JJ. Differential activation of DNA-PK based on DNA strand orientation and sequence bias. Nucleic Acids Res. 2005;33:152–161. doi: 10.1093/nar/gki157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehman JA, Hoelz DJ, Turchi JJ. DNA-Dependent Conformational Changes in the Ku Heterodimer. Biochemistry. 2008;47:4359–4368. doi: 10.1021/bi702284c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bitan G. Structural study of metastable amyloidogenic protein oligomers by photo-induced cross-linking of unmodified proteins. Methods Enzymol. 2006;413:217–236. doi: 10.1016/S0076-6879(06)13012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naue N, Fedorov R, Pich A, Manstein DJ, Curth U. Site-directed mutagenesis of the chi subunit of DNA polymerase III and single-stranded DNA-binding protein of E. coli reveals key residues for their interaction. Nucleic Acids Res. 2011;39:1398–1407. doi: 10.1093/nar/gkq988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cary RB, Peterson SR, Wang J, Bear DG, Bradbury EM, Chen DJ. DNA looping by Ku and the DNA-dependent protein kinase. Proc Natl Acad Sci USA. 1997;94:4267–4272. doi: 10.1073/pnas.94.9.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang D, Yoo S, Dynan WS, Jung M, Dritschilo A. Ku proteins join DNA fragments as shown by atomic force microscopy. Cancer Res. 1997;57:1412–1415. [PubMed] [Google Scholar]
- 20.Singleton BK, Priestley A, Steingrimsdottir H, Gell D, Blunt T, Jackson SP, Lehmann AR, Jeggo PA. Molecular and biochemical characterization of xrs mutants defective in Ku80. Mol Cell Biol. 1997;17:1264–1273. doi: 10.1128/mcb.17.3.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spagnolo L, Rivera-Calzada A, Pearl LH, Llorca O. Three-dimensional structure of the human DNA-PKcs/Ku70/Ku80 complex assembled on DNA and its implications for DNA DSB repair. Mol Cell. 2006;22:511–519. doi: 10.1016/j.molcel.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Grob P, Zhang TT, Hannah R, Yang H, Hefferin ML, Tomkinson AE, Nogales E. Electron microscopy visualization of DNA-protein complexes formed by Ku and DNA ligase IV. DNA Repair (Amst) 2012;11:74–81. doi: 10.1016/j.dnarep.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]