Abstract
Our adaptive immune system induces distinct responses to different pathogens because of the functional plasticity of dendritic cells (DCs); however, how DCs program unique responses remains unclear. Here, we found that the cytokine thymic stromal lymphopoietin (TSLP) potently transduced a unique T helper type 2 (TH2)–inducing compound signal in DCs. Whereas activation of nuclear factor κB (predominantly p50) drove DCs to produce OX40L to induce TH2 differentiation, the activation of signal transducer and activator of transcription 6 (STAT6) triggered DCs to secrete chemokines necessary for the recruitment of TH2 cells. In addition, TSLP signaling limited the activation of STAT4 and interferon regulatory factor 8 (IRF-8), which are essential factors for the production of the TH1-polarizing cytokine interleukin-12 (IL-12). By contrast, Toll-like receptor ligands and CD40 ligand did not activate STAT6 in myeloid DCs, but instead increased the abundance of STAT4 and IRF-8 to induce TH1 responses through the production of IL-12. Therefore, we propose that the functional plasticity of DCs relies on elaborate signal codes that are generated by different stimuli.
INTRODUCTION
Dendritic cells (DCs) are professional antigen-presenting cells (APCs), which are characterized by a strong ability to stimulate the proliferation of T cells and by a functional plasticity in the induction of distinct T helper cell responses to different types of invading pathogens (1). Certain microbial components, such as ligands for Toll-like receptors (TLRs), and CD40 ligand (CD40L), which is on activated T cells, induce the maturation of DCs, a process by which immature DCs differentiate into fully competent APCs capable of priming T cell responses, and the production of the cytokine interleukin-12 (IL-12). IL-12 is indispensable for mounting T helper type 1 (TH1) responses to eradicate most intracellular microbes by inducing the production of interferon-γ (IFN-γ) (2, 3). By contrast, extracellular pathogens, such as helminths, and allergens induce distinct immune responses called TH2 responses, which cause eosinophilic inflammation; however, the underlying molecular mechanisms that determine the functional plasticity of DCs are poorly understood (4–8).
Thymic stromal lymphopoietin (TSLP) is an IL-7–like cytokine that is a key molecule for initiating TH2 responses (9). In humans, TSLP is produced predominantly by epithelial cells and activates myeloid DCs (mDCs) to induce TH2 responses in T cells, which is associated with allergic inflammation (9). Inhibiting the function of TSLP in vivo has provided a promising therapeutic effect for allergic diseases (10, 11). The ability of TSLP-activated mDCs (TSLP-mDCs) to induce TH2 responses is directly linked to three unique features of these cells: (i) the secretion of chemokines that specifically attract TH2 cells; (ii) the presence of the TH2-polarizing molecule OX40 ligand (OX40L); and (iii) the inability to produce the TH1-polarizing cytokine IL-12 (12–14). We wished to understand how TSLP receptor (TSLPR) signaling induced TH2 responses so that we could try to uncover the molecular mechanisms responsible for the functional plasticity of DCs.
RESULTS
TSLP activates a distinct set of STAT proteins to program TH2-inducing mDCs
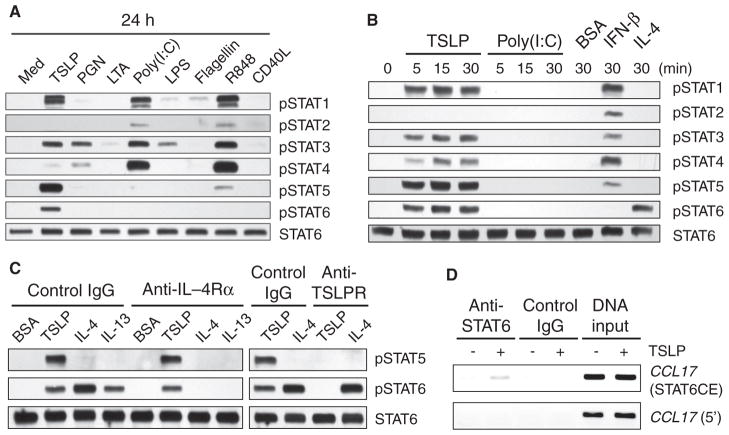
Previous studies of cell lines that have the TSLPR complex, which consists of TSLPR and the α chains of the IL-7 receptor (IL-7Rα), showed that TSLP activates the transcription factors signal transducer and activator of transcription 3 (STAT3) and STAT5 (15, 16). However, activation of these ubiquitous signaling molecules is unlikely to explain the unique features of TSLP-mDCs. STAT6 regulates the production of the TH2 cell–attracting CC chemokine CCL17 [also known as thymus and activation-regulated chemokine (TARC)] in T cells and macrophages (17, 18). We therefore examined the activation status of all of the STAT proteins in human mDCs after 24 hours of culture with TSLP, ligands for different TLRs, or CD40L (Fig. 1A). Phosphorylation of STAT1 and STAT3 was widely induced by TSLP and certain TLR ligands. In addition, TSLP induced the preferential phosphorylation of STAT5 and STAT6, which are involved in TH2 responses (19), whereas the TLR3 agonist polyinosinic:polycytidylic acid [poly(I:C)] and the TLR7 and TLR8 agonist R848, two major stimuli of the production of IL-12p70 in human primary mDCs (20), induced the preferential phosphorylation of STAT2 and STAT4, key transcription factors that are involved in TH1 responses (5). We occasionally observed that R848 also induced much weaker phosphorylation of STAT5 than did TSLP (fig. S1). CD40L did not induce detectable phosphorylation of any STAT protein.
Fig. 1.
TSLP directly activates STAT6 in mDCs. (A) Western blotting analysis was performed to compare the activation of different STAT proteins in mDCs cultured for 24 hours with medium alone (Med), TSLP (25 ng/ml), PGN, LTA, poly(I:C), LPS, flagellin, R848, or CD40L. (B) Western blotting analysis was performed to compare the early time course of activation of STAT proteins in mDCs stimulated with TSLP (25 ng/ml), poly(I:C), vehicle control (BSA), IFN-β (25 ng/ml), or IL-4 (25 ng/ml). (C) Effect of neutralizing antibodies against IL-4Rα or TSLPR on the activation of the indicated STAT proteins in mDCs by TSLP, IL-4, and IL-13. Cells were preincubated with the indicated neutralizing or isotype-matched control antibodies (10 μg/ml) for 30 min at 37°C and then stimulated with 10 ng/ml of TSLP, IL-4, or IL-13 or with vehicle control (BSA) for 10 min at 37°C. (A to C) Western blots were incubated with antibodies specific for the indicated phosphorylated STAT proteins or with an antibody against STAT6 to confirm equal loading of all lanes. Data are representative of three independent experiments. (D) ChIP assays demonstrating the enrichment of STAT6 at the region around the STAT6 consensus elements (STAT6CE) of the promoter of CCL17 in response to TSLP. A region ~6000 bp upstream of the initiator codon of the gene was used as negative control. Data are displayed as inverted images for easier visibility and are representative of three independent experiments. IgG, immunoglobulin G.
To determine whether TSLP directly activated multiple STATs, mDCs were stimulated for up to 30 min with TSLP, poly(I:C), IFN-β, IL-4 (a cytokine that drives TH2 responses), or bovine serum albumin (BSA) as a negative control (Fig. 1B). TSLP induced the phosphorylation of STAT1, -3, -4, -5, and -6 within 5 min, whereas poly(I:C) did not stimulate the phosphorylation of any STAT protein within 30 min, which suggests that poly(I:C) indirectly induces phosphorylation of STAT proteins at later time points, possibly through an autocrine pathway that involves IFN-β (Fig. 1A) (21). Indeed, IFN-β induced the phosphorylation of STAT1, -2, -3, -4, and -5, whereas IL-4 stimulated the phosphorylation of STAT6 within 30 min, as was reported for other cell types (22). Phosphorylation of STAT6 is typically induced by IL-4Rα upon binding to IL-4 or IL-13 (23). TSLP-mediated phosphorylation of STAT6 was blocked by neutralizing antibodies against TSLPR but not by antibodies against IL-4Rα, whereas IL-4– and IL-13–mediated phosphorylation of STAT6 was blocked by antibodies against IL-4Rα but not by antibodies against TSLPR, indicating that TSLPR mediates the activation of STAT6 independently of IL-4Rα (Fig. 1C). Chromatin immunoprecipitation (ChIP) assays revealed the binding of TSLP-activated STAT6 to the promoter of CCL17 (Fig. 1D), suggesting that the production of CCL17 by TSLP-mDCs (9) depends on TSLP-mediated activation of STAT6.
TSLP induces robust and sustained activation of Janus kinase 1 and 2 in mDCs
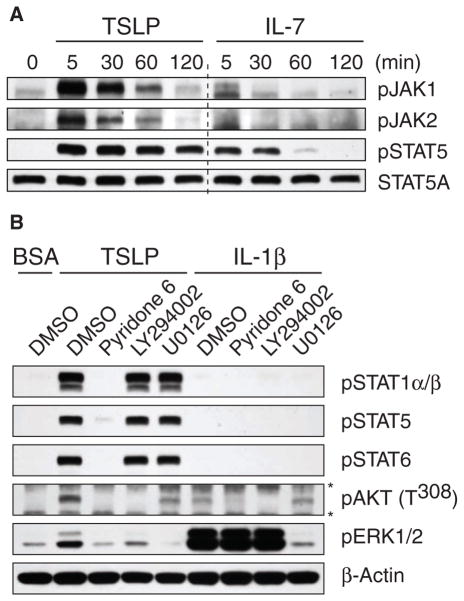
Cytokine-dependent activation of STATs is primarily mediated by Janus kinases (JAKs) (22–24); however, JAKs are not involved in TSLP signaling in mice (15). We therefore examined whether JAKs were activated by TSLP in human primary mDCs. Whereas IL-7 stimulated a weak and transient (<5 min) phosphorylation of JAK1, TSLP induced robust and sustained (~1 hour) phosphorylation of JAK1 and JAK2 in mDCs (Fig. 2A). This finding may explain why TSLP, but not other cytokines such as IL-7, could induce sustained (>2 hours) and broad activation of STATs (Figs. 1B and 2A and fig. S2). Because JAKs have the potential to activate several signaling pathways in addition to those involving STATs (24), we examined the activation of the phosphoinositide 3-kinase (PI3K)–Akt pathway and of mitogen-activated protein kinases (MAPKs) in response to TSLP and found that phosphorylation of Akt and the MAPKs extracellular signal–regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) was rapidly induced by TSLP (Fig. 2B and fig. S3).
Fig. 2.
Strong activation of JAK mediates sustained and broad TSLP-dependent signaling. (A) Western blotting analysis was performed to compare the kinetics of TSLP- and IL-7–mediated phosphorylation of JAK1, JAK2, and STAT5 in mDCs. Western blots were incubated with specific antibodies against the indicated phosphorylated proteins or with an antibody against STAT5A to confirm equal loading of all lanes. (B) Western blotting analysis was performed to demonstrate the contributions of JAK, PI3K, and ERK to TSLP-mediated signals. mDCs were pretreated with the pan-JAK inhibitor pyridone 6, the PI3K inhibitor LY294002, the MEK (ERK) inhibitor U0126, or DMSO (0.1%) for 30 min at 37°C and then were stimulated with 10 ng/ml of TSLP or IL-1β or with BSA for 10 min at 37°C. (*) Nonspecific bands. (A and B) The data shown are representative of three independent experiments.
To confirm the role of JAKs in mediating the range of TSLP-dependent signals, mDCs were pretreated with the pan-JAK inhibitor pyridone 6, the PI3K inhibitor LY294002, the MEK (ERK) inhibitor U0126, or with di-methyl sulfoxide (DMSO) as the vehicle control and were then stimulated with TSLP or IL-1β, a cytokine that stimulates JAK-independent signaling. We found that the pan-JAK inhibitor completely blocked the TSLP-dependent phosphorylation of STATs, Akt, and ERK, whereas it did not block the IL-1β–dependent phosphorylation of ERK and only partially blocked the phosphorylation of Akt, indicating that the robust and broad signals in response to TSLP were dependent on the activation of JAKs (Fig. 2B). The PI3K inhibitor LY294002 did not block TSLP-dependent phosphorylation of STATs but blocked the phosphorylation of Akt and ERK in response to TSLP. In contrast, whereas LY294002 blocked the IL-1β–dependent phosphorylation of Akt, it did not block the phosphorylation of ERK in response to IL-1β, demonstrating that the TSLP-dependent activation of ERK required activation of the PI3K-Akt pathway. The MEK inhibitor blocked the phosphorylation of ERK in response to either TSLP or IL-1β, but it did not inhibit the TSLP-dependent phosphorylation of STAT and Akt, nor did it block the IL-1β–dependent phosphorylation of Akt. Collectively, these data suggest that JAKs constitute critical mediators of the broad signaling of TSLP in human mDCs.
TSLP induces the production of OX40L in mDCs by the sustained activation of p50 and RelB
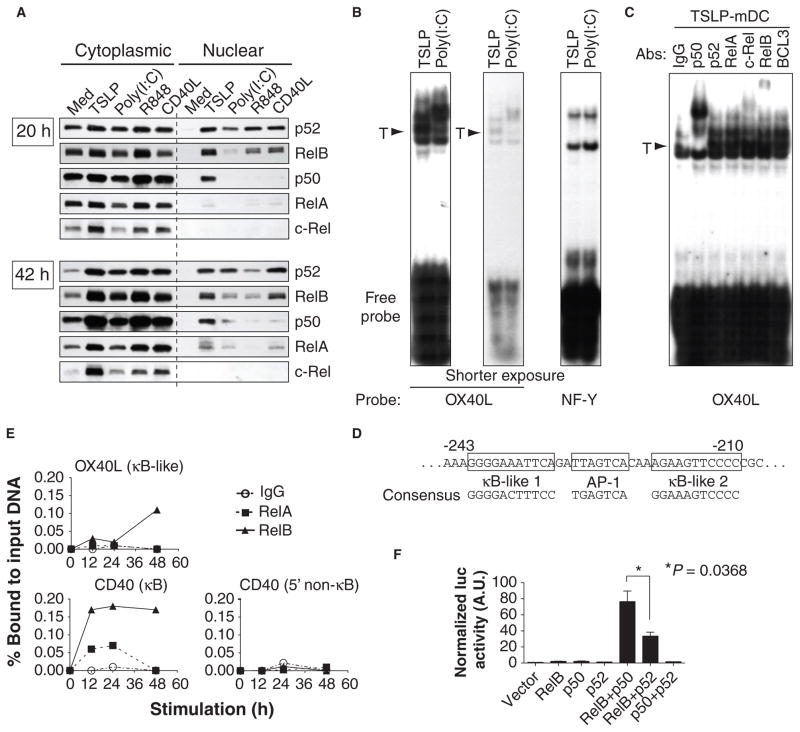
Upon activation with TSLP, mDCs increase the abundance on their cell surface of molecules such as major histocompatibility complex II (MHC II), CD40, CD80, and CD86 in 24 hours and that of OX40L, a potent TH2-polarizing molecule, in 48 to 72 hours (9, 12, 13). This increase in the abundance of OX40L is the second unique feature of TSLP-mDCs. Because the promoter of OX40L contains two potential nuclear factor κB (NF-κB) binding sites (25) and because TSLP increases the expression of several NF-κB target genes in mDCs (fig. S4), we hypothesized that the NF-κB pathway might play a role in the increased production of OX40L that is triggered by TSLP but not by other stimuli (12). To delineate the differential activation of NF-κB by different stimuli, we compared the nuclear translocation of NF-κB molecules in mDCs cultured with medium alone, TSLP, poly(I:C), R848, or CD40L for 20 hours, when OX40L messenger RNA (mRNA) is undetectable, and for 42 hours, when OX40L mRNA is detectable (12) (Fig. 3A). TSLP induced a comparable nuclear translocation of the NF-κB molecules p52 and RelB to that induced by poly(I:C), R848, and CD40L at both time points. In addition, TSLP induced a robust nuclear translocation of p50 at both time points.
Fig. 3.
TSLP activates NF-κB–dependent pathways that lead to the increased abundance of OX40L at the cell surface of mDCs. (A) Western blotting analysis was performed to demonstrate the cytoplasmic and nuclear localization of NF-κB molecules in mDCs 20 and 42 hours after culture with medium alone (Med), TSLP, poly(I:C), R848, or CD40L. (B) EMSA showing the binding of nuclear proteins to probes for the κB-like sequence of the promoter of OX40L and the binding sequence of the “housekeeping” transcription factor NF-Y in mDCs cultured for 60 hours with TSLP or poly(I:C). For the experiment with the OX40L probe, an autoradiograph of the same gel after a shorter exposure time is added to show the band separation. T, TSLP-predominant bands. (C) Super-shift assays were performed with antibodies against the indicated NF-κB molecules and BCL3 to demonstrate the composition of the nuclear proteins bound to the κB-like sequence probe of OX40L from mDCs cultured for 60 hours with TSLP. (D) Nucleotide sequence of the region surrounding the potential NF-κB binding sites within the 5′-flanking region of OX40L. The nucleotide positions indicate the relative distance from the initiator codon. The sequences of the consensus κB site and the activating protein 1 (AP-1) site are depicted. (E) ChIP assays demonstrating the kinetics of the recruitment of RelA and RelB to the promoters of OX40L and CD40 in TSLP-treated mDCs. Data are expressed as the percentage binding of either protein to the input DNA amount by quantifying the intensities of PCR-amplified bands. (A to C, and E) Data are representative of three independent experiments. (F) NF-κB–dependent activation of the OX40L promoter in HEK 293T cells was determined by luciferase reporter assay. Error bars represent the SEM from three independent experiments.
To determine whether the NF-κB components were capable of binding to the κB-like sequences of the OX40L promoter, we performed electrophoretic mobility shift assays (EMSAs) at 60 hours after treatment with TSLP, when the expression of OX40L and the production of OX40L protein reach maximal levels (12). TSLP and poly(I:C) induced distinct patterns of nuclear protein complexes bound to the κB-like sequences of the OX40L promoter, whereas they induced an identical pattern of nuclear protein complexes bound to the control probe containing the NF-Y binding site (Fig. 3B). Supershift assays demonstrated that the protein complexes bound to the κB-like sequences of the OX40L promoter observed in TSLP-mDCs contained predominantly p50 and, to a lesser extent, RelB and c-Rel (Fig. 3C). The findings that TSLP did not induce the accumulation of detectable amounts of nuclear c-Rel in mDCs (Fig. 3A) and that RelAwas not detected in the protein complexes bound to the κB-like sequences of the OX40L promoter (Fig. 3C) suggest that p50 and RelB may be responsible for the activation of the OX40L promoter in TSLP-mDCs.
To demonstrate the physiological binding of RelB to the OX40L promoter, we performed ChIP assays in primary human mDCs cultured with TSLP. The recruitment of RelB to the κB-like sequences of the OX40L promoter (Fig. 3D) was detected at 12 hours and was further increased in intensity at 48 hours (Fig. 3E and fig. S5). No substantial recruitment of RelA was detected. As a control, we observed that TSLP induced weak and transient binding of RelA (~24 hours) but stronger and more sustained binding of RelB (>48 hours) to the classical NF-κB binding site within the CD40 promoter (26). To test whether p50 and RelB could activate the OX40L promoter, we performed luciferase reporter gene assays in human embryonic kidney (HEK) 293T cells. RelB, p50, or p52 alone did not activate the OX40L promoter, whereas RelB and p50, and to a lesser extent RelB and p52, did (Fig. 3F). Because p52 was not detected among the protein complexes that bound to the OX40L promoter (Fig. 3C), these data indicate that TSLP induced the nuclear translocation of p50, which formed a transcriptionally active complex with RelB to induce the expression of OX40L in mDCs.
Failure of TSLP to stimulate the production of IRF-8 and STAT4 underlies the uncoupling of DC maturation from IL-12 production
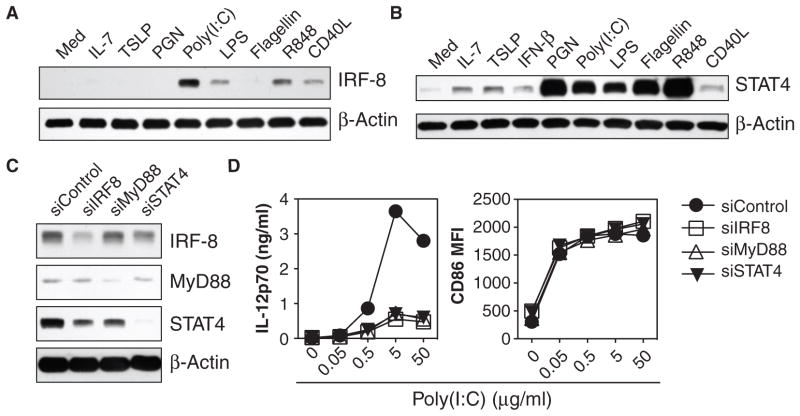
The third important feature that distinguishes TSLP-mDCs from TLR-activated DCs is that maturation of TSLP-DCs is uncoupled from the production of IL-12, an essential cytokine required for induction of TH1 immune responses (9, 12). Activation of PI3K-Akt and ERK pathways inhibits the production of IL-12 in DCs (27, 28); however, poly(I:C)-induced production of IL-12 could not be dampened by concurrent stimulation with TSLP, which suggested that TSLP did not use dominant-negative regulators to inhibit the production of IL-12 (fig. S6). We therefore examined the potential roles of two stimulators of the production of IL-12, interferon regulatory factor 8 (IRF-8) (29, 30), and STAT4 (31). Whereas TSLP did not increase the abundance of IRF-8 in mDCs, poly(I:C), R848, lipopoly-saccharide (LPS), and CD40L did (Fig. 4A). TSLP weakly induced an increase in the abundance of STAT4 and a subtle change in the extent of STAT4 phosphorylation, whereas poly(I:C) and R848 strongly induced increases in both the abundance of STAT4 and the extent of its phosphorylation (Figs. 1, A and B, and 4B).
Fig. 4.
TSLP does not stimulate the production of IRF-8 or STAT4, essential factors for the production of IL-12 by mDCs. (A) Western blotting analysis was performed to compare the differential regulation of the abundance of IRF-8 in mDCs that were activated for 24 hours by the indicated stimuli, as described in Fig. 1A with the inclusion of IL-7 (25 ng/ml) and the exclusion of LTA. (B) Western blotting analysis was performed to compare the abundance of STAT4 in mDCs after 24 hours of activation by the indicated stimuli, as described in (A) with the inclusion of IFN-β (25 ng/ml).(C) Western blotting analysis was performed to show the efficiency of knockdown of the indicated proteins in poly(I:C) (5 μg/ml)-stimulated mDCs transfected with siRNAs specific for IRF-8, MyD88, or STAT4 or with a nontargeting control siRNA (siControl). (A to C) Western blots were incubated with antibody against β-actin to demonstrate equivalent loading of all lanes. (D) siRNA-transfected mDCs were stimulated with poly(I:C) for 24 hours and the concentration of IL-12p70 in the culture supernatants and the mean fluorescent intensity (MFI) of cell-surface CD86 are depicted. Data are representative of three (A and B) or five (C and D) independent experiments.
To directly demonstrate the role of IRF-8 and STAT4 in the production of IL-12 in human mDCs, IRF-8, STAT4, and, as a positive control, the adaptor protein myeloid differentiation marker 88 (MyD88) (32) were knocked down in human primary mDCs by small interfering RNAs (siRNAs) (Fig. 4C), and we examined the poly(I:C)-dependent production of IL-12 and the increased abundance of cell-surface CD86 in these cells. Reducing the abundance of IRF-8, STAT4, or MyD88 strongly suppressed poly(I:C)-induced production of IL-12p70 without affecting the increase in the abundance of CD86 (Fig. 4D), as was previously suggested in other cell types (29–32). These data demonstrate that the production of IL-12 can be uncoupled from DC maturation (as assessed by the increased abundance of CD86) and that IRF-8 and STAT4 mediate the production of IL-12 but not the maturation of DCs. The inability of TSLP to increase the abundance of IRF-8 and STAT4 in human primary mDCs (Fig. 4, A and B) may therefore explain the absence of IL-12 production by TSLP-mDCs.
DISCUSSION
Here, we demonstrated that TSLP induces a unique compound signal that programs mDCs to induce TH2 responses that is distinct from those signals induced by other known activators of mDCs such as poly(I:C), R848, LPS, peptidoglycan (PGN), and CD40L, which normally activate mDCs to induce TH1-type responses. In experiments with human primary mDCs we found that TSLP induced broad and robust JAK-dependent signaling. In particular, TSLP directly activated STAT6, which explains the unique ability of TSLP-mDCs to produce the TH2-attracting chemokine CCL17. Previous studies on TSLP signaling with TSLPR-expressing cell lines failed to detect the activation of JAKs and STAT6, which underscores the importance of analyzing primary cells. The human mDCs used in this study are in vivo–derived mDCs that represent about 0.5% of total peripheral blood mononuclear cells. We did not use mDCs generated in vitro from blood monocytes or from CD34+ hematopoietic progenitor cells because they do not respond to TSLP (33). The yield of mDCs per buffy coat sample (which represents about 500 ml of human peripheral blood) is about 500,000 cells. For typical experiments, we used 2 × 106 to 5 × 106 mDCs (at >99% purity) that were isolated by cell sorting of 4 to 10 human blood buffy coat samples. Our study thus shows the feasibility of conducting comprehensive cell signaling studies in rare primary human DCs.
The second most important feature of TSLP signaling in human primary mDCs is that it increases the abundance at the cell surface of OX40L, a key molecule for polarizing naïve CD4+ T cell differentiation into inflammatory TH2 cells through the activation of the NF-κB pathway. Notably, TSLP-induced nuclear p50 and RelB to contribute to the expression of OX40L in mDCs (12, 13). However, OX40L is distinct from many of the typical NF-κB–regulated genes, such as those that encode MHC II and the costimulatory molecules CD40, CD80, and CD86, whose promoter regions contain the well-conserved consensus NF-κB binding sites (κB sites). The OX40L promoter, on the other hand, contains two atypical NF-κB binding sites that consist of 11 base pairs (bp) (κB-like sequences; Fig. 3D), which may have relatively low binding affinity for canonical NF-κB dimers (25). It was postulated that the p50 homodimer preferably recognizes an 11-bp DNA sequence, whereas the RelA homodimer and the RelA-p50 heterodimer prefer 9- and 10-bp DNA sequences, respectively (34). This unique feature of the OX40L promoter region may explain (i) why TSLP, but not other stimuli such as poly(I:C) and CD40L, efficiently induces the expression of OX40L, and (ii) why it takes 48 to 72 hours for TSLP to induce the expression of OX40L as opposed to its faster (~24-hour) induction of the expression of the genes encoding MHC II, CD40, CD80, and CD86 in mDCs. Our study also suggests that the type of nuclear NF-κB components or complexes may dictate the ability to activate the OX40L promoter in mDCs. The nuclear p50-RelB complex induced by TSLP is relatively more efficient in binding to the two atypical κB-like sequences in the OX40L promoter and therefore in activating the expression of OX40L than are the typical NF-κB complexes. During immune responses to pathogens, the production of different costimulatory and coinhibitory molecules on APCs have different kinetics (35). Our study suggests that the nature of the NF-κB binding sites within the promoter regions of the genes that encode these molecules may represent “molecular timers” of their expression.
The third important feature of TSLP signaling in human primary mDCs is that TSLP does not increase the abundance of IRF-8 or STAT4, the two critical transcription factors required for the production of IL-12 in mDCs. The maturation of mDCs by various TLR ligands and CD40L is coupled with the production of IL-12 by the DCs (4–8). However, maturation of mDCs induced by TSLP, including the increased abundance of MHC II, CD40, CD80, and CD86, is uncoupled from the production of IL-12 (9, 12). Given that the activation of NF-κB is a critical signal for DC maturation (36), our study suggests that the uncoupling of mDC maturation from the production of IL-12 is due to the ability of TSLP to activate multiple NF-κB components without the apparent induction of the expression of IRF-8 or STAT4. Because the activation of NF-κB is involved in the TLR- or CD40L-mediated expression of STAT4 in monocyte-derived DCs (37), TSLP-mediated activation of NF-κB may be qualitatively different from that induced by TLR ligands or CD40L.
In conclusion, this study demonstrates that TSLP programs human mDCs to induce TH2 responses by activating multiple signal pathways in a unique manner. The functional plasticity of mDCs to induce distinct T helper cell responses relies on elaborate signal codes generated by the activation of different receptors on mDCs. This study also highlights the importance and the feasibility of studying signal transduction in rare human primary cells.
MATERIALS AND METHODS
Reagents
PGN from Bacillus subtilis (5 μg/ml); lipoteichoic acid (LTA, 1 μg/ml), poly(I:C) (25 μg/ml, unless otherwise stated), LPS from Escherichia coli O111:B4 (1 μg/ml), flagellin from Salmonella typhimurium (5 μg/ml), and R848 (1 μg/ml) were purchased from InvivoGen. Recombinant human IL-2 and IFN-β were purchased from PeproTech. Recombinant human IL-1β, IL-4, IL-7, and neutralizing antibodies against IL-4Rα were purchased from R&D Systems. Recombinant human TSLP was either purchased from R&D Systems or expressed in HEK 293A cells and purified as described previously (12). These two independent recombinant TSLP proteins exhibited identical signal transduction capabilities. All recombinant cytokines were resuspended in phosphate-buffered saline containing 0.1% BSA, which was used as a vehicle control, where applicable. Neutralizing mouse antibodies against TSLPR (clone 1F11) were developed in our laboratory. Recombinant soluble CD40L (1 μg/ml) and Enhancer Solution (1 μg/ml) (Alexis Biochemicals) were used to stimulate CD40. The pan-JAK inhibitor pyridone 6 (38) (16 μM for the treatment of DCs), the PI3K inhibitor LY294002 (32.5 μM), and the MEK (ERK) inhibitor U0126 (10 μM) were purchased from EMD Biosciences and dissolved in DMSO before use.
Purification and culture of mDCs
The Institutional Review Board (IRB) for Human Research at The University of Texas M. D. Anderson Cancer Center approved this study. CD11c+lineage− mDCs were isolated from the blood of healthy donors with a FACSAria (BD Biosciences) as previously described (12). Sorted CD11c+ mDCs with purity >99% were cultured in RPMI 1640 medium (Invitrogen) containing 5% fetal calf serum (FCS) (Atlanta Biologicals). For short-term stimulation, freshly isolated mDCs were cultured in medium alone for 18 hours before stimulation.
Western blotting analysis
Cultured mDCs were collected and lysed with the PhosphoSafe Extraction Buffer (EMD Biosciences) supplemented with proteinase inhibitors [1 mM Pefabloc, 10 μM leupeptin, and 5 mM EDTA (Thermo Fisher Scientific)] for 10 min at room temperature. In some experiments, cytoplasmic and nuclear proteins were separated with the NE/PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific). Cell lysates were mixed with SDS sample buffer and boiled for 5 min. Samples were subjected to Western blotting analysis with antibodies against phosphorylated STAT1 (pSTAT1) (Tyr701), pSTAT2 (Tyr690), pSTAT3 (3E2) (Tyr705), pSTAT5 (Tyr694), pSTAT6 (Tyr641), STAT4 (C46B10), pJak1 (Tyr1022/1023), pAkt (Thr308), Akt (5G3), pERK1/2, pp38 MAPK, inhibitor of NF-κBα (IκBα; Cell Signaling Technology); pSTAT4 (Tyr693) (BD Biosciences); STAT1 (E-23), STAT3 (C-20), STAT6 (S-20 or M-20), pJak2 (Tyr1007/1008), p50 (E-10), RelA (C-20), RelB (C-19), c-Rel (N), IRF-4 (N-18), IRF-8 (C-19), BCL3 (C-14) (Santa Cruz Biotechnology); p52 (#05-361) (Millipore); active JNK (Promega); STAT5A (R&D Systems); MyD88 (Invitrogen); or β-actin (Sigma-Aldrich). To detect different proteins on the same membrane, bound antibodies were stripped off by incubating the membrane in either the Restore PLUS Western Blot Stripping Buffer (Thermo Fisher Scientific) or 62.5 mM tris-HCl (pH 6.5), 2% SDS, 0.68% (v/v) β-mercaptoethanol for 5 min at room temperature, or 30 min at 55°C, respectively, and then intensive washing.
ChIP assays
ChIP assays were performed with the ChIP assay kit (Millipore) with slight modifications to the manufacturer’s instructions. To detect the binding of STAT6 to DNA, we used 0.4% formaldehyde for cross-linking as reported (17). To detect the binding of RelA or RelB to DNA, we conducted a two-step cross-linking method as previously reported (39). Antibodies against STAT6 (M-20), RelA (C-20), and RelB (C-19) (Santa Cruz Biotechnology) were used for the immunoprecipitations. Primers for the detection of precipitated DNA by polymerase chain reaction (PCR) assay were as follows: CCL17 (TARC), surrounding region of STAT6 binding site, 5′-CAG CTG TGC GTG GAG GCT TTT CA-3′ and 5′-TCC TTC CCT AGA CCA GTG AAG TTC GAA GA-3′; 6000-bp upstream region, 5′-ACA AGT GGG CAG AGA GGA AA-3′ and 5′-TCA GTT GCA CTG CCA TCT TC-3′); TNFSF4 (OX40L), surrounding region of κB-like sequences, 5′-CCT GTTAGC CCA GAG GAA AA-3′ and 5′-CCA GGG CCA GAG ATA AAA GG-3′); and TNFRSF5 (CD40) (26), surrounding region of proximal κB site, 5′-GAG GTG GGA TGG AAT GGA AT-3′ and 5′-GTA TGG GGA GGC GTT TCA AG-3′; 2500-bp upstream non-κB region, 5′-TTG CTG AAC CCA CTC ATT CA-3′ and 5′-AGC ATT TAG CAT GCC AGC TC-3′.
EMSAs
Nuclear extracts were subjected to EMSAs with a 32P-radiolabeled OX40L promoter κB-like sequence oligonucleotide probe (5′-AAA GGG GAA ATT CAG ATT AGT CAC AAA GAA GTT CCC CCG-3′) or a control probe bound by the constitutive transcription factor NF-Y (5′-AAA AGA TTA ACC AAT CAC GTA CGG TCT-3′). Antibodies against p50 (NLS), p52 (C-5), RelA (A), c-Rel (N), RelB (C-19), and BCL3 (C-14) (Santa Cruz Biotechnology) were used for supershift assays.
Luciferase reporter assays
All plasmids were constructed by standard molecular biology techniques. HEK 293T cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% FCS. Cells were transiently transfected with a 1000-bp OX40L promoter-inserted firefly luciferase reporter plasmid (pGL-4.10, Promega), a constitutive Renilla luciferase expression vector phRL-TK (Promega), and expression vectors for NF-κB molecules (RelB, p50, and p52) by Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Promoter activity was measured with the DLR kit (Promega) on a Sirius luminometer (Berthold Detection Systems) and expressed as arbitrary units (firefly luciferase activity normalized by Renilla luciferase activity). Statistical analysis was performed with the two-tailed Student’s t test with Prism software (GraphPad).
Transfection of mDCs with siRNAs
Freshly isolated mDCs were precultured with granulocyte-macrophage colony-stimulating factor (GM-CSF, R&D Systems, 50 ng/ml) for 18 hours, and cells were transfected with siRNAs at a final concentration of 120 nM with Lipofectamine 2000-CD (Invitrogen) according to the manufacturer’s instructions. One day after transfection, cells were resuspended in fresh medium and stimulated with poly(I:C) for 24 hours. The following Dharmacon ON-TARGETplus SMARTpool siRNA reagents (Thermo Fisher Scientific) were used: IRF-8 (Dharmacon Catalog # L-011699-00), STAT4 (L-011784-00), MYD88 (L-004769-00), and Non-targeting pool (D-001810-10).
ELISAs
The amount of human IL-12p70 in culture supernatants was measured by DuoSet ELISA development kit (R&D Systems) according to the manufacturer’s instructions.
Flow cytometry
The abundance of CD86 on the surface of mDCs was analyzed by a FACSCalibur with a fluorescein isothiocyanate–conjugated antibody against CD86 (BD Biosciences), and the geometric means of the fluorescent signals were used to express the results in terms of mean fluorescence intensity (MFI).
Supplementary Material
Fig. S1. R848 is a weaker inducer of STAT5 phosphorylation than is TSLP.
Fig. S2. TSLP, but not IL-7 or IL-4, is capable of inducing the activation of a range of STAT proteins in mDCs.
Fig. S3. TSLP induces the phosphorylation of ERK, JNK, and Akt in mDCs.
Fig. S4. TSLP increases the abundance of NF-κB target molecules in mDCs.
Fig. S5. PCR gel electrophoresis data for the ChIP assays shown in Fig. 3E.
Fig. S6. TSLP is not a dominant-negative regulator of the production of IL-12.
Acknowledgments
We thank S. S. Watowich for critical reading of the manuscript; M. J. Wentz for preparation of the manuscript; Y.-L. Li-Yuan, W. Cao, and Y.-H. Wang for helpful suggestions; and K. Ramirez and Z. He for assistance with cell sorting. Our cell sorting facility is supported by grant P30CA16672 from the National Cancer Institute. Y.-J.L. is supported by M. D. Anderson Cancer Center Foundation and the National Institute of Allergy and Infectious Diseases (grants AI061645 and U19 AI071130).
REFERENCES AND NOTES
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 3.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaliński P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: The concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 5.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 6.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 7.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: Lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–298. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 8.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O’Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: Dependency on antigen dose and differential Toll-like receptor ligation. J Exp Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 10.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, Diehl L, Austin CD, Meng YG, Tan M, Bullens SL, Seeber S, Fuentes ME, Labrijn AF, Graus YM, Miller LA, Schelegle ES, Hyde DM, Wu LC, Hymowitz SG, Martin F. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y-J, Soumelis V, Watanabe N, Ito T, Wang YH, de Waal Malefyt R, Omori M, Zhou B, Ziegler SF. TSLP: An epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 15.Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG. Thymic stromal lymphopoietin: A cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 16.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, de Waal Malefyt R, Kastelein RA, Bazan JF. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 17.Wirnsberger G, Hebenstreit D, Posselt G, Horejs-Hoeck J, Duschl A. IL-4 induces expression of TARC/CCL17 via two STAT6 binding sites. Eur J Immunol. 2006;36:1882–1891. doi: 10.1002/eji.200635972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liddiard K, Welch JS, Lozach J, Heinz S, Glass CK, Greaves DR. Interleukin-4 induction of the CC chemokine TARC (CCL17) in murine macrophages is mediated by multiple STAT6 sites in the TARC gene promoter. BMC Mol Biol. 2006;7:45. doi: 10.1186/1471-2199-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 21.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. A type I interferon autocrine–paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard WJ, O’Shea JJ. Jaks and STATs: Biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 23.Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–188. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Ihle JN. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 25.Ohtani K, Tsujimoto A, Tsukahara T, Numata N, Miura S, Sugamura K, Nakamura M. Molecular mechanisms of promoter regulation of the gp34 gene that is trans-activated by an oncoprotein Tax of human T cell leukemia virus type I. J Biol Chem. 1998;273:14119–14129. doi: 10.1074/jbc.273.23.14119. [DOI] [PubMed] [Google Scholar]
- 26.Tone M, Tone Y, Babik JM, Lin CY, Waldmann H. The role of Sp1 and NF-κB in regulating CD40 gene expression. J Biol Chem. 2002;277:8890–8897. doi: 10.1074/jbc.M109889200. [DOI] [PubMed] [Google Scholar]
- 27.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 28.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 29.Giese NA, Gabriele L, Doherty TM, Klinman DM, Tadesse-Heath L, Contursi C, Epstein SL, Morse HC., III Interferon (IFN) consensus sequence-binding protein, a transcription factor of the IFN regulatory factor family, regulates immune responses in vivo through control of interleukin 12 expression. J Exp Med. 1997;186:1535–1546. doi: 10.1084/jem.186.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein–deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J Exp Med. 1997;186:1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokumasa N, Suto A, Kagami S, Furuta S, Hirose K, Watanabe N, Saito Y, Shimoda K, Iwamoto I, Nakajima H. Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-γ production and the induction of Th1 cell differentiation. Blood. 2007;110:553–560. doi: 10.1182/blood-2006-11-059246. [DOI] [PubMed] [Google Scholar]
- 32.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 33.Akamatsu T, Watanabe N, Kido M, Saga K, Tanaka J, Kuzushima K, Nishio A, Chiba T. Human TSLP directly enhances expansion of CD8+ T cells. Clin Exp Immunol. 2008;154:98–106. doi: 10.1111/j.1365-2249.2008.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YQ, Ghosh S, Ghosh G. A novel DNA recognition mode by the NF-κB p65 homodimer. Nat Struct Biol. 1998;5:67–73. doi: 10.1038/nsb0198-67. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Chen L. T lymphocyte co-signaling pathways of the B7-CD28 family. Cell Mol Immunol. 2004;1:37–42. [PubMed] [Google Scholar]
- 36.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Den-dritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remoli ME, Ragimbeau J, Giacomini E, Gafa V, Severa M, Lande R, Pellegrini S, Coccia EM. NF-κB is required for STAT-4 expression during dendritic cell maturation. J Leukoc Biol. 2007;81:355–363. doi: 10.1189/jlb.0506319. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR, Cameron PM, Meinke PT, Liverton N, Weng Y, DeMartino JA. Photochemical preparation of a pyridone containing tetracycle: A Jak protein kinase inhibitor. Bioorg Med Chem Lett. 2002;12:1219–1223. doi: 10.1016/s0960-894x(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 39.Tian B, Nowak DE, Jamaluddin M, Wang S, Brasier AR. Identification of direct genomic targets downstream of the nuclear factor-κB transcription factor mediating tumor necrosis factor signaling. J Biol Chem. 2005;280:17435–17448. doi: 10.1074/jbc.M500437200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. R848 is a weaker inducer of STAT5 phosphorylation than is TSLP.
Fig. S2. TSLP, but not IL-7 or IL-4, is capable of inducing the activation of a range of STAT proteins in mDCs.
Fig. S3. TSLP induces the phosphorylation of ERK, JNK, and Akt in mDCs.
Fig. S4. TSLP increases the abundance of NF-κB target molecules in mDCs.
Fig. S5. PCR gel electrophoresis data for the ChIP assays shown in Fig. 3E.
Fig. S6. TSLP is not a dominant-negative regulator of the production of IL-12.