Abstract
The purpose of the study was to investigate the role of altered proprioception on anticipatory (APAs) and compensatory (CPAs) postural adjustments and their interaction. Nine healthy adults were exposed to external perturbations induced at the shoulder level while standing with intact or altered proprioception induced by bilateral Achilles tendon vibration. Visual information was altered (eyes open or closed) in both the conditions. Electrical activity of the eight trunk and leg muscles and center of pressure (COP) displacements were recorded and quantified within the time intervals typical for APAs and CPAs. The results showed that when proprioceptive information was altered in eyes open conditions, anticipatory muscle activity was delayed. Moreover, altered proprioceptive information resulted in smaller magnitudes of compensatory muscle activity as well as smaller COP displacements after the perturbation in both eyes open and eyes closed conditions. The outcome of the study provides information on the interaction between APAs and CPAs in the presence of altered proprioception.
Keywords: postural control, proprioception, vision
INTRODUCTION
Human posture is controlled by the integration of information from the vestibular, proprioceptive, and visual systems. Each sensory system detects an error indicating deviation of body orientation from a certain reference position, individual error signals are summed and an appropriate corrective torque is generated as a function of this summed signal (Peterka 2002). Numerous studies have demonstrated the importance of individual sensory systems in balance maintenance. They have confirmed that stimulation of visual (Bronstein 1986; Dijkstra et al. 1994), vestibular (Nashner and Wolfson 1974; Hlavacka and Njiokiktjien 1985; Pavlik et al. 1999), and proprioceptive (Allum 1983; Kavounoudias et al. 1999) systems evoke body sway.
A number of techniques are used to alter proprioception while studying control of posture. Among them are a local anesthesia (Kjaergard et al. 1984), cuff compression (Mauritz and Dietz 1980; Demura et al. 2008) or lower legs cooling (Fujiwara et al. 2003; Stal et al. 2003). Another relatively easy-to-implement way of altering proprioception involves vibrating the muscle tendons (Roll et al. 1993; Gurfinkel and Kireeva 1995; Gurfinkel et al. 1996). For example, the effect of a vibratory stimulus has been studied by vibrating the neck (Courtine et al. 2003), trunk (Schmid et al. 2005), or ankle tendons (Thompson et al. 2007). It had been demonstrated that vibration applied to the ankle muscles produces body tilts in stance without any effect on gait (Ivanenko et al. 2000; Courtine et al. 2001; Verschueren et al. 2002). Such a vibration, if applied at the proper frequency and amplitude, activates mainly the muscle spindles connected to the primary Ia afferents. The CNS interprets this vibration as a stretching of that muscle (Burke et al. 1976; Roll and Vedel 1982; Roll et al. 1989). This results in an interpretation of proprioceptive information which does not match with the actual body position. Consequently, the body starts tilting in the direction of the vibrated muscles (Hayashi et al. 1981) which is accompanied by the increased body sway (Eklund 1973; Gurfinkel and Kireeva 1995). Such a vibration has been shown to change spatial body orientation very fast (Roll et al. 1989; Ceyte et al. 2007; Thompson et al. 2007) resulting in a postural response known as a ‘vibration-induced falling’ (Eklund 1973; Hayashi et al. 1981).
It was shown that if the effect of vibration-induced changes in proprioception is combined with erroneous signals from visual and/or vestibular systems, the control of vertical posture becomes yet more complex. Thus, it was reported that bilateral Achilles tendon vibration applied in the absence of vision resulted in the backward lean of the body with trunk extension, posterior pelvic tilt and flexion of hips and knees (Thompson et al. 2007). When vibration was applied to the Soleus muscles simultaneously with a moving visual scene which compromised visual information, the angles of “body –tilt” that were induced by the vibration were modulated by the motion of the visual scene (Adamcova and Hlavacka 2007). In contrast to this, it has been shown that body instability diminishes the effect of vibration applied to the Achilles tendon (Ivanenko et al. 1999) or tensor fascia latae muscles (Gurfinkel et al. 1996). Based on the outcome of these studies it was suggested that when tendon vibration is combined with body instability, the vibration-induced artificial afferent flow is blocked (Ivanenko et al. 1999). At the same time, it was demonstrated that increased body stability, produced by a back support combined with the vibration-induced changes in proprioception, did not affect the body tilt induced by the Achilles tendon vibration (Talis and Solopova 2000).
Humans commonly experience perturbations applied to their body resulting in the displacement of the body’s center of mass (COM) closer to or beyond the boundaries of the base of support (Maki and McIlroy 1996), thus compromising balance (Macpherson et al. 1989; Henry et al. 1998). The central nervous system (CNS) uses two main strategies to restore balance if it is distorted by a perturbation: (1) feed forward control, which is the anticipatory postural adjustments (APA) prior to the expected body perturbations (Belenkiy et al. 1967; Massion 1992) and (2) feedback control, which is the compensatory postural adjustments (CPA) that are initiated by the sensory feedback signals after the perturbations (Horak et al. 1996) (Park et al. 2004; Alexandrov et al. 2005). There is a clear difference between the two strategies: APAs serve to minimize the displacement of the body’s COM prior to a perturbation (Bouisset and Zattara 1987; Aruin and Latash 1995) while CPAs serve as a mechanism of restoration of the position of COM after a perturbation has already occurred (Macpherson et al. 1989; Maki and McIlroy 1996).
Information on the role of altered proprioception or proprioceptive stimulation on anticipatory and compensatory components of postural control is limited. Nevertheless, it was reported that vibration of Achilles tendons induced an increase in APAs in rectus femoris, biceps femoris and erector spinae muscles prior to the fast arm movements and load release (Slijper and Latash 2004). It was also demonstrated that the latency of the anticipatory activation of biceps femoris in the experiments with the fast arm flexion movement is modulated depending upon the application of the vibratory stimuli. When vibration was applied to Tibialis anterior, early activation of Biceps femoris was elicited 30 ms earlier than when applied to the Soleus muscle (Kasai et al. 2002). The outcome of our recent experiments with altered vision (wearing glasses with negative lenses) revealed that activation of the trunk and leg muscles observed prior to the body perturbation induced by a swinging pendulum is delayed and reduced as the pendulum was perceived positioned further away (Mohapatra et al. 2011). However, it is still unknown if the backward body lean induced by vibration of Achilles tendons (that might lead to the pendulum being perceived as positioned further away) would associated with similar delayed and reduced APAs.
Studies of the effect of altered proprioceptive information on compensatory postural control revealed that bilateral Achilles tendon vibration affects body kinematics and COM and COP displacements (Thompson et al. 2011). At the same time it has been shown that the patterns of adaptation to the rotational movements of the support surface were not affected by the application of bilateral Achilles tendon vibration (Hatzitaki et al. 2004).
Sensory deficit is a well-known consequence of many neurological disorders. While sensory deficit is commonly seen in the entire limb, still there are cases of patchy and localized loss of proprioception (Lephart et al. 1997; Ducic et al. 2004; Harati Y 2008; Eun et al. 2011). As such investigating the effect of altered proprioceptive information in control of posture has the potential to help enhance balance rehabilitation. Moreover, little is known about how changes in proprioceptive information affect the generation of anticipatory and compensatory corrections used to maintain balance before and after an external perturbation. In addition, it is virtually unknown how changes in proprioceptive information combined with changes in visual information modify the relationship between anticipatory and compensatory components of postural control. These deficits in the research limit the development of rehabilitation approaches that are centered on APA-based interventions that seek to restore balance abilities in individuals with altered proprioceptive.
In this study we aimed to investigate how changes in proprioception, induced by a vibration applied to Achilles tendons in condition with and without vision influence the APAs and CPAs. We hypothesized that: 1) APA patterns will be different between conditions with intact and altered proprioception. In particular, due to the backward body tilt and increased sway we expect to see delays in the anticipatory activation of muscles. 2) In conditions with altered proprioception, CPA patterns will differ when compared to conditions with intact proprioception irrespective of the availability of vision.
METHODS
Subjects
Nine healthy subjects (3 males and 6 females) without any known neurological, musculoskeletal or balance disorders participated in the experiment. The mean age of the subjects was 25 ± 0.9 years; mean body mass 64.2 ± 4 kg; and mean height 1.67 ± 0.03 m. They all signed a written informed consent approved by the Institutional Review Board.
Procedure
The subjects maintained a comfortable posture with their feet placed shoulder width apart while standing barefoot on the force platform being positioned in front of an aluminum pendulum attached to the ceiling. An additional load (mass = 5% of subject’s body weight) was fixed to the pendulum at its lower end. The width of the padded hitting surface of the pendulum was adjusted to match the subject’s shoulder width. The pendulum was released by an experimenter at an initial angle of 30 degree to the vertical and at a distance of 0.6m from the subject. Unidirectional (anterior to posterior) perturbations were applied by the pendulum on the shoulders of the subjects; the pendulum’s padded surfaces minimized a possible discomfort associated with the pendulum impact. The subjects were instructed to maintain their balance after the perturbation. The four experimental conditions were: (1) eyes open (EO), (2) eyes open with tendon vibration (VEO), (3) eyes closed (EC), and (4) eyes closed with tendon vibration (VEC) (Fig. 1). In eyes closed conditions the subjects were wearing an eye mask. A chalk was used to mark the subject’s foot position on the top of the force platform at the start of the experiment. This foot position was checked by the experimenter throughout the experimental conditions.
Fig. 1.
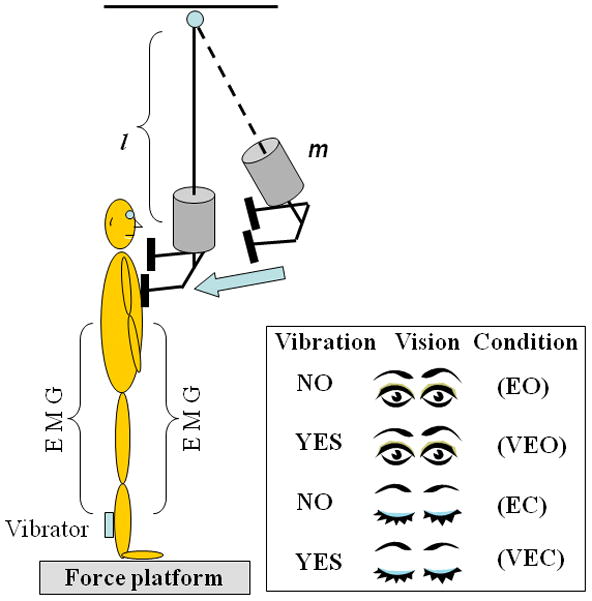
Schematic diagram of the experimental setup. The subjects stood with their arms at their sides, and the pendulum impact was on their shoulders while proprioception and visual conditions were manipulated. l is the length and m is an additional mass (5% of subject’s body weight).
Two custom-built miniature tendon vibrators were securely strapped bilaterally to the Achilles tendons of the subject. The vibrator produced a vibration at the frequency of 90 Hz with 1 mm amplitude. The vibrators were switched on one minute prior and were kept on throughout the five trials in each of the two conditions involving tendon vibration. The results of a pilot experiment involving two subjects demonstrated that vibration induces a small backward body tilt however, both the subjects were able to keep their balance throughout the experimental condition that lasted for about 30 s. After the recording, the subjects were required to open their eyes and vibrators were turned off for at least one minute. During this time the subjects performed dynamic movements of ankle (for example, walking in place.) to minimize the effect of vibration (Thompson et al. 2007).
The subjects wore wireless headphones playing music throughout all of the conditions to mask any kind of auditory information which may alert them about the moment of release of the pendulum. For safety, the participants remained in a harness with two straps attached to the ceiling during the experiment. The subjects performed two to three practice trials in each experimental condition prior to the start of data collection. Five trials, each of 5s in duration, were performed in each experimental condition and the order of the conditions was randomized across subjects.
Instrumentation
Electrical activity of muscles (EMGs) were recorded unilaterally (right side) from the following muscles: tibialis anterior (TA), Soleus (SOL), biceps femoris (BF), rectus femoris (RF), gluteus medius (GMed), rectus abdominis (RA), external oblique (EOb) and erector spinae (ES). These specific trunk and leg muscles were selected because of their involvement in control of vertical posture while dealing with symmetrical perturbations induced in the sagittal plane and were previously used to study anticipatory and compensatory control of posture (Latash et al. 1995; Aruin and Latash 1996; Santos et al. 2010a). After the skin area was cleaned with alcohol wipes, disposable electrodes (Red Dot 3M) were attached to the muscle belly of each of the above mentioned muscles. Such placement was based on recommendations reported in the literature (Basmajian 1980). A ground electrode was attached to the anterior aspect of the leg over the tibial bone after similar skin preparations. The EMG signals were collected, filtered and amplified (10–500 Hz, gain 2000) with a commercially available EMG system (Myopac, RUN Technologies, USA).
Ground reaction forces and moments of forces were recorded using a force platform (Model OR-5, AMTI, USA). An accelerometer (Model 208CO3, PCB Piezotronics Inc, USA) was attached to the subject’s proximal clavicle; its signal was used to record the moment of pendulum impact. The forces, moments of forces, EMG and accelerometer signals were digitized with a 16 bit resolution at 1000 Hz by means of customized LabVIEW 8.6.1 software (National Instruments, Austin TX, USA).
Data processing
The data were analyzed off-line using the MATLAB (Math Works, Natick, MA) program. First, the accelerometer signal was corrected for offset, and ‘time-zero’ (T0=0, the moment of pendulum impact) was acquired by a computer algorithm as a point in time at which the signal exceeded 5% of the maximum acceleration. This value was confirmed through visual inspection by an experienced researcher. Then, all trials were aligned to T0. EMG signals were then rectified and filtered with a 50 Hz low-pass, 2nd order, zero-lag Butterworth filter, while the reaction forces and moments were filtered with a 20 Hz low-pass, 2nd order, zero-lag Butterworth filter. Data in the range from −600 ms (before T0) to +500 ms (after T0) were selected for further analysis from which −600 ms to −450 ms of the data were taken for baseline activity. Integrals of EMG, muscle latencies, and center of pressure displacements were calculated.
The averaged EMG signals for each muscle and each subject were integrated (IntEMGi) with 150 ms time windows for a total of 4 time windows representing the −250 ms to +350 ms of the data. Each of the time windows were further corrected by the averaged 150 ms baseline activity:
| (1) |
In equation 1, IntEMGi is the integral of EMG activity of muscles inside each 150 ms interval which was corrected by the baseline activity. Then the integrals of EMG for each muscle for each subject were normalized to peak magnitude across all of the conditions (equation 2).
| (2) |
Due to the normalization, all of the IEMGNORM values were within the range from +1 to −1 (Krishnan et al. 2011). Four epochs were selected (each of 150 ms in duration) in relation to T0. The four epochs were: (1) from −250 ms to −100 ms (anticipatory, APA1); (2) from −100 ms to +50 ms (anticipatory, APA2); (3) from +50 ms to +200 ms (compensatory reactions, CPA1); and (4) +200 ms to +350 ms (late compensatory reactions, CPA2) (Santos et al. 2010b; Santos et al. 2010a).
Muscle latencies were detected in a time window from −250 ms to +250 ms in relation to T0 by a combination of both a computer algorithm and the visual inspection of the individual trials for each muscle. The latency for a specific muscle was defined as the instant lasting for at least 50 ms when its EMG amplitude was greater (activation) or smaller (inhibition) than the mean plus 2 SD of the baseline (−500 ms to −400 ms with respect to T0).
Time-varying COP displacement was calculated using the following approximation (Winter et al. 1996):
| (3) |
Where Mx is the moment in the sagittal plane, Fz and Fy are the vertical and anterior-posterior components of the ground reaction force, and dz is the distance from the origin of the platform to the surface (0.038 m). Since the perturbations were induced symmetrically, only COP displacements in the anterior-posterior direction were recorded. The COPsignals were corrected by its respective baseline and the COP data windows were shifted 50 ms forward to account for the electro-mechanical delay (Cavanagh and Komi 1979; Howatson et al. 2009). We calculated the peak magnitude of the COP, the time of the peak magnitude, and the magnitude of COP at the moment of perturbation (T0).
Statistics
Multiple repeated measures ANOVAs were performed with two within subject factors: visual conditions (EO, VEO, EC and VEC) and epochs (APA1, APA2, CPA1 and CPA2). The dependent variables were IEMGNORMs, latency of trunk and leg muscles, peak magnitude of the COP, time of the COP peak magnitude, and magnitude of COP at the moment of perturbation (T0). A post hoc analysis with Bonferroni correction was further done to compare between conditions, epochs and their interactions. For all tests, statistical significance was set at p < 0.05. Statistical analysis was performed in SPSS 17 for Windows 7 (SPSS Inc., Chicago, USA).
RESULTS
EMG Profiles
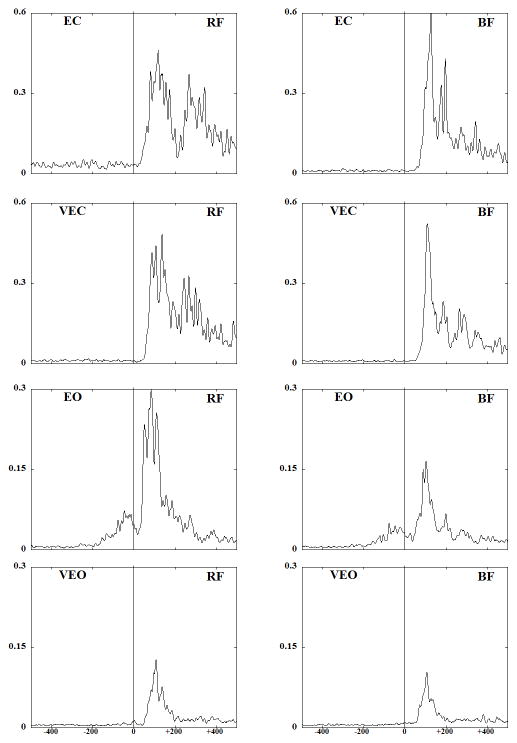
Fig. 2 shows EMG traces obtained from the anterior (RF) and the posterior (BF) muscles of a representative subject performing the experimental task under four different conditions. Anticipatory activity, seen as bursts in the background EMG activity, was present in the two conditions with eyes open. Quite to the contrary, anticipatory activity was negligible in the no vision conditions. Moreover, in conditions with tendon vibration the activation of RF and BF were delayed in the eyes open conditions. EMG activity after the perturbation was larger in EC condition as compared to EO condition where anticipatory activity was present. In addition, peaks of EMG activity during the compensatory period were smaller in conditions with tendon vibration (VEO and VEC).
Fig. 2.
EMG patterns (averaged across 5 trials) for a representative subject for the rectus femoris (RF) and biceps femoris (BF) muscles are presented across all the experimental conditions. Vertical line at T0 indicates the moment of pendulum impact.
Integrals of EMG activity
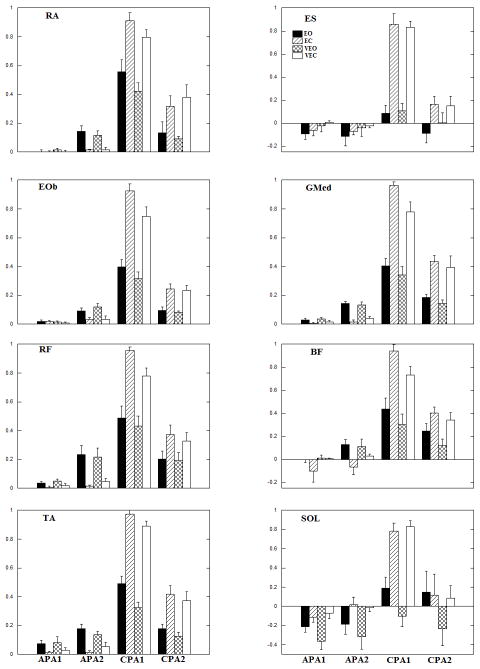
The anticipatory and compensatory IEMGNORM of the trunk and leg muscles averaged across subjects are shown in Fig. 3. In general, the anticipatory integrals of EMG (APA1 and APA2) are seen in all anterior muscles, (RA, RF, and TA) in the conditions where vision was available. In VEO condition, the anticipatory integrals of EMG across all anterior muscles stayed relatively the same as in the EO condition whereas the compensatory integrals of EMG were reduced in the VEO as compared to EO condition. Anticipatory integrals of EMG for these muscles were absent or negligible in conditions when eyes were closed (EC and VEC). As a result, compensatory IEMGNORM in the two blindfolded conditions were larger compared to eyes open conditions. When tendon vibration was applied in the conditions with eyes closed, compensatory IEMGNORM were smaller compared to the eyes closed condition with no tendon vibration. Overall, the largest IEMGNORM for all anterior muscles during the CPA1 were seen in the EC, followed by VEC, EO and VEO conditions. Table 1 shows the repeated measures ANOVA results for conditions, epochs and their interaction. The statistical analysis revealed a significant effect of the conditions, epochs and their interactions. Pairwise comparisons of conditions showed that RA IEMGNORM calculated for the EC-EO and VEO-VEC conditions were significantly different (p<0.05). Moreover, TA IEMGNORM for the, EO-EC, EO-VEC, EC-VEO and VEO-VEC pairs were statistically significant (p<0.05). Pairwise comparisons of epochs showed that APA1-CPA1, APA1-CPA2, APA2-CPA1 and CPA1-CPA2 pairs to be significantly different (p<0.05) in all the anterior muscles. The interactions revealed that IEMGNORM were smaller with application of vibration in both the CPA1 and CPA2 epochs for both EO and EC conditions.
Fig 3.
Mean EMG integrals during the four experimental conditions across subjects. Each column represents the IntEMGi for 150 ms epochs (APA1, APA2, CPA1 and CPA2) with its standard error bars.
Table 1.
Repeated measures ANOVA of IEMGNORM for conditions, epochs and their interactions. The results are shown for the four experimental conditions: EO, EC, VEO, VEC and four epochs: APA1, APA2, CPA1 and CPA2.
| Muscles | Conditions | Epochs | Conditions * Epochs | |||
|---|---|---|---|---|---|---|
| [F3,24] | p | [F3,24] | p | [F9,72] | p | |
| TA | 16.83 | <0.0001 | 203.33 | <0.0001 | 41.55 | <0.0001 |
| SOL | 17.13 | <0.0001 | 16.05 | <0.0001 | 6.99 | <0.0001 |
| RF | 3.60 | 0.03 | 141.48 | <0.0001 | 23.74 | <0.0001 |
| BF | 3.04 | 0.04 | 76.27 | <0.0001 | 33.61 | <0.0001 |
| GMed | 16.34 | <0.0001 | 184.72 | <0.0001 | 28.93 | <0.0001 |
| EOb | 18.89 | <0.0001 | 257.41 | <0.0001 | 27.60 | <0.0001 |
| RA | 8.43 | 0.001 | 72.17 | <0.0001 | 13.07 | <0.0001 |
| ES | 17.03 | <0.0001 | 78.55 | <0.0001 | 22.69 | <0.0001 |
The anticipatory activity (APA1 and APA2) represented by integrals of EMG is also seen in all posterior muscles (ES, BF, and SOL) in conditions with vision available. In the VEO condition, the compensatory integrals of EMG were reduced when compared to EO condition for all posterior muscles except ES. Anticipatory integrals of EMG for these muscles were absent or negligible in conditions when eyes were closed (EC, VEC). As a result, compensatory IEMGNORM in the two blindfolded conditions were larger compared to eyes open conditions. However, in conditions with the tendon vibration (VEC) the compensatory IEMGNORM in all posterior muscles with the exception of SOL were smaller comparedto EC condition. The repeated measures ANOVA results revealed a significant effect of the conditions, epochs and their interactions (Table 1). Pairwise comparisons of conditions discovered that ES IEMGNORM were statistically significant for the following conditions: EO-EC, EO-VEC, EC-VEO and VEO-VEC (p<0.05). Moreover, the SOL IEMGNORM were statistically significant between the EO-VEO, EC-VEO, and VEO-VEC conditions (p<0.05). Pairwise comparisons of epochs showed APA1-CPA1 and APA2-CPA1 pairs to be significantly different (p<0.05) in all the posterior muscles. IEMGNORM were smaller with application of vibration in both the CPA1 and CPA2 epochs for both EO and EC conditions which is supported by significant interactions.
The anticipatory integrals of EMG (APA1 and APA2) are seen in both the lateral muscles (GMed and EOb) in conditions with vision available. On the contrary, anticipatory integrals of EMG for these muscles were absent or negligible in conditions when eyes were closed. When tendon vibration was present in VEO condition, the anticipatory integrals of EMG stayed relatively the same as in the EO condition whereas the compensatory integrals of EMG were reduced in the VEO condition in both the lateral muscles. Similar pattern was also seen in these muscles when VEC and EC conditions were compared. Overall, the largest IEMGNORM during CPA1 was seen in the EC, followed by VEC, EO and VEO conditions for both the lateral muscles. Table 1 shows the repeated measures ANOVA results for conditions, epochs and their interaction. The statistical analysis revealed that there was a significant effect of the conditions, epochs and their interactions. Pairwise comparisons of conditions discovered that GMed IEMGNORM during EO-EC, EO-VEC and EC-VEO conditions were significantly different (p<0.05). Moreover, EOb IEMGNORM during EO-EC, EO-VEC, EC-VEO and VEO-VEC conditions pairs were statistically significant (p<0.05). Pairwise comparisons of epochs showed all the pairs to be significantly different (p<0.05) from each other for both the muscles. The interactions revealed that IEMGNORM were smaller with application of vibration in both the CPA1 and CPA2 epochs for both EO and EC conditions.
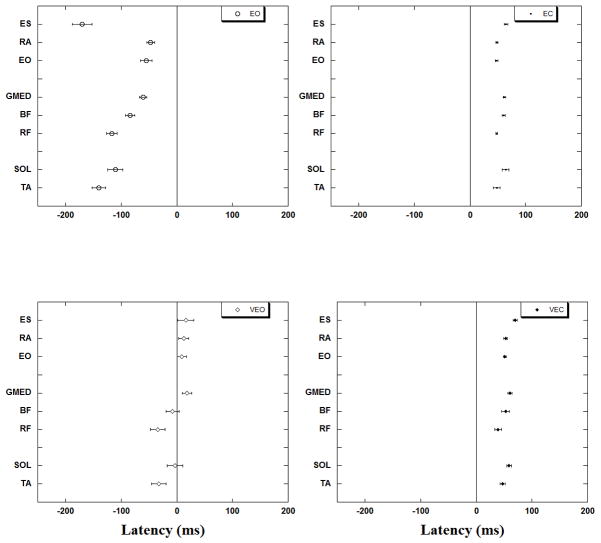
Onset of EMG activity
The onset of EMG activity of all studied muscles for each of the four experimental conditions is presented in Fig. 4. In the EO condition all the muscles showed anticipatory activation, with SOL and ES showing inhibition before the perturbation. When tendon vibration was induced in the VEO condition, the onset of all leg and trunk muscles was significantly delayed (p<0.0001). It is interesting to note that when tendon vibration was applied in conditions with full vision, the onset of the lateral (GMed and EOb) and trunk (RA and ES) muscle activity was further delayed so the muscles became active only after the perturbation. Thus, in the EO condition TA became active first at −140 ± 12 ms followed by BF (−85 ± 8 ms), GMed (−61 ± 6 ms) and RA (−47 ± 7 ms) before the perturbation. In VEO condition TA was active at −33 ± 13 ms followed by BF (−8 ± 11 ms) before the perturbation whereas, GMed and RA were active +17 ± 8 ms and +12 ± 8 ms respectively after perturbation.
Fig. 4.
Muscle latencies with standard error bars are represented for the four experimental conditions. Note the delay of muscle activation in VEO.
In both the EC and VEC conditions the onset of muscle activity for all muscles was seen only after perturbation. ANOVA revealed no difference between the onset of muscle activation involving the EC and VEC conditions.
Changes in the COP displacement
The perturbation-related COP displacements were all in the backward direction irrespective of the condition. The anticipatory displacement of COP at the moment of perturbation (T0) was the smallest for the EC followed by VEC, EO and was the largest for the VEO condition (Fig 5). Statistically significant differences (p<0.01) were found for the anticipatory COP displacement (at T0) between EO-EC, EO-VEC, EC-VEO and VEO-VEC conditions.
Fig. 5.
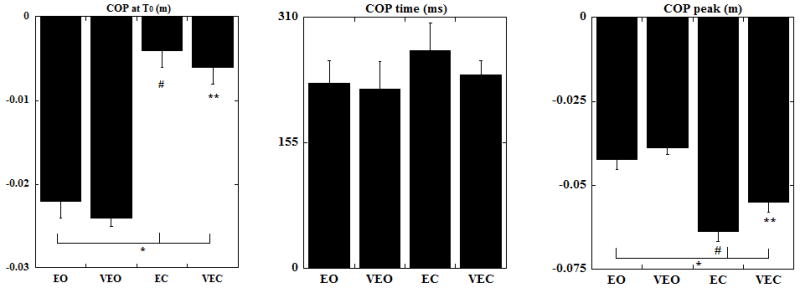
The magnitude of COP displacement at T0, the time of the COP peak and the magnitude of peak COP displacement are shown as mean with standard errors. COP positive values correspond to displacements in the direction opposite to the perturbation. * indicates statistical significance between EO-EC and EO-VEC, # indicates statistical significance between EC-VEO and ** indicates statistical significance between VEO-VEC conditions.
The time to the peak COP (Fig. 5) was reduced in conditions with vibration (VEO and VEC). The COP peak was seen earliest in VEO followed by EO and VEC whereas it was most delayed in the EC condition. This difference in time however was not statistically significant. The magnitude of the COP displacement after perturbation (Fig. 5) was the highest for the EC followed by VEC, EO and it was the smallest for the VEO condition. Statistically significant differences were found between EO-EC, EO-VEC, EC-VEO and VEO-VEC conditions (p<0.05).
DISCUSSION
This study was designed to investigate how the changes in proprioception, induced by vibratory stimuli to the Achilles tendon, influence APAs and CPAs in terms of muscular activity and COP displacements. The outcome of the experiments supports the first hypothesis that the alteration of the lower leg proprioception in the presence of vision resulted in significant delays of anticipatory activation of muscles (APA). The results of the experiments also support the second hypothesis because they demonstrated that irrespective of the availability of vision, altered proprioception was associated with a reduction of compensatory (CPA) activity of muscles and lesser COP displacements after the perturbation. Moreover, vibration-related changes in CPAs were larger when vision was not available.
I. The effect of altered proprioception
The important role of proprioceptive information in control of posture is well documented. For example, it is reported that in a well-lit environment with a firm base of support, healthy individuals rely on a combination of somatosensory (70%), vision (10%) and vestibular (20%) information in order to maintain their upright posture (Peterka 2002). Furthermore, intact muscles around the ankle joint have traditionally been considered the source of muscle proprioceptive information, responsible for signaling changes in body position (Nakagawa et al. 1993; Barbieri et al. 2008). Thus, changes in the accuracy of proprioceptive information could affect the ability of an individual to control his posture (Barbieri et al. 2008).
The role of altered proprioceptive information in control of perturbed posture is of a special interest because many individuals with neurological conditions have diminished proprioceptive information and are frequently exposed to external perturbations. For example, it was shown that diminished proprioceptive information in individuals with diabetic neuropathy leads to greater body sway around equilibrium point than in healthy adults (Nardone and Schieppati 2004). It has been established by epidemiological studies that a reduction of leg proprioception sense is a risk factor for falls in the elderly and patients with peripheral neuropathy (Richardson et al. 1992; Lord et al. 1996).
Alterations in the proprioceptive information in the current study were induced by bilateral vibration of the Achilles tendons which produced body tilt in the direction of the vibrated tendon which is in line with the literature (Polonyova and Hlavacka 2001; Ceyte et al. 2007). Such a vibration resulted in delayed anticipatory activity of the leg and trunk muscles in VEO as compared to EO (no vibration condition). There are several possible explanations for this observation. First, given the fact that somatosensory information is a driving force in balance control (Peterka 2002) and that vibration of muscles or their tendons induces powerful discharge of muscle spindle primary afferents (Bove et al. 2003), it is not surprising that alterations in the proprioceptive input clearly affected muscle activation patterns and COP displacements even in the presence of vision. This outcome is in line with previous literature (Kasai et al. 2002; Slijper and Latash 2004). Second, a delay in the activation of the leg and trunk muscles could be explained by changes in the position of the body with application of vibration. Indeed, like what is described in previous literature (Ceyte et al. 2007; Thompson et al. 2007), application of vibratory stimuli to the Achilles tendon induces tilt of the body backwards. The subjects in the current study also exhibited a leaning backward, moving away from the pendulum. As such, the time from the pendulum release (which was known by the subjects since their vision was not obstructed) until the pendulum hits the body, should increase. We speculate that based on the expectation of a delayed perturbation impact, the CNS selected a strategy of delayed APAs. Given the fact that the subjects were healthy adults, capable of fast changes in the strategy if needed, it may suggest that delays in APAs were a way the CNS dealt with erroneous signals from ankle proprioceptors. It is important to note that vibration-induced alteration in the proprioceptive information induces body tilt and increased body sway (Eklund 1973; Gurfinkel and Kireeva 1995), however it is difficult to distinguish between the body lean and increased body sway without assessing the body kinematics. Another explanation could be that altered proprioceptive information did not allow the regular pattern of APAs to be triggered in a timely manner resulting in the delays of muscle activation. The possibility of the delays in triggering of APAs has been previously described in studies with simple reaction time instructions (De Wolf et al. 1998). Differences in the baseline activity of the postural muscles between conditions with no vibration and with vibration (that is associated with body tilt) could be another reason for the observed dissimilarity in the anticipatory EMG activity. We believe this was not the case since the baseline activity from −600 ms to −450 ms for both with and without vibration conditions was subtracted from the EMG signal prior to its integration. As such, the reported changes in the EMG patterns represent changes in the anticipatory activation of muscles associated with the altered proprioceptive information and not the body tilt.
Tendon vibration in the current study resulted in decreased compensatory EMG activity in all the trunk and leg muscles which resulted in smaller compensatory COP displacements. Such a decrease in compensatory IEMGNORM could be explained by the increase of the anticipatory IEMGNORM as was shown previously (Santos et al. 2010a; Mohapatra et al. 2011). However, no difference in the anticipatory IEMGNORM was observed between EO and VEO conditions in the current study. This suggests that the decrease in compensatory EMG activity is due to the effect of tendon vibration rather than the effect of APAs on CPAs.
II. The effect of vision
The significance of visual information in postural control is well recognized. It has been documented that body sway around equilibrium point is increased with decrease in visual acuity; and sway is maximal in blindfolded conditions (Uchiyama and Demura 2008). It has also been demonstrated that peripheral rather than central vision contributes to maintaining a stable standing posture, with postural sway being influenced more in the direction of stimulus observation, or head/gaze direction, than in the direction of trunk orientation (Berencsi et al. 2005).
The results of the current study indicate that when vision is not available, APAs are not generated. A lack of robust anticipatory postural adjustments in blindfolded conditions relates to massive compensatory postural adjustments seen in increased EMG activity and COP displacements after the perturbation, indicating a decreased postural stability. These results are in line both with the previously reported fact that when a perturbation is unpredictable, APAs are diminished leading to huge CPAs (Santos et al. 2010b; Santos et al. 2010a) as well as with the outcome of our recent study which revealed that altering visual acuity using differently powered glasses results in diminished APAs and increased CPAs (Mohapatra et al. 2011).
III. Combined effect of altered proprioceptive and visual information
When proprioceptive information was altered simultaneously with the altering of visual information (VEC), IEMGNORM calculated during both CPA1 and CPA2 epochs were smaller in the majority of the studied muscles when compared to blindfolded conditions with no altered proprioception (EC). Such a decrease in the activation of muscles resulted in smaller compensatory COP displacements after the perturbation.
These results are consistent with the conclusion of previous studies that focused on the individual as well as the combined effect of the alteration of two sources of information in control of posture. When one system is challenged in two different ways (proprioception altered by Achilles tendon vibration and by reduced postural stability), the effect of Achilles tendon vibration diminishes (Gurfinkel et al. 1996; Ivanenko et al. 1999). Moreover, when information from somatosensory and vestibular systems were altered separately, postural movement strategies were appropriately selected for their environmental contexts (Horak et al. 1990). Furthermore, when proprioceptive information was altered in vestibular-loss individuals there were delays in the activation of the paraspinalis muscles and decrease in the magnitude of quadriceps muscle activity (Allum and Honegger 1998).
In our study information from two sensory systems, vision and proprioception, was altered before applying external perturbations to the upper body of the subjects. The findings revealed that in conditions with altered proprioception and a lack of vision there were smaller activation of muscles and smaller COP displacements during the CPA epochs. There are two possible explanations to such a reduction in activity of muscles and COP displacement during the CPA. First is based on the outcome of previous studies that show that generation of optimal APAs could significantly minimize CPAs (muscle activation after a perturbation) (Santos et al. 2010b; Santos et al. 2010a). However, no substantial change in APAs was observed in no vision conditions with tendon vibration suggesting that variation in the magnitude of APAs between the no vibration and vibration conditions could not explain the decreased EMG activity during the CPA epochs. The second explanation relates to the effect of a backward lean of the body as a result of the application of Achilles tendon vibration. In the VEC condition, the backward displacement of the COP measured at 500 ms before T0 was about 20% of the backward shift of the COP at T0. As such, smaller EMG activity and COP displacements observed during the restoration of posture after the perturbation onset could be a result of the tendon vibration-related backward body lean.
There are study limitations that should be considered. First, the Achilles tendon vibration induces backward body lean as well as body sway and instability. As such the delayed and decreased APAs observed in the current study during the Achilles tendon vibration reveal the combined effect of the lean of the body and the increased body instability. Future studies are needed to estimate the contribution of each element in the postural control. Second, since the study was conducted on healthy young subjects with altered proprioception induced by vibratory stimulation, its outcome could not be directly applied to individuals with impaired propriocerption. As such additional studies involving individuals with deficient proprioception are needed.
CONCLUSION
Altered proprioceptive information from the ankle joints resulted in delayed generation of anticipatory postural adjustments prior to the external perturbation and the diminished compensatory postural adjustments. The outcome of the study sheds light on the interplay between APAs and CPAs in healthy individuals in the presence of altered proprioception.
Acknowledgments
This study was supported in part by NIH grant HD-064838.
References
- Adamcova N, Hlavacka F. Modification of human postural responses to soleus muscle vibration by rotation of visual scene. Gait Posture. 2007;25:99–105. doi: 10.1016/j.gaitpost.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S. Feedback equilibrium control during human standing. Biol Cybern. 2005;93:309–322. doi: 10.1007/s00422-005-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allum JH. Organization of stabilizing reflex responses in tibialis anterior muscles following ankle flexion perturbations of standing man. Brain Res. 1983;264:297–301. doi: 10.1016/0006-8993(83)90828-4. [DOI] [PubMed] [Google Scholar]
- Allum JH, Honegger F. Interactions between vestibular and proprioceptive inputs triggering and modulating human balance-correcting responses differ across muscles. Exp Brain Res. 1998;121:478–494. doi: 10.1007/s002210050484. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp Brain Res. 1995;106:291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Anticipatory postural adjustments during self-initiated perturbations of different magnitude triggered by a standard motor action. Electroencephalogr Clin Neurophysiol. 1996;101:497–503. doi: 10.1016/s0013-4694(96)95219-4. [DOI] [PubMed] [Google Scholar]
- Barbieri G, Gissot AS, Fouque F, Casillas JM, Pozzo T, Perennou D. Does proprioception contribute to the sense of verticality? Exp Brain Res. 2008;185:545–552. doi: 10.1007/s00221-007-1177-8. [DOI] [PubMed] [Google Scholar]
- Basmajian JV. Electromyography--dynamic gross anatomy: a review. Am J Anat. 1980;159:245–260. doi: 10.1002/aja.1001590302. [DOI] [PubMed] [Google Scholar]
- Belenkiy V, Gurfinkel V, Pal’tsev Y. Elements of control of voluntary movements. Biofizika. 1967;10:135–141. [PubMed] [Google Scholar]
- Berencsi A, Ishihara M, Imanaka K. The functional role of central and peripheral vision in the control of posture. Hum Mov Sci. 2005;24:689–709. doi: 10.1016/j.humov.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomechanics. 1987;20:735–742. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Bove M, Nardone A, Schieppati M. Effects of leg muscle tendon vibration on group Ia and group II reflex responses to stance perturbation in humans. J Physiol. 2003;550:617–630. doi: 10.1113/jphysiol.2003.043331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AM. Suppression of visually evoked postural responses. Exp Brain Res. 1986;63:655–658. doi: 10.1007/BF00237488. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976;261:673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Ceyte H, Cian C, Zory R, Barraud PA, Roux A, Guerraz M. Effect of Achilles tendon vibration on postural orientation. Neurosci Lett. 2007;416:71–75. doi: 10.1016/j.neulet.2007.01.044. [DOI] [PubMed] [Google Scholar]
- Courtine G, Papaxanthis C, Laroche D, Pozzo T. Gait-dependent integration of neck muscle afferent input. Neuroreport. 2003;14:2365–2368. doi: 10.1097/00001756-200312190-00015. [DOI] [PubMed] [Google Scholar]
- Courtine G, Pozzo T, Lucas B, Schieppati M. Continuous, bilateral Achilles’ tendon vibration is not detrimental to human walk. Brain Res Bull. 2001;55:107–115. doi: 10.1016/s0361-9230(01)00504-4. [DOI] [PubMed] [Google Scholar]
- De Wolf S, Slijper H, Latash ML. Anticipatory postural adjustments during self-paced and reaction-time movements. Exp Brain Res. 1998;121:7–19. doi: 10.1007/s002210050431. [DOI] [PubMed] [Google Scholar]
- Demura S, Yamaji S, Kitabayashi T, Yamada T, Uchiyama M. Attention of postural control on foot somatosensor disturbance caused by the compression of blood vessels. J Hum Ergol (Tokyo) 2008;37:91–102. [PubMed] [Google Scholar]
- Dijkstra TM, Schoner G, Gielen CC. Temporal stability of the action-perception cycle for postural control in a moving visual environment. Exp Brain Res. 1994;97:477–486. doi: 10.1007/BF00241542. [DOI] [PubMed] [Google Scholar]
- Ducic I, Short KW, Dellon AL. Relationship between loss of pedal sensibility, balance, and falls in patients with peripheral neuropathy. Ann Plast Surg. 2004;52:535–540. doi: 10.1097/01.sap.0000122654.65588.f0. [DOI] [PubMed] [Google Scholar]
- Eklund G. Further studies of vibration-induced effects on balance. Ups J Med Sci. 1973;78:65–72. [PubMed] [Google Scholar]
- Eun MY, Kang CH, Kim BJ. Tibial neuropathy associated with Behcet’s disease. Am J Phys Med Rehabil. 2011;90:432–433. doi: 10.1097/PHM.0b013e3181f71214. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Asai H, Miyaguchi A, Toyama H, Kunita K, Inoue K. Perceived standing position after reduction of foot-pressure sensation by cooling the sole. Percept Mot Skills. 2003;96:381–399. doi: 10.2466/pms.2003.96.2.381. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS, Kireeva TB. Maintenance of vertical posture during simultaneous vibration of the calf and anterior tibial muscles. Fiziol Cheloveka. 1995;21:106–120. [PubMed] [Google Scholar]
- Gurfinkel VS, Kireeva TB, Lebik Iu S. Effect of postural muscle vibration on equilibrium maintenance in the frontal plane at various levels of stability. Fiziol Cheloveka. 1996;22:83–92. [PubMed] [Google Scholar]
- Harati YBE, editor. Disorders of peripheral nerves. Butterworth-Heinemann Elsevier; Philadelphia: 2008. [Google Scholar]
- Hatzitaki V, Pavlou M, Bronstein AM. The integration of multiple proprioceptive information: effect of ankle tendon vibration on postural responses to platform tilt. Exp Brain Res. 2004;154:345–354. doi: 10.1007/s00221-003-1661-8. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Miyake A, Jijiwa H, Watanabe S. Postural readjustment to body sway induced by vibration in man. Exp Brain Res. 1981;43:217–225. doi: 10.1007/BF00237767. [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. EMG responses to maintain stance during multidirectional surface translations. J Neurophysiol. 1998;80:1939–1950. doi: 10.1152/jn.1998.80.4.1939. [DOI] [PubMed] [Google Scholar]
- Hlavacka F, Njiokiktjien C. Postural responses evoked by sinusoidal galvanic stimulation of the labyrinth. Influence of head position. Acta Otolaryngol. 1985;99:107–112. doi: 10.3109/00016488509119152. [DOI] [PubMed] [Google Scholar]
- Horak FB, MacPherson JM, Peterson BW. Postural orientation and equilibrium. Published for the American Physiological Society by Oxford University Press; New York: 1996. [Google Scholar]
- Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82:167–177. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- Howatson G, Glaister M, Brouner J, van Someren KA. The reliability of electromechanical delay and torque during isometric and concentric isokinetic contractions. J Electromyogr Kinesiol. 2009;19:975–979. doi: 10.1016/j.jelekin.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Lacquaniti F. Influence of leg muscle vibration on human walking. J Neurophysiol. 2000;84:1737–1747. doi: 10.1152/jn.2000.84.4.1737. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Talis VL, Kazennikov OV. Support stability influences postural responses to muscle vibration in humans. Eur J Neurosci. 1999;11:647–654. doi: 10.1046/j.1460-9568.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- Kasai T, Yahagi S, Shimura K. Effect of vibration-induced postural illusion on anticipatory postural adjustment of voluntary arm movement in standing humans. Gait Posture. 2002;15:94–100. doi: 10.1016/s0966-6362(01)00177-1. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Gilhodes JC, Roll R, Roll JP. From balance regulation to body orientation: two goals for muscle proprioceptive information processing? Exp Brain Res. 1999;124:80–88. doi: 10.1007/s002210050602. [DOI] [PubMed] [Google Scholar]
- Kjaergard H, Korsgaard Larsen T, Rasmussen PS, Brondum L. Impairment of postural stability following perivascular axillary block with mepivacaine. Acta Anaesthesiol Scand. 1984;28:508–510. doi: 10.1111/j.1399-6576.1984.tb02108.x. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Aruin AS, Latash ML. Two stages and three components of the postural preparation to action. Exp Brain Res. 2011;212:47–63. doi: 10.1007/s00221-011-2694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Aruin AS, Neyman I, Nicholas JJ. Anticipatory postural adjustments during self inflicted and predictable perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1995;58:326–334. doi: 10.1136/jnnp.58.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart SM, Pincivero DM, Giraldo JL, Fu FH. The role of proprioception in the management and rehabilitation of athletic injuries. Am J Sports Med. 1997;25:130–137. doi: 10.1177/036354659702500126. [DOI] [PubMed] [Google Scholar]
- Lord SR, Lloyd DG, Li SK. Sensori-motor function, gait patterns and falls in community-dwelling women. Age Ageing. 1996;25:292–299. doi: 10.1093/ageing/25.4.292. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Horak FB, Dunbar DC, Dow RS. Stance dependence of automatic postural adjustments in humans. Exp Brain Res. 1989;78:557–566. doi: 10.1007/BF00230243. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. Postural control in the older adult. Clin Geriatr Med. 1996;12:635–658. [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Mauritz KH, Dietz V. Characteristics of postural instability induced by ischemic blocking of leg afferents. Exp Brain Res. 1980;38:117–119. doi: 10.1007/BF00237939. [DOI] [PubMed] [Google Scholar]
- Mohapatra S, Krishnan V, Aruin AS. The effect of decreased visual acuity on control of posture. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Ohashi N, Watanabe Y, Mizukoshi K. The contribution of proprioception to posture control in normal subjects. Acta Otolaryngol Suppl. 1993;504:112–116. doi: 10.3109/00016489309128134. [DOI] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. Group II spindle fibres and afferent control of stance. Clues from diabetic neuropathy. Clin Neurophysiol. 2004;115:779–789. doi: 10.1016/j.clinph.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Wolfson P. Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Res. 1974;67:255–268. doi: 10.1016/0006-8993(74)90276-5. [DOI] [PubMed] [Google Scholar]
- Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res. 2004;154:417–427. doi: 10.1007/s00221-003-1674-3. [DOI] [PubMed] [Google Scholar]
- Pavlik AE, Inglis JT, Lauk M, Oddsson L, Collins JJ. The effects of stochastic galvanic vestibular stimulation on human postural sway. Exp Brain Res. 1999;124:273–280. doi: 10.1007/s002210050623. [DOI] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Polonyova A, Hlavacka F. Human postural responses to different frequency vibrations of lower leg muscles. Physiol Res. 2001;50:405–410. [PubMed] [Google Scholar]
- Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc. 1992;40:1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- Roll JP, Popov K, Gurfinkel V, Lipshits M, Andre-Deshays C, Gilhodes JC, Quoniam C. Sensorimotor and perceptual function of muscle proprioception in microgravity. J Vestib Res. 1993;3:259–273. [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 1982;47:177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res. 1989;76:213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol. 2010a;20:388–397. doi: 10.1016/j.jelekin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J Electromyogr Kinesiol. 2010b;20:398–405. doi: 10.1016/j.jelekin.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, De Nunzio AM, Schieppati M. Trunk muscle proprioceptive input assists steering of locomotion. Neurosci Lett. 2005;384:127–132. doi: 10.1016/j.neulet.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash ML. The effects of muscle vibration on anticipatory postural adjustments. Brain Res. 2004;1015:57–72. doi: 10.1016/j.brainres.2004.04.054. [DOI] [PubMed] [Google Scholar]
- Stal F, Fransson PA, Magnusson M, Karlberg M. Effects of hypothermic anesthesia of the feet on vibration-induced body sway and adaptation. J Vestib Res. 2003;13:39–52. [PubMed] [Google Scholar]
- Talis VL, Solopova IA. Vibration–induced postural reaction continues after the contact with additional back support. Motor Control. 2000;4:407–419. doi: 10.1123/mcj.4.4.407. [DOI] [PubMed] [Google Scholar]
- Thompson C, Belanger M, Fung J. Effects of bilateral Achilles tendon vibration on postural orientation and balance during standing. Clin Neurophysiol. 2007;118:2456–2467. doi: 10.1016/j.clinph.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Thompson C, Belanger M, Fung J. Effects of plantar cutaneo-muscular and tendon vibration on posture and balance during quiet and perturbed stance. Hum Mov Sci. 2011;30:153–171. doi: 10.1016/j.humov.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Demura S. Low visual acuity is associated with the decrease in postural sway. Tohoku J Exp Med. 2008;216:277–285. doi: 10.1620/tjem.216.277. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Swinnen SP, Desloovere K, Duysens J. Effects of tendon vibration on the spatiotemporal characteristics of human locomotion. Exp Brain Res. 2002;143:231–239. doi: 10.1007/s00221-001-0987-3. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]