Abstract
Alteration of hemodynamic loading induces remodeling that includes changes in myocardial properties and extracellular matrix structure. We investigated the hypothesis that cardiac hypertrophy due to volume overload produces changes in myocardial diastolic mechanics and stiffness that are in part due to alterations in advanced glycation end-product (AGE) collagen cross-linking. Rats developed volume overload induced by arteriovenous fistula (AVF). To assess the dependence of AGE cross-linking on mechanics, we prevented AGE formation by administering the drug aminoguanidine (AG) to one group of AVF rats (AG+AVF). Volume overload did not modify collagen concentration. Right ventricular AGE cross-links were modestly elevated in AVF hearts but were significantly reduced by AG. AVF rats exhibited significantly increased septal AGE cross-links that were inhibited in the AG+AVF group. AVF -induced increases in left ventricular longitudinal stiffness and septal circumferential stiffness were prevented in AG+AVF hearts. Volume overload appears to regionally modify AGE collagen cross-linking and stiffness, and AG treatment prevented these increases, demonstrating that AGE cross-linking plays a role in mediating diastolic compliance in volume-overload hypertrophy.
Keywords: ventricle, strain, stress, material properties
DURING MYOCARDIAL HYPERTROPHY, the heart remodels in response to altered loading, and this remodeling includes changes in ventricular geometry and tissue properties (14). Volume-overload hypertrophy increases ventricular stiffness (11, 17) without significant changes in collagen content (11, 25). Collagen plays a significant role in passive cardiac muscle stiffness (21), and some studies implicate collagen cross-links as a significant determinant of chamber stiffness in the heart (1, 28). Previous studies reported alterations in aortic compliance and ventricular chamber stiffness in association with the inhibition of enzymatic collagen cross-linking (6, 19). Investigators also observed diminished arterial and chamber compliance that correlated with an increase in nonenzymatic glycated collagen cross-links (2, 36). In addition, there is evidence that myocardial collagen cross-links may be elevated in response to volume-overload hypertrophy (15, 17).
Collagen formation includes enzymatic processes that create intra- and intermolecular cross-links. However, glucose and other sugars also act as cross-linking agents of the extracellular matrix (ECM). Collagen has a long biological half-life, and the level of nonenzymatic glycosylation, or glycation, increases gradually with aging (3) or in hyperglycemic conditions such as diabetes (24). Reducing sugars (glucose, fructose, etc.) bond to free protein amino groups and go through a series of reactions to form a class of heterogeneous, nonenzymatic sugar-amino adducts that are called advanced glycation end-products (AGE) (3). AGE exist in a non-reactive form as an irreversible, intermolecular covalent cross-link with other glucose-modified proteins (5, 34).
AGE modify and damage tissues in various ways in addition to forming cross-links (34). These modifications, and the cross-linking actions of AGE, contribute to numerous clinical complications associated with aging, diabetes, atherosclerosis, neuropathy, retinopathy, and renal failure (8,34). Therefore, the investigation of disrupting AGE cross-links is of particular interest to the scientific and medical community. Currently, AGE disruption may be achieved either by preventing their formation or by cleaving existing AGE cross-links through specific drug interactions (8). One example is the compound aminoguanidine (AG), which prevents formation of fluorescent AGE products and the accumulation of AGE collagen cross-links (5, 26).
This research investigated the hypothesis that cardiac hypertrophy due to volume overload induces changes in myocardial mechanics and stiffness that are in part due to alterations in nonenzymatic AGE collagen cross-linking. Experimental rats developed volume-overload hypertrophy due to arteriovenous fistula (AVF). To test the dependence of nonenzymatic cross-linking on cardiac mechanics, we prevented the formation of AGE cross-links by administering AG to rats for 6 wk. We found that inhibition of AGE cross-links prevented some of the alterations in myocardial stiffness and passive mechanics associated with volume overload.
MATERIALS AND METHODS
Volume overload and drug administration
All studies were performed according to the National Institutes of Health Institute Guide for the Care and Use of Laboratory Animals, and all experimental protocols were reviewed and approved by the University of California-San Diego Animal Subjects Committee. Each rat was anesthetized via intraperitoneal injection (100 mg/kg ketamine HCI, 8 mg/kg xylazine, and 2 mg/kg morphine). Chronic volume overload was produced in 10-wk-old rats weighing ~300 g by creating an AVF between the aorta and vena cava in the abdomen (also termed an aortocaval fistula) (11). The aorta and vena cava were isolated and exposed through a large abdominal incision (~5.0 cm) under sterile conditions. They were cross-clamped with clips posterior and anterior to the site of incision below the renal vessels. After an aortic incision, a fistula was created through the common wall by passing a micro-surgical suture through the shared wall and resecting a small piece of the vessel. The aortic incision was sutured, and the clamps were removed. The mixture of arterial and venous blood in the vena cava was visualized to check for shunt patency. The visceral organs were restored, and the abdominal muscle and skin openings were surgically closed. This procedure increases the preload on the heart by increasing the filling volume and pressure and results in volume-overload hypertrophy. The chronic volume overload progressed over a 6-wk period. A comparative group of AVF rats were given the AGE inhibitor AG (25 mg·kg body wt–1·day–1) in daily doses via intraperitoneal injections. Weight-matched rats were also included in the study as a control group.
Heart isolation
Rat hearts were excised and isolated 6 wk after the AVF surgery for mechanical testing and biochemical analysis. After anesthesia administration, the rats were ventilated with room air and ECG leads were inserted. A Millar catheter (1.4-Fr) was inserted through the carotid artery and advanced retrograde toward the heart and into the left ventricle (LV) to measure in vivo arterial and myocardial hemodynamics. The heart was then arrested and excised by opening the chest via thoracotomy and injecting cardioplegic arrest solution [4.0 g/l NaCl, 4.44 g/l KCl, 1.0 g/l NaHC02, 2.0 g/l sucrose, 3.0 g/l 2,3-butanedione monoximine, and 1,000 units heparin (10 ml/l)] into the LV apex. The heart was immediately rinsed in cold cardioplegic solution, trimmed, and weighed.
Isolated heart inflation and data collection
The experimental rats were divided into two subgroups to investigate the mechanics of either the LV or the septum. The left atrium was trimmed, and a small pointed, flared tube was inserted and pushed through the apex of the LV to drain the ventricle. The heart was slowly perfused with an aortic cannula under low pressure with additional cardioplegic solution to flush the remaining blood. A balloon connected to a closed inflation system was placed in the LV (11). The mechanics of the ventricle were recorded by passively inflating isolated hearts and recording the pressure-volume (P-V) and pressure-strain relations with video-imaged surface markers (11) (composed of titanium oxide; no. T8141, Sigma, St. Louis, MO). In the LV study group, these markers were placed on the epicardial LV free wall. Alternatively, in the septal study group, the right ventricular (RV) free wall was excised and markers were placed on the exposed septum. The hearts were kept moist during the studies. Pressure, volume, and timing signals were recorded online directly to a computer, and surface markers were imaged on videotape.
Strain and stress calculation
Deformation of the ventricle was analyzed by calculating the homogenous strain on the surface of the LV or septal wall. The methods are given in detail in previous publications (11). Two-dimensional finite strain was computed during passive inflation either on the septal or LV free wall surface. The resulting strains, Eij, are the three independent components of the two-dimensional Lagrangian strain tensor referred to a zero-pressure state. The reference coordinate system is chosen to align with the long axis of the heart; thus E11 represents circumferential strain, E22 represents longitudinal strain, and E12 represents in-plane shear strain.
Diastolic wall stress was calculated with an analytic model. The geometry was modeled as an axisymmetric truncated ellipsoid using a three-dimensional prolate spheroidal shell and dimensions measured from excised hearts. The reference state was the unloaded ventricle with a finite initial volume and zero pressure. P-V data and epicardial strain ratios were used to simulate the passive inflation of each rat heart. The average midwall circumferential (T11) and longitudinal (T22) wall stresses were calculated with a Mirsky formulation for midwall stress in an ellipsoid (23), where a and b are, respectively, the midwall semimajor and semiminor axes of the prolate ellipsoid, P is the pressure, and h is the wall thickness
Stress-strain data were fit with an exponential curve (T AekE, where A is the stress intercept at zero strain) for each rat in each direction, and the myocardial stiffness coefficient (k) was taken as the exponential coefficient of the fitted trend, which is the slope of the linearized stress-strain relation (28, 37).
Measurement of AGE
AGE content was determined based on the fluorescence assay methods of Monnier et al. (24) and Jyothirmayi et al. (18). First, frozen myocardial tissue was thawed and weighed. The tissue was minced and homogenized in 3 ml of PBS. The homogenate was then centrifuged (3,000 g for 20 min), and the pellet was washed in 3 ml of PBS. After two repetitions, the pellet was extracted in 2 ml of 0.5 M acetic acid overnight at 4°C. After being spun again, the precipitate was extracted in 2 ml of 1% pepsin in 0.5 M acetic acid overnight at 4°C. The pepsin extraction was performed three times. After the samples were spun, the remaining precipitate was extracted in 2 ml of a solution containing 0.05% proteinase K and 0.1% SDS overnight with low-speed shaking at 37°C. The sample precipitates were then extracted in 300 μl of 0.05% collagenase VII (Sigma) in PBS. In addition, 1 μl each of chloroform and toluene were added. These samples were incubated for 24 h at 37°C. The samples were centrifuged (1,000 g for 5 min), and the super-natant was removed for analysis. Three 40-μl aliquots of the final supernatant were each diluted with 100 μl of water for fluorescence measurement with a SPECTRAmax microplate spectrofluorometer (Molecular Devices, Sunnyvale, CA). All fluorescence measures (370 nm excitation, 440 nm emission) were corrected by subtracting the blank collagenase VII sample fluorescence and averaged over the three readings. The remaining final supernatant was used for a colorimetric hydroxyproline measurement, after which AGE content was calculated as arbitrary fluorescence units per milligram of collagen, and then normalized to the average of control measurements for each group of samples.
Measurement of collagen content
Hydroxyproline concentration (a marker of collagen content) was determined by the colorimetric assay of Woessner (35). The volume of the remaining supernatant or the mass of the lyophilized tissue was recorded and hydrolyzed in 20× volumes of6 N HCl and left overnight (18–24 h) in a heat block incubator (Fisher Scientific, Pittsburgh, PA) at 100°C. After being cooled in a dark place, the liquid sample was filtered into a 5- to 25-ml volumetric flask with Whatman no. 1 filter paper and brought to volume with methanol. A portion of the sample (1 ml) was placed in a porcelain dish and evaporated in a 60°C water bath. The dried sample was solubilized in 1 ml of methanol and then evaporated two more times. Dried samples were then resolubilized in 5–10 ml of water. After subsequent additions of 1 ml each of chloramine T solution, 3.15 M perchloric acid, and para-dimethylaminobenzaldehyde solution, each sample and five hydroxyproline standards were incubated in a water bath for 20 min. Hydroxyproline concentrations of the samples were calculated from the absorbance at a wavelength = 557 nm and the computed hydroxyproline standard curve by a spectrophotometer (Beckman Coulter, Fullerton, CA). Collagen concentration was calculated by assuming that collagen weighs 7.25 times hydroxyproline and has a molecular weight of 300,000.
Measurement of hydroxylysyl pyridinoline cross-links
The remaining hydrolyzate from the hydroxyproline procedure was prepared for hydroxylysyl pyridinoline (HP) cross-link determination by following the methods of Skinner (31). Briefly, the remaining hydrolyzate was concentrated on a rotoevaporator, suspended in a slurry of cellulose and mobile phase, and applied to a cellulose column. HP cross-links absorb into a cellulose bed and can be separated from other amino acids by washing with mobile phase. HP was eluted from the column with water and again concentrated on a rotoevaporator, and pyridoxamine hydrochloride was added as an internal standard. The degree of collagen cross-linking was assessed with the reverse-phase HPLC methods of Eyre et al. (12). Pyridoxamine and HP were detected by fluorescence spectrophotometry (excitation 295 nm, emission 395 nm), and cross-link values are reported as moles of HP per mole of collagen.
Statistics
Results presented reflect means ± SE per group. To test for significance between groups, one- or two-way ANOVA or ANOVA repeated-measures analysis was performed with Fisher's protected least-significant-difference post hoc comparisons.
RESULTS
Surgery statlstlcs and body weights
Volume overload was successfully induced in 67% of the rats in the chronic study. The rats with closed fistulas at the time of postmortem inspection (33%) were discarded from the study. There was no difference in initial weight between AG+AVF (303 ± 5 g) and AVF (303 ± 4 g) rats. AG+AVF rats weighed less at the end of the 6-wk study (AG+AVF 367 ± 5 g, AVF 413 ± 10 g; P < 0.01). Subsequently, AG+AVF rats gained considerably less weight compared with AVF rats (AG+AVF 21 ± 2%, AVF 37 ± 4%; P < 0.01).
Hypertrophy and geometry
The overall heart weight-to-body weight ratio increased as expected because of chronic volume overload (Table 1; P < 0.01). Significant myocardial enlargement was observed in the LV, septum, and RV for both AVF groups compared with controls (Table 1). Volume overload produced the greatest relative hypertrophy in the RV (76% larger than controls), followed by the LV (52%) and the septum (34%). AG did not alter the hypertrophic response of the RV, septum, or LV to AVF (Table 1). Apex-to-base dimension increased in both groups after AVF, and the wall thickness-to-radius ratio decreased in both groups after AVF. However, wall thickness was similar among all three groups.
Table 1.
Myocardial weights and geometry
| Control | AVF | AG+AVF | |
|---|---|---|---|
| No. of animals | 18 | 14 | 11 |
| HW/BW, mg/g | 3.99 ± 0.08 | 5.94 ± 0.29* | 6.14 ± 0.35* |
| LV/BW, mg/g | 1.65 ± 0.05 | 2.42 ± 0.10* | 2.55 ± 0.14* |
| Sep/BW, mg/g | 0.82 ± 0.03 | 1.07 ± 0.05* | 1.17 ± 0.06* |
| RV/BW, mg/g | 0.68 ± 0.02 | 1.13 ± 0.06* | 1.23 ± 0.07* |
| Apex-base, cm | 1.71 ± 0.02 | 1.83 ± 0.04* | 1.92 ± 0.04* |
| r, cm | 0.25 ± 0.01 | 0.32 ± 0.01* | 0.33 ± 0.01* |
| h, cm | 0.32 ± 0.01 | 0.32 ± 0.01 | 0.33 ± 0.01 |
| h / r | 1.27 ± 0.06 | 1.02 ± 0.06* | 1.01 ± 0.08* |
Values are means ± SE. AVF, arteriovenous fistula; AG, amino-guanidine; HW, heart weight; BW, body weight; LV, left ventricle; Sep, septum; RV, right ventricle, r, inner radius; h, wall thickness.
P < 0.01 vs. control.
Hemodynamics
LV end-diastolic pressure more than doubled from control pressures in both AVF groups (control 2.0 ± 0.2 mmHg, AVF 4.9 ± 0.6 mmHg, AG+AVF 6.3 ± 0.7 mmHg; P < 0.01) and was also accompanied by an increase in peak systolic pressure (control 78.4 ± 4.9 mmHg, AVF 90.4 ± 4.4 mmHg, AG+AVF 97.6 ± 6.1 mmHg; P < 0.05). Pulse pressure measured in the aorta was nearly twice as large in AVF and AG+AVF rats compared with controls (control 21.0 ± 2.1 mmHg, AVF 39.2 ± 1.4 mmHg, AG+AVF 44.8 ± 3.6 mmHg; P < 0.01). There was no effect of AG on hemodynamics between the AVF groups.
Collagen and cross-linking
Results of the collagen concentration analysis are shown in Table 2. There was no difference in LV or septum collagen concentration among the three experimental groups. RV collagen concentration was similar between control and AVF tissue but reduced in AG+AVF animals (P < 0.01). In all rats and groups, the RV contained the highest concentration of collagen, followed by the septum and then the LV (P < 0.01). Absolute values of total hydroxyproline are also included in Table 2.
Table 2.
Collagen concentration and total hydroxyproline in rat myocardium
| Group |
||||
|---|---|---|---|---|
| Control | AVF | AG+AVF | All Groups | |
| LV, %collagen | 1.77 ± 0.08 | 1.62 ± 0.10 | 1.58 ± 0.08 | 1.67 ± 0.05 |
| Sep, %collagen | 2.16 ± 0.14 | 2.11 ± 0.13 | 2.02 ± 0.22 | 2.09 ± 0.09‡ |
| RV, %collagen | 2.96 ± 0.18 | 3.18 ± 0.15 | 2.29 ± 0.17*† | 2.91 ± 0.12†§ |
| LV OHPro, mg | 1.57 ± 0.09 | 2.19 ± 0.23* | 2.01 ± 0.11 | 1.87 ± 0.10 |
| Sep OHPro, mg | 1.06 ± 0.09 | 1.37 ± 0.10* | 1.27 ± 0.08 | 1.23 ± 0.06‡ |
| RV OHPro, mg | 0.97 ± 0.05 | 1.95 ± 0.20* | 1.43 ± 0.08† | 1.49 ± 0.12†§ |
Values are means ± SE; %collagen is unitless [(100%) × (mg collagen)/(mg dry tissue weight)]. OHPro, hydroxyproline.
P < 0.05 vs. control
P < 0.05 vs. AVF
P < 0.05 vs. LV
P < 0.05 vs. Sep.
Two types of collagen cross-linking were measured. LV enzymatic cross-linking (HP cross-linking) was similar among the three groups (control 3.16 ± 0.28 mol HP/mol collagen, AVF 2.95 ± 0.39 mol HP/mol collagen, AG+AVF 3.33 ± 0.22 mol HP/mol collagen). However, the regional variation in nonenzymatic AGE cross-linking is illustrated in Fig. 1. LV nonenzymatic AGE cross-linking was similar in all groups. However, the AVF-induced increase in septal AGE cross-links (P < 0.05 vs. controls) was prevented in the AG+AVF group (P < 0.01 vs. AVF). RV AGE cross-links (data not shown) were slightly elevated in AVF hearts, but AGE accumulation was significantly reduced by AG treatment (P < 0.01 vs. AVF, P < 0.05 vs. control).
Fig. 1.
Regional advanced glycation end-product (AGE) cross-linking in experimental groups. AGE cross-links were elevated in the septum of rats with arteriovenous fistula (AVF) but inhibited with aminoguanidine (AG) treatment. LV, left ventricle. †P < 0.05 vs. controls; ‡P < 0.01 vs. AVF.
P-V curves
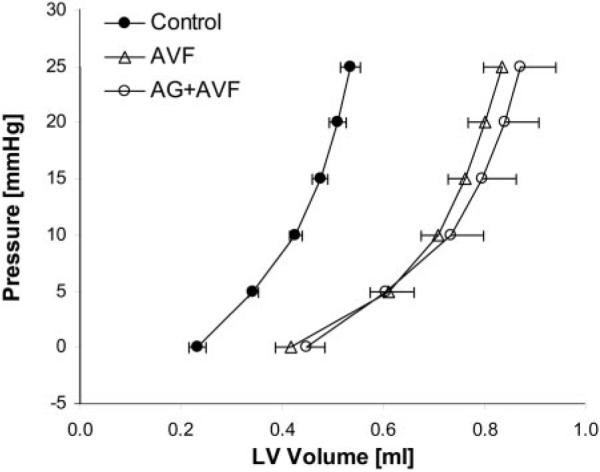
AVF and AG+AVF hearts exhibited LV P-V curves that were significantly shifted to the right of controls (Fig. 2; P < 0.01), depicting their larger LV volumes. There was no difference between nontreated AVF and AG-treated AVF hearts with respect to their diastolic P-V characteristics.
Fig. 2.
LV chamber pressure-volume (P-V) curves measured during passive inflation. P-V values for AVF and AG+AVF hearts are similarly enlarged compared with the control curve (P < 0.01).
Stress, strain, and stiffness
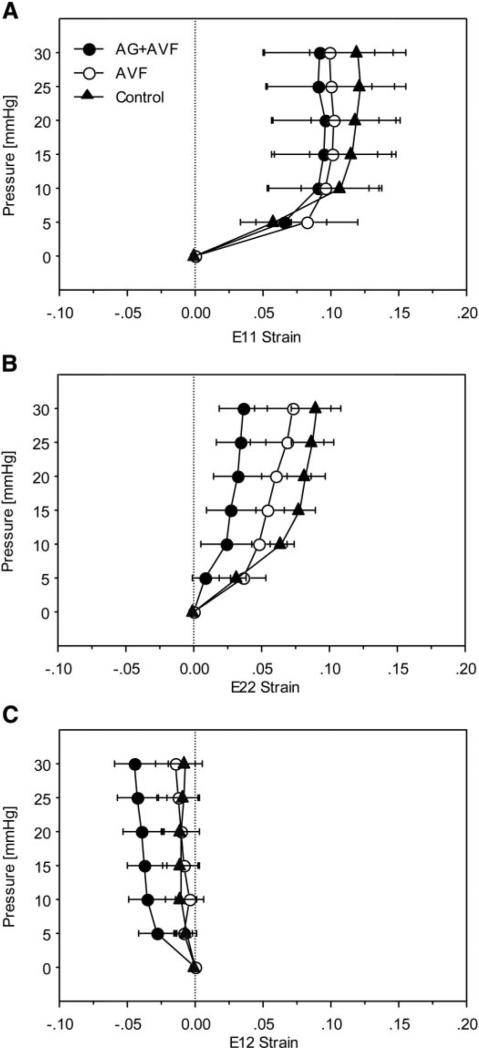
Because the P-V curves represent global ventricular mechanics but the biochemical analysis showed alterations in AGE cross-links only on the septal wall, we measured pressure-strain relations during passive LV filling on the RV surface of the septum (Fig. 3). AVF surgery with or without AG treatment had no statistically significant effect compared with hearts of sham-operated rats on the relationship between pressure and strain in the circumferential, longitudinal, or shear directions on the septal wall.
Fig. 3.
Strains measured on the septal wall during passive inflation. Circumferential (E11, A), longitudinal (E22, B), and shear (E12, C) strains were similar among experimental groups.
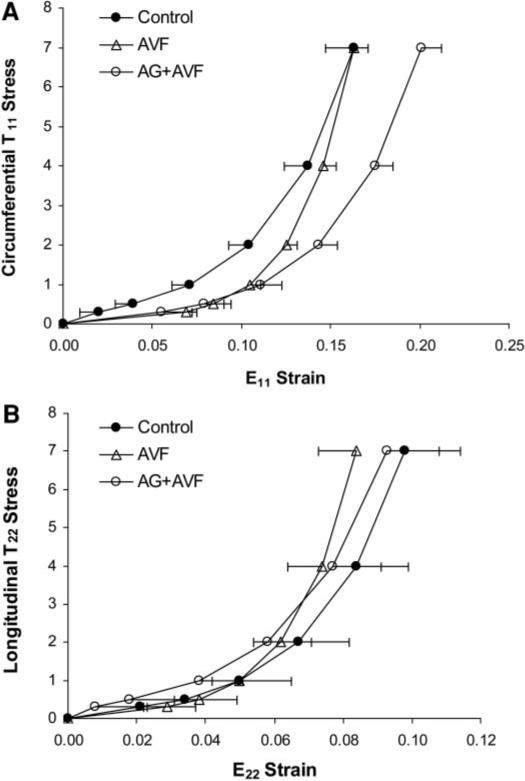
Because P-V and pressure-strain relations reflect the combined effects of changes in ventricular geometry and material properties, we used regional parameters (geometry, pressure, and strains) measured on the septum and LV free wall to estimate local stress-strain relations. Passive septal circumferential (T11-E11 stress-strain curves exhibited significant differences among all three groups (Fig. 4). AG+AVF curves were shifted to the right of control curves. However, AVF stress-strain curves fell between the other two groups and exhibited greater stiffness coefficients (Table 3; P < 0.01). Analysis of septal longitudinal (T22-E22) stress-strain curves showed that the AVF group exhibited a larger longitudinal stiffness coefficient (P < 0.01). The circumferential myocardial stiffness coefficient was increased in AVF rats compared with controls and attenuated in the AG+AVF group (Table 3). Likewise, longitudinal septal stiffness mirrored the trends in T22 curves, but these changes in longitudinal compliance were not statistically significant. Septal T11-E11 curves had larger initial linear slopes in the control group compared with the AVF and AG+AVF groups (P < 0.01), possibly because of AVF-induced structural remodeling that reduced circumferential compliance at low loads.
Fig. 4.
Diastolic stress-strain curves from the septal wall. The AVF circumferential stress-strain (T11-E11) curve (A) is significantly steeper (P < 0.01) and modestly shifted compared with the control curve. AG+AVF curves were also shifted (P < 0.05) but exhibited a shape similar to that of control curves. Longitudinal stress-strain (T22-E22) values (B) were consistent among all groups, but AVF curves were again significantly steeper than both control and AG+AVF curves (P < 0.05).
Table 3.
Myocardial stiffness coefficients
| Control | AVF | AG+AVF | |
|---|---|---|---|
| LV free wall | |||
| Circumferential stiffness | 25.7 ± 1.5 | 22.0 ± 3.3 | 22.7 ± 1.0 |
| Longitudinal stiffness | 73.0 ± 7.3 | 184.2 ± 51.2*† | 87.5 ± 15.4 |
| Sep | |||
| Circumferential stiffness | 22.7 ± 2.9 | 34.6 ± 2.5*† | 23.4 ± 3.2 |
| Longitudinal stiffness | 47.6 ± 8.3 | 63.7 ± 8.8 | 40.7 ± 6.8 |
Values are means ± SE.
P < 0.05 AVF vs. control
P < 0.05 AVF vs. AG+AVF.
In contrast to LV longitudinal stiffness, LV circumferential myocardial stiffness was unaffected by AVF or AG treatment (Table 3). The longitudinal stiffness coefficient in the LV free wall was significantly increased in AVF rats compared with controls, and this change in myocardial compliance was almost completely prevented in AG+AVF hearts (P < 0.05; Table 3). There was no difference in initial linear slopes of LV stress-strain curves.
DISCUSSION
Summary of results
The purpose of this study was to investigate the hypothesis that nonenzymatic AGE collagen cross-links play a role in mediating myocardial stiffness in volume-overload hypertrophy in rats. Specifically, we postulated that inhibition of AGE cross-links would prevent decreases in compliance of the LV and septum in rats with volume overload. The main findings of this study are that 1) volume overload appears to induce regional upregulation of AGE cross-linking in the septum without modifying collagen concentration; 2) concomitant increases in regional tissue stiffness in the septum may be a result of these increased cross-links; and 3) inhibition of AGE formation by AG prevented alterations in myocardial compliance. Thus it appears that nonenzymatic AGE cross-links are at least partly responsible for increased myocardial stiffness in volume-overload hypertrophy induced by AVF.
Collagen and cross-linking in volume overload
Collagen concentration values in volume overload were similar to those previously published for rats (11, 25). AG treatment (AG+AVF group) had no effect on collagen concentration in the LV and septum but did reduce collagen in the RV. This modification of RV collagen may be due to the fact that the RV sustains a large portion of the increased loading due to the overload and thus undergoes more extensive ECM remodeling. This augmented RV remodeling is also evident in the fact that the RV exhibited the largest relative hypertrophy in both AVF groups.
AGE cross-linking was significantly increased in the septum and modestly elevated in the RV but did not change in the LV. This heterogeneous cross-link remodeling may be due to the regional variation in loading experienced in the heart because of AVF-induced volume-overload hypertrophy. Although accumulation of AGE is usually associated with prolonged exposure to hyperglycemic conditions such as diabetes or aging, other studies have observed AGE cross-link changes stimulated by altered hemodynamic loading in euglycemic conditions. For example, Norton and colleagues (28) concluded that alterations in collagen cross-links were responsible for the stiff myocardium associated with hypertension in spontaneously hypertensive rats when treatment increased collagen solubility (an indication of reduced cross-links) and diminished myocardial stiffness without modifying collagen concentration.
Hypertrophy and remodeling
Our results suggest that AG did not affect global or regional hypertrophy compared with that in nontreated AVF rats. Previous chronic studies reported mixed results concerning the effect of AG on heart weight or the heart weight-to-body weight ratio in rats (9, 20, 27). This variation may be due to dosage, length of exposure, or additional cardiac assaults in the rats. Our results also showed that AG had no effect on dilation or wall thinning compared with those in nontreated AVF rats. However, AG+AVF weight gain was considerably impaired over the 6-wk study compared with that in nontreated AVF rats and may have influenced the calculated weight ratios.
Influence of cross-linking on cardiac mechanics
Previous studies demonstrated that myocardial compliance is altered by AGE cross-linking. Diabetes produced increased myocardial stiffness and AGE accumulation in rats; however, both stiffness and AGE were inhibited with the administration of AG (27). Likewise, in diabetic dogs, elevated AGE accompanied increased LV chamber stiffness but drug administration with either AG (2) or metformin (18) prevented AGE formation and consequently decreased chamber stiffness.
In our study, AGE cross-linking appears to play a role in determining cardiac mechanics as evidenced by the alterations in myocardial stress, strain, and stiffness that occur because of changes in cross-linking. Passive P-V characteristics in rats with volume overload were not altered because of the inhibition of AGE cross-links. On the basis of the regional measures of ECM structure and function made in the present study, it is not surprising that the P-V curves were not sensitive to local changes in ECM or passive mechanics. Because P-V curves represent a global description of ventricular mechanics, local changes in structure and function may not be reflected in measures of global passive properties.
LV circumferential strain was significantly increased in AVF rats compared with controls, but this increase was completely prevented with cross-link inhibition (data not shown). These changes in mechanics do not appear, at first glance, to be associated with cross-linking because AGE cross-links were unchanged in the LV free wall. However, the changes in septal compliance may explain changes observed in the LV free wall. For example, in AVF rats, the increase in circumferential septal stiffness may cause the increased distension of the LV free wall (evidenced by elevated E11 strains) as a compensatory reaction to the volume overload. Moreover, circumferential septal stiffness returns to normal with AG treatment, as does the E11 strain in the LV free wall in AG+AVF rats. The changes in septal and LV mechanics with the onset of volume overload and the reactions to cross-link inhibition demonstrate both the local and regional compensatory interactions that occur in the heart.
There was a significant reduction in AGE cross-linking, along with a modest decrease in longitudinal stiffness (P = 0.06), in the septum of AG+AVF rats compared with the AVF group. Volume overload increased LV longitudinal stiffness in AVF rats, and this change in compliance was also prevented by AG treatment. However, there was no change in AGE cross-links in the LV, so this effect appears to be due to the mechanical interaction of the other regions of the heart, namely, the septum, in a manner similar to that described above.
Regional mechanics and ECM
Volume overload appears to regionally modify the loading on the heart and produce dissimilar remodeling responses throughout the myocardial tissue and ECM. For example, previous studies reported changes in tension development and relaxation that occurred in RV papillary muscles, but not in LV muscles, from rats with volume overload (22). It is well known that RV and LV filling affect the pressure and compliance of the other ventricle in normal and diseased states (30, 33). This LV-RV interaction may be largely dependent on septal stiffness (13). However, abnormal loading in the heart can change septal, as well as ventricular, mechanics (30). In clinical hypertrophic cardiomyopathy, diastolic septal stiffness was found to be slightly higher than that of the LV posterior wall (4). In addition, aortic regurgitation resulted in LV volume overload and regional ischemia that produced septal hypertrophy and increased metabolism that was in response to greater stress development in the septum compared with the LV (10).
Regional changes in mechanics may be due to regional remodeling of the ECM. Echocardiography showed that patients with hypertrophy exhibited abnormal motion in the septum and posterior wall and that these regional abnormalities were strongly associated with altered myocardial properties most likely due to the ECM (29). Spinale and colleagues (32) observed regional differences in collagen extractability within the normal LV free wall and regional variations in cross-link remodeling during the progression and regression of tachycardia-induced cardiomyopathy.
Therefore, the heterogeneous response of AGE cross-links and myocardial remodeling reported in this study agree with previous observations of regional remodeling and support the hypothesis that altered loading in the heart initiates local myocardial and ECM remodeling that subsequently modifies regional mechanics, such as strain and wall stress, in a nonhomogeneous manner.
Actions of AG on hemodynamics
AG treatment did not change hemodynamic parameters from those in nontreated AVF rats. In general, the influence of AG on the cardiovascular system may be complicated because two of its targets generate contrasting effects on hemodynamics, namely, collagen cross-links, which may stiffen cardiovascular tissue, and nitric oxide (NO), which acts as a vasorelaxant. AG inhibition of the enzyme inducible nitric oxide synthase could theoretically prevent NO from contributing beneficial effects on hemodynamics. In fact, there is growing evidence that AG does not affect parameters associated with this type of NO activity and actually may improve hemodynamic parameters itself (7, 16).
Study limitations
The results of this study are subject to some limitations. An analysis of RV mechanics was not obtained, and this prevented a complete investigation of AGE cross-linking influence in volume-overload hypertrophy. The experimental and computational difficulties of obtaining RV stresses hindered this analysis. Stress in the LV and septum was calculated as average wall stress based on an analytic model using a symmetric prolate ellipsoid. Factors such as residual stress were not included in this simple model analysis, and they could play a role in altering the values of calculated wall stress.
There was no control group of animals treated only with AG in this study; however, others have already published such results. Previous chronic studies of 4, 6, and 18 mo reported no changes in body weight, kidney weight, mortality, aortic collagen and elastin content, myocardial hydroxyproline concentration, or myocardial collagen fluorescence between normal and AG-treated rats (9, 20, 27). More importantly, these same studies observed no difference in mean blood pressure, systolic and diastolic pressure, cardiac output, heart rate, and, most notably, LV elastic stiffness. However, inconsistent results were reported in regard to changes in heart weight, LV weight, and the heart weight-to-body weight ratio, as mentioned above.
Another limitation was the lack of blood glucose data, which prohibited a thorough description of the glycemic conditions present in the experimental animals and impeded identification of a possible candidate for AGE stimulation. Statistical strength of the data analyses, in general, was weakened because of the modest number of animals in the experimental groups. Finally, there were no sham-operated animals, and therefore any effect of the surgery and long-term housing compared with chronic AVF rats could not be accounted for.
In conclusion, this study provides a description of regional collagen and AGE cross-linking composition in volume-overload hypertrophy. We investigated the hypothesis that regional variations in passive myocardial mechanics are modulated by changes in local collagen cross-linking. Specifically, we postulated that the inhibition of nonenzymatic AGE cross-links would prevent changes in diastolic mechanics and myocardial stiffness due to volume-overload hypertrophy. Our results show that volume overload appears to induce heterogeneous alterations in AGE formation and myocardial stiffness in the myocardium. Furthermore, inhibition of AGE formation by AG in rats with volume overload prevented these decreases in myocardial compliance. Thus it appears that nonenzymatic AGE cross-links are at least partly responsible for increased myocardial stiffness in volume-overload hypertrophy.
Acknowledgments
The authors thank Dr. David Arniel and Fred Harwood [Department of Orthopaedics, University of California-San Diego (UCSD)] for assistance with the HP cross-link analysis. In addition, we are grateful to Dr. James Covell, Rish Pavelec, Zhuangjie Li, Troy Kalscheur, and Rachel Alexander (Department of Medicine, UCSD) for assistance with the chronic animal experiments and data analysis.
This study was supported by National Heart, Lung, and Blood Institute Grants HL-64321 and HL-54686 and a Whitaker Foundation Biomedical Graduate Fellowship.
REFERENCES
- 1.Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci USA. 2000;97:2809–2813. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avendano GF, Agarwal RK, Bashey RI, Lyons MM, Soni BJ, Jyothirmayi GN, Regan TJ. Effects of glucose intolerance on myocardial function and collagen-linked glycation. Diabetes. 1999;48:1443–1447. doi: 10.2337/diabetes.48.7.1443. [DOI] [PubMed] [Google Scholar]
- 3.Bailey AJ, Sims TJ, Avery NC, Halligan EP. Non-enzymic glycation of fibrous collagen: reaction products of glucose and ribose. Biochem J. 1995;305:385–390. doi: 10.1042/bj3050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonandi L, Hess OM, Grimm J, Krayenbuhl HP. Relaxation and regional myocardial stiffness in obstructive hypertrophic cardiopathy patients: effect of verapamil. G Ital Cardiol. 1984;14:77–84. In Italian. [PubMed] [Google Scholar]
- 5.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Arninoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 6.Bruel A, Ortoft G, Oxlund H. Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis. 1998;140:135–145. doi: 10.1016/s0021-9150(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 7.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerami A, Ulrich P. Pharmaceutical intervention of advanced glycation endproducts. Novartis Found Symp. 2001;235:202–212. doi: 10.1002/0470868694.ch16. [DOI] [PubMed] [Google Scholar]
- 9.Corman B, Duriez M, Poitevin P, Heudes D, Bruneval P, Tedgui A, Levy BI. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc Natl Acad Sci USA. 1998;95:1301–1306. doi: 10.1073/pnas.95.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn RB. Regional blood flow and metabolite levels in the left ventricular free wall and septum during aortic insufficiency: implications for the development of asymmetric septal hypertrophy. J Am Coll Cardiol. 1986;8:1182–1188. doi: 10.1016/s0735-1097(86)80399-0. [DOI] [PubMed] [Google Scholar]
- 11.Emery JL, Omens JH. Mechanical regulation of myocardial growth during volume-overload hypertrophy in the rat. Am J Physiol Heart Circ Physiol. 1997;273:H1198–H1204. doi: 10.1152/ajpheart.1997.273.3.H1198. [DOI] [PubMed] [Google Scholar]
- 12.Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984;137:380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 13.Farrar DJ, Woodard JC, Chow E. Pacing-induced dilated cardiomyopathy increases left-to-right ventricular systolic interaction. Circulation. 1993;88:720–725. doi: 10.1161/01.cir.88.2.720. [DOI] [PubMed] [Google Scholar]
- 14.Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med. 1980;69:576–584. doi: 10.1016/0002-9343(80)90471-4. [DOI] [PubMed] [Google Scholar]
- 15.Harper J, Harper E, Covell JW. Collagen characterization in volume-overload- and pressure-overload-induced cardiac hypertrophy in minipigs. Am J Physiol Heart Circ Physiol. 1993;265:H434–H438. doi: 10.1152/ajpheart.1993.265.2.H434. [DOI] [PubMed] [Google Scholar]
- 16.Hirono S, Islam MO, Nakazawa M, Yoshida Y, Kodama M, Shibata A, Izumi T, Imai S. Expression of inducible nitric oxide synthase in rat experimental autoimmune myocarditis with special reference to changes in cardiac hemodynamics. Circ Res. 1997;80:11–20. doi: 10.1161/01.res.80.1.11. [DOI] [PubMed] [Google Scholar]
- 17.Iimoto DS, Covell JW, Harper E. Increase in cross-linking of type I and type III collagens associated with volume-overload hypertrophy. Circ Res. 1988;63:399–408. doi: 10.1161/01.res.63.2.399. [DOI] [PubMed] [Google Scholar]
- 18.Jyothirmayi GN, Soni BJ, Masurekar M, Lyons M, Regan TJ. Effects of metformin on collagen glycation and diastolic dysfunction in diabetic myocardium. J Cardiovasc Pharmacol Ther. 1998;3:319–326. doi: 10.1177/107424849800300407. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Spinale FG, Tanaka R, Johnson W, Cooper G, IV, Zile MR. Inhibition of collagen cross-linking: effects on fibrillar collagen and ventricular diastolic function. Am J Physiol Heart Circ Physiol. 1995;269:H863–H868. doi: 10.1152/ajpheart.1995.269.3.H863. [DOI] [PubMed] [Google Scholar]
- 20.Li YM, Steffes M, Donnelly T, Liu C, Fuh H, Basgen J, Bucala R, Vlassara H. Prevention of cardiovascular and renal pathology of aging by the advanced glycation inhibitor aminoguanidine. Proc Natl Acad Sci USA. 1996;93:3902–3907. doi: 10.1073/pnas.93.9.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacKenna DA, Omens JH, McCulloch AD, Covell JW. Contribution of collagen matrix to passive left ventricular mechanics in isolated rat hearts. Am J Physiol Heart Circ Physiol. 1994;266:H1007–H1018. doi: 10.1152/ajpheart.1994.266.3.H1007. [DOI] [PubMed] [Google Scholar]
- 22.Micheletti R, Giacalone G, Bianchi G. Effect of propionyl-l-carnitine on the mechanics of right and left papillary muscles from volume-overloaded rat hearts. J Cardiovasc Pharmacol. 1996;27:52–57. doi: 10.1097/00005344-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Mirsky I. Handbook of Physiology. The Cardiovascular System. The Heart. I. Am. Physiol. Soc.; Bethesda, MD: 1979. Elastic properties of the myocardium: a quantitative approach with physiological and clinical applications. pp. 497–531. sect. 2. chapt. 14. [Google Scholar]
- 24.Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986;314:403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- 25.Namba T, Tsutsui H, Tagawa H, Takahashi M, Saito K, Kozai T, Usui M, Imanaka-Yoshida K, Imaizumi T, Takeshita A. Regulation of fibrillar collagen gene expression and protein accumulation in volume-overloaded cardiac hypertrophy. Circulation. 1997;95:2448–2454. doi: 10.1161/01.cir.95.10.2448. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson BO. Biological effects of aminoguanidine: an update. Inflamm Res. 1999;48:509–515. doi: 10.1007/s000110050495. [DOI] [PubMed] [Google Scholar]
- 27.Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation. 1996;93:1905–1912. doi: 10.1161/01.cir.93.10.1905. [DOI] [PubMed] [Google Scholar]
- 28.Norton GR, Tsotetsi J, Trifunovic B, Hartford C, Candy GP, Woodiwiss AJ. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation. 1997;96:1991–1998. doi: 10.1161/01.cir.96.6.1991. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro LM, Moore RB, Logan-Sinclair RB, Gibson DG. Relation of regional echo amplitude to left ventricular function and the electrocardiogram in left ventricular hypertrophy. Br Heart J. 1984;52:99–105. doi: 10.1136/hrt.52.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibbald WJ, Driedger AA. Right ventricular function in acute disease states: pathophysiologic considerations. Crit Care Med. 1983;11:339–345. doi: 10.1097/00003246-198305000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Skinner SJ. Rapid method for the purification of the elastin cross-links, desmosine and isodesmosine. J Chromatogr. 1982;229:200–204. doi: 10.1016/s0378-4347(00)86052-1. [DOI] [PubMed] [Google Scholar]
- 32.Spinale FG, Zellner JL, Johnson WS, Eble DM, Munyer PD. Cellular and extracellular remodeling with the development and recovery from tachycardia-induced cardiomyopathy: changes in fibrillar collagen, myocyte adhesion capacity and proteoglycans. J Mol Cell Cardiol. 1996;28:1591–1608. doi: 10.1006/jmcc.1996.0150. [DOI] [PubMed] [Google Scholar]
- 33.Taylor RR, Covell JW, Sonnenblick EH, Ross J., Jr Dependence of ventricular distensibility on filling of the opposite ventricle. Am J Physiol. 1967;213:711–718. doi: 10.1152/ajplegacy.1967.213.3.711. [DOI] [PubMed] [Google Scholar]
- 34.ffirich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing proportions of this amino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 36.Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, ffirich P, Cerami A, Levy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA. 1998;95:4630–4634. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodiwiss AJ, Norton GR. Exercise-induced cardiac hypertrophy is associated with an increased myocardial compliance. J Appl Physiol. 1995;78:1303–1311. doi: 10.1152/jappl.1995.78.4.1303. [DOI] [PubMed] [Google Scholar]