Abstract
Background: Although the importance of adequate zinc intake has been known for decades, the estimated global prevalence of zinc deficiency remains high. This substantiates the need for a specific and sensitive status assessment tool.
Objective: The objective was to evaluate erythrocyte zinc transporters as candidate molecules with the potential of being a biomarker of dietary zinc status in humans.
Design: A 24-d observational study with acclimation (7 d, 10.4 mg Zn/d), zinc-depletion (10 d, 0.3 mg Zn/d), and zinc-repletion (7 d, 29.5 mg Zn/d) phases was conducted in healthy men (n = 9). Proteomic approaches including Western blot analyses and tandem mass spectrometry were implemented to identify the zinc responsiveness of selected red blood cell membrane proteins.
Results: Zinc transporter 1 (ZnT1) and Zrt/Irt-like proteins ZIP8 and ZIP10 were detected in human erythrocyte membranes. No effects of short-term dietary zinc depletion were observed on the amounts of these proteins. However, changes in a cytoskeletal protein, dematin, by zinc depletion were identified through the nonspecific signals produced by an anti-ZIP8 antibody. This response was further validated by a dematin-specific antibody and with erythrocytes collected from mice fed a zinc-deficient diet.
Conclusions: The presence of ZnT1, ZIP8, and ZIP10 in human red blood cells implicates their role in the regulation of cellular zinc metabolism in the human erythroid system. The zinc responsiveness of membrane dematin suggests its capability to serve as a biomarker for dietary zinc depletion and its involvement in impaired erythroid membrane fragility by zinc restriction. This trial was registered at clinicaltrials.gov as NCT01221129.
INTRODUCTION
The homeostatic regulation of zinc is crucial during the maturation of erythroid progenitor cells. The majority of zinc in erythrocytes is present as a component of metalloenzymes, which include carbonic anhydrase and Cu/Zn-superoxide dismutase (1), and lesser amounts are associated with metallothionein (2). Recently, we identified the presence of zinc transporters 1 (ZnT1)4, Zrt/Irt-like protein 8 (Zip8), and Zrt/Irt-like protein 10 (Zip10) in the plasma membranes of murine erythrocytes (3). ZnT1 and Zip10 were differentially responsive to dietary zinc in mice. Similarly, the metallothionein content in erythrocytes of zinc-restricted and zinc-supplemented humans was lower and higher, respectively (2, 4). Metallothionein and zinc transporters are important components that are necessary for cellular zinc homeostasis in all cell types including red blood cells (RBCs).
The functional outcomes of metabolic changes in RBCs produced by altered dietary zinc intake have not been extensively investigated. With respect to the zinc transporters in RBC membranes, their temporal expression patterns are constant with higher zinc import and export during the early compared with late stages of terminal erythroid differentiation in mice (3). This may help to limit cellular zinc availability during the terminal phase of erythropoiesis, which, when in excess, interferes with iron incorporation during hemoglobin biosynthesis (5). Similarly, zinc is important for maintenance of membrane integrity of erythrocytes. Dietary zinc intake has been reported to influence fragility of RBCs in studies of rodents (6) and in humans (7). Collectively, the literature suggests that erythroid cells are influenced by zinc nutritional status.
The study described in this article was conducted to determine whether erythroid ZnT1, ZIP8, and ZIP10 expression is responsive to zinc in humans and to assess the potential of these transporters as status assessment tools of human dietary zinc deficiency (8). The novel, to our knowledge, finding reported here is that a protein recognized nonspecifically by the Zip8 antibody in the plasma membrane was identified as zinc responsive, indicating its potential as a zinc biomarker. The zinc-responsive protein, dematin, is a cytoskeletal protein involved in the maintenance of the cellular morphology, motility, and membrane structural integrity (9, 10). Hence, our findings may relate to the decades-old observation that zinc influences RBC membrane fragility.
SUBJECTS AND METHODS
Subjects
Healthy male adults (aged 21–35 y) were recruited to participate in the study (Table 1). Exclusion criteria for the dietary regimen included the following: a body weight <50 kg, cigarette smoking, alcohol abuse, dependence on medications, use of denture cream (11) or dietary zinc supplements, and history of any chronic disease or allergic reaction. A 24-h dietary recall followed by calculations with the Nutrition Data System for Research was conducted, and blood was collected to estimate habitual dietary zinc concentrations in each subject. The study protocol was reviewed and approved by both the University of Florida Institutional Review Board and the University of Florida Clinical Research Center. All subjects provided written, informed consent before enrollment. The study was registered at clinicaltrials.gov as NCT01221129.
TABLE 1.
Characteristics of and serum zinc concentration in the participants (n = 9)1
| Study phase |
|||
| Screening | After acclimation (baseline) | End of depletion | |
| Age (y) | 24 ± 2 | — | — |
| Height (cm) | 173.9 ± 6.6 | — | — |
| Weight (kg) | 77.1 ± 12.5 | 76.7 ± 12.6 | 76.3 ± 11.8 |
| Energy needs (kcal/d) | 3,043 ± 193 | — | — |
| Zinc intake (mg Zn/d) | 13.9 ± 7.3 | 10.415 | 0.296 |
| Serum zinc (μg/dL) | 91.9 ± 17.9 | 86.6 ± 8.1 | 69.1 ± 13.4* |
All values are means ± SDs. *Different from baseline, P < 0.001 (Student-Newman-Keuls test for pairwise comparisons).
Acute dietary zinc depletion
The 24-d dietary regimen was composed of 3 phases of dietary zinc treatment. During the first 7-d period, subjects consumed meals composed of a basal mixed diet (2-d cycle menu), which provided 2700 kcal and ∼11 mg Zn/d, to establish a defined baseline condition (acclimation). Supplemental zinc-free energy shakes were used to adjust the daily energy contents of the diet to ensure body weight maintenance. During the subsequent depletion phase (10 d), the subjects consumed a strawberry-flavored, egg white–based liquid formula (The Hershey Company), which provided <0.5 mg Zn/d. Energy and mineral contents of this diet were comparable to those used in previous dietary zinc-depletion studies (2, 12) and are described in detail elsewhere (13). Soft candies (<2.50 μg Zn/g; Starburst; Mars) and the supplemental energy shake, used in the depletion phase, were provided for energy adjustment. The zinc content of all dietary components was measured by inductively coupled plasma atomic emission spectrometry. Sodium phytate from rice (1.4 g/d; Sigma-Aldrich) was supplemented to the liquid formula to minimize the bioavailability of zinc in the diet, and carboxymethyl cellulose (2 g/d; TIC Gums) was added to prevent bowel discomfort attributable to the extensive consumption of a liquid diet (14). A multivitamin supplement (CVS Pharmacy) was given as a source of other vitamins. Biotin (2 mg/d; CVS Pharmacy) was provided as separate doses. Distilled water (Nestlé Waters North America Inc) and sugar-free carbonated beverages (Diet Pepsi and Sierra Mist; PepsiCo), in which zinc contents were undetectable by flame atomic absorption spectrophotometry, were provided throughout the first 2 phases of the study. After completion of the zinc-depletion phase (on day 17), subjects were allowed to return to their self-selected habitual diet and consumed 15 mg Zn/d (Jarrow Formulas) to replenish their zinc status. An anonymous questionnaire of compliance was completed by each subject on completion of the study, and serum zinc concentrations were monitored during participation to assess the compliance of each subject and the effectiveness of the experimental diets. There were no major deviations from the protocol reported or identified.
Sample collection and processing
On day 7 (end of acclimation) and day 17 (end of depletion period) of the study, after an overnight fast, whole blood was drawn into EDTA-treated tubes (Vacutainer; BD Diagnostics). Plasma and peripheral blood mononuclear cells were removed from whole blood by using a Ficoll gradient (Histopaque-1077; Sigma-Aldrich). Briefly, whole blood was carefully layered on an equal volume of the density gradient medium and fractionated at 400 × g for 30 min. RBC pellets were washed with HEPES buffer (154 mmol NaCl/L, 10 mmol HEPES/L, 1 g bovine serum albumin/L) and filtered through a column composed of α-cellulose (Sigma-Aldrich) and microcrystalline cellulose (Sigmacell Type 50; Sigma-Aldrich) for leukocyte depletion (15). Residual platelets were removed by 2 additional washes of the RBC eluate at 200 × g for 10 min with phosphate-buffered saline. Additional blood was collected on the first day and at days 10, 15, 20, and 24. Serum samples were isolated, and zinc concentrations were measured by atomic absorption spectrophotometry as described earlier (13).
For erythrocyte membrane isolation, purified RBCs were lysed by using a hypotonic buffer [5 mmol Na2HPO4/L (pH 7.4)] with a protease inhibitor cocktail (Thermo Scientific). Lysates were collected by centrifugation at 12,000 × g for 10 min at 4°C; this process was repeated until the supernatant and pellets lost their red color. After a wash at 20,000 × g, membrane pellets were solubilized with 5 mmol Tris-HCl/L 0.5% Triton X-100 (Sigma-Aldrich)–containing protease inhibitors and were stored at −80°C.
Western blot analysis of RBC zinc transporters
The expression of zinc transporters ZnT1, Zip8, and Zip10 in the erythrocyte plasma membrane was previously identified in mouse models (3). Western blot analysis using affinity-purified rabbit polyclonal antibodies was conducted to confirm the presence of these transporters in human RBCs. All primary antibodies, in addition to those for the detection of human (ab89161; Abcam) and mouse (sc-135881; Santa Cruz Biotechnology) dematin, were designed for previous studies (3, 16, 17). Specificity of signals produced by each in-house-made antibody was determined by preabsorption with respective antigenic peptides specific to the target proteins of interest. The glycosylation status of each protein was evaluated by incubation of protein samples with peptide-N-glycosidase F (New England BioLabs) at 37°C for 2 h before Western blot analysis. Erythrocyte proteins were separated by 7.5∼10% acrylamide SDS-PAGE and were transferred to nitrocellulose membranes. Efficient transfer and equal loading were visualized by Ponceau staining. Membranes were blocked by Tris-buffered saline containing 5% skim milk for 1 h, and primary antibodies were added at a final concentration of 1∼2 μg/mL. After incubation with the primary antibody for 1∼2 h, blots were washed with Tris-buffered saline and then treated with anti-IgG antibody conjugated to horseradish peroxidase for 1 h. Signals indicating abundance of the protein were visualized by using an enhanced chemiluminescent substrate (SuperSignal WestPico; Thermo Scientific) and autoradiographic films. Blots were incubated in stripping buffer (Restore PLUS Western Blot Stripping Buffer; Thermo Scientific) for 15 min when reprobing with subsequent primary antibody was needed.
Immunoprecipitation and tandem mass spectrometry
Erythrocyte membrane fractions were immunoprecipitated by using the anti-human ZIP8 polyclonal antibody (17) to enrich the abundance of proteins targeted by the antibody. Briefly, 1 mg protein was incubated overnight in 1 vol radioimmunoprecipitation assay buffer containing 4 μg antibody at 4°C and purified by using protein A/G-conjugated agarose beads (Thermo Scientific). After extensive wash steps, protein bound to the agarose beads was released by 10 min incubation at 100°C with Laemmli buffer. Immunoprecipitated proteins were separated by using SDS-PAGE. After the gel was divided into 2 halves, each was subjected to either gel staining or Western blot analysis. Proteins were stained by incubation in Coomassie Blue staining solution [0.1% Coomassie Brilliant Blue R-250 (Thermo Scientific) (10% acetic acid, 50% methanol and 40% water)] for 1 h at room temperature, and background stains were removed by a destaining solution composed of 10% acetic acid, 50% methanol, and 40% water. The stained gel was stored in the destaining solution, which was diluted 1:4 with water, until protein digestion for mass spectrometry. Western blot analysis was conducted for the identification of IgG-detectable bands and the protein producing a nonspecific band by the anti-human ZIP8 primary antibody. The Western blot image was then matched with the Coomassie-stained gel to determine the position of the nonspecific band in the gel, which was excised with methanol-treated blades for liquid chromatography–tandem mass spectrometry (LC-MS/MS). Protein digestion and LC-MS/MS were conducted at the proteomics core of the Interdisciplinary Center for Biotechnology Research of the University of Florida. The nature of identified peptides was determined by using Scaffold 3 (Proteome Software).
Animal experiments
Young male C57BL/6J mice were fed an egg white–based AIN76 diet (Research Diets), which contained <1 mg Zn/kg diet or 30 mg Zn/kg diet, for 21 d (3). The dietary restriction protocol for the animal models was previously described (3, 18) and was approved by the University of Florida Institutional Animal Care and Use Committee.
Statistical analyses
Power estimates from previous dietary zinc studies in humans (2, 19) predicted that n = 9 would be sufficient for the detection of a within-subject difference with 80% power at a 2-sided P value of <0.05. For values from the human study, repeated-measures ANOVA with Student-Newman-Keuls multiple comparisons posttest or paired t test was conducted, and baseline values (day 7) served as the control. Statistical analyses for the animal experiments were performed by using Student's t test. All statistical analyses were conducted by using the InStat 3 software (GraphPad Software), and changes were considered significant if P < 0.05.
RESULTS
Erythrocyte zinc transporter expression during low zinc intake
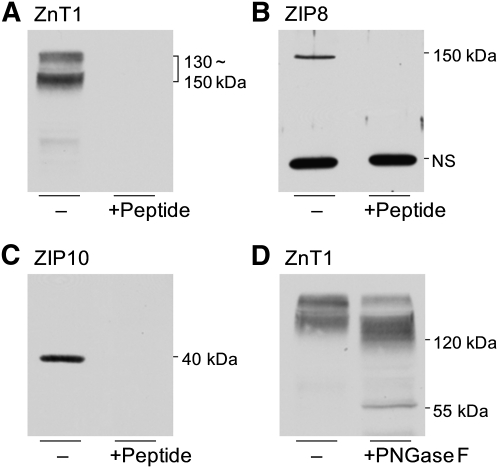
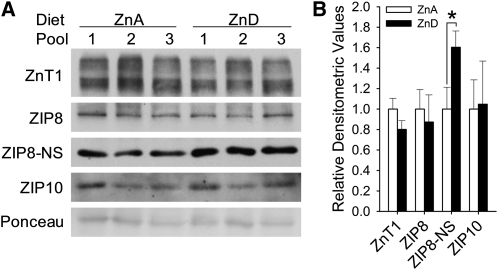
Western blot analyses using in-house-designed antibodies against human ZnT1, ZIP8, and ZIP10 successfully produced positive signals from erythrocyte samples (Figure 1), which confirmed the screening results from the previous animal experiments (3). The specificity of signals was determined by preabsorption controls, which indicated that the estimated molecular weights of human ZnT1, ZIP8, and ZIP10 in erythrocytes were 130∼150 kDa, 150 kDa, and 40 kDa, respectively. The prominent band at 50 kDa produced by the ZIP8 antibody did not disappear by peptide competition and thus was considered nonspecific. A shift in the migration of ZnT1 by peptide-N-glycosidase F treatment indicates the glycosylation of this transporter in human erythrocytes. In contrast to our findings from experiments in mice (3), there were no significant changes in the zinc transporters by dietary zinc depletion (Figure 2). However, signal intensities from the nonspecific band produced by the ZIP8 antibody were significantly higher in the membrane fraction of erythrocytes collected from subjects at the end of the depletion phase.
FIGURE 1.
Zinc transporter expression in the plasma membrane of human erythrocytes. The presence of ZnT1(A), ZIP8 (B), and ZIP10 (C) in erythrocyte ghosts was detected by Western blot analysis. The specificity of signals was determined by preincubating each primary antibody with their respective antigenic peptides. D: Glycosylation of ZnT1 was identified by PNGaseF treatment. NS, nonspecific band; PNGaseF, peptide-N-glycosidase F; ZnT1, zinc transporter 1; ZIP8, Zrt/Irt-like protein 8; ZIP10, Zrt/Irt-like protein 10.
FIGURE 2.
Effects of acute dietary zinc depletion on zinc transporter expression in human erythrocytes. A: The amounts of each transporter before (ZnA) and after (ZnD) 10 d of dietary zinc depletion were determined by Western blot analysis by using affinity-purified antibodies. B: Signal intensities were quantified by densitometric analysis, and mean (±SD) values were normalized to baseline values. *Significantly different at P < 0.05 (paired t test, n = 3 subjects in each pooled sample). ZIP8, Zrt/Irt-like protein 8; ZIP10, Zrt/Irt-like protein 10; ZIP8-NS, the nonspecific band detected with the ZIP8 antibody; ZnA, zinc adequate; ZnD, zinc deficient; ZnT1, zinc transporter 1.
Identification of dematin as a zinc-responsive erythrocyte membrane protein
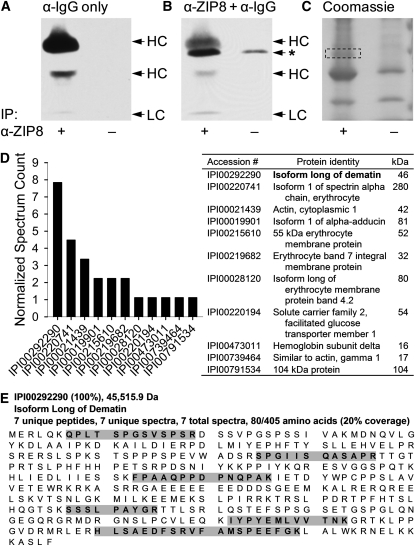
The zinc-responsive protein nonspecifically detected by the human ZIP8 antibody was further characterized by proteomic approaches including immunoprecipitation and mass spectrometry. After enriching the protein of interest by immunoprecipitation with the human ZIP8 antibody, protein samples were subjected to SDS-PAGE and subsequent Western blot analyses or gel staining with Coomassie Blue. Because the zinc-responsive protein band had a molecular weight of ∼50 kDa, on the basis of the known size of the IgG heavy chain (55 kDa), it was important to confirm the separation between these coimmunoprecipitated proteins before band excision for protein identification. Western blot analysis by using the secondary antibody against rabbit IgG identified 3 bands originating from the ZIP8 antibody used for immunoprecipitation, of which 2 represented heavy chains (40 and 55 kDa) and another identified the light chain (25 kDa) (Figure 3A). Reprobing the membrane with the ZIP8 antibody and its respective secondary antibody enabled the discrimination of the signal produced by the protein of interest (50 kDa) from that originating from the heavy chain of IgG (Figure 3B, identified by an asterisk). By matching with the Western blot on the basis of molecular weight markers, the region corresponding to the size of the nonspecific band was excised from a counterpart gel stained with Coomassie for protein identification by LC-MS/MS (Figure 3C, as shown within the box).
FIGURE 3.
Identification of the protein nonspecifically detected by the human ZIP8 antibody. A: Detection of IgG incorporated into the protein sample during immunoprecipitation with the ZIP8 antibody (α-ZIP8) by an anti-IgG secondary antibody (α-IgG). B: Position of the zinc-responsive band nonspecifically produced by α-ZIP8 band on a Western blot. The nonspecific band is indicated by an asterisk. C: Coomassie stain of a counterpart gel of the Western blots shown in panels A and B. The region migrating at the size of the nonspecific protein is indicated by a dashed box and was excised from the stained gel for LC-MS/MS. D: The peptide profile of the isolated proteins determined by LC-MS/MS. Dematin, with an expected molecular weight of 46 kDa, showed the highest normalized spectrum count among the 11 identified proteins. E: Peptide sequences unique for dematin identified by LC-MS/MS are highlighted in gray. HC, heavy chain; IP, immunoprecipitation; LC, light chain; LC-MS/MS, liquid chromatography–tandem mass spectrometry; ZIP8, Zrt/Irt-like protein 8.
The protein profile identified from the mass spectrum of the digested sample was composed of 11 proteins (Figure 3D). Among these, the long isoform of dematin was detected with the highest normalized spectrum counts. The predicted molecular weight of 46 kDa was closest to that of the nonspecific band size detected by Western blot analysis by using the ZIP8 antibody (50 kDa). Approximately 20% of the complete amino acid sequence of dematin was covered by 7 associated tryptic peptides identified by the mass spectrum (Figure 3E). This coverage rate was highest among all proteins detected, which suggested that the unknown protein producing nonspecific bands by the ZIP8 antibody was dematin.
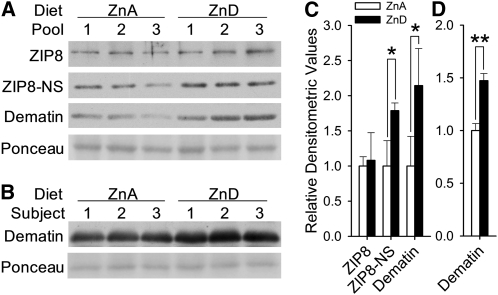
The protein identity determined by mass spectrometry and the presence of dematin in the membrane fraction of human erythrocyte were validated by Western blot analysis of samples immunoprecipitated with an antibody designed to target dematin. A strong signal corresponding to a molecular weight of 50 kDa was produced by probing the anti-dematin immunoprecipitated samples by the human ZIP8 antibody (Supplemental Figure 1 under “Supplemental data” in the online issue). Western blot analyses of blood samples collected before and after dietary zinc depletion confirmed the zinc responsiveness of membrane dematin amounts in human erythrocytes (Figure 4A). The estimated molecular weight and the magnitude of response determined by the dematin antibody were comparable to those detected by the nonspecific signals from the human ZIP8 antibody. There was no change in ZIP8 amounts by dietary treatment, which confirmed the data shown in Figure 2.
FIGURE 4.
Upregulation of erythrocyte membrane dematin by zinc-deprived conditions. A: Response of dematin to 10 d of dietary zinc depletion in humans (n = 3 subjects per pooled sample). Dematin amount was measured by either the signals produced by the nonspecific signals from the ZIP8 antibody or those from a dematin-specific antibody. B: Changes in erythrocyte membrane dematin amounts in mice (n = 3) after being fed a low-zinc diet for 21 d. Signal intensities of Western blots produced by human (C) and mouse (D) samples were quantified by densitometric analysis, and mean (±SD) values were normalized to their respective control values. *,**Significantly different (paired t test and Student's t test for human and mouse samples, respectively): *P < 0.05, **P < 0.005. ZIP8, Zrt/Irt-like protein 8; ZIP8-NS, nonspecific band detected with the ZIP8 antibody; ZnA, zinc adequate; ZnD, zinc depleted.
To further validate our findings and to determine the effect of moderate dietary zinc depletion on concentrations of erythroid membrane dematin, a dietary study with a 21-d period of zinc depletion was conducted in mice. Corresponding to the mode of response observed in the human subjects, an increase in dematin amounts was measurable with the plasma membrane fraction of the mouse erythrocytes in mice fed the low-zinc diet (Figure 4B). The responses of membrane dematin amounts in both human and mouse RBCs to low zinc conditions were significant with fold changes of 2.0 and 1.5, respectively (Figure 4, C and D).
DISCUSSION
The presence of ZnT1, Zip8, and Zip10 in the plasma membrane of mouse erythrocytes was previously identified by using a battery of in-house-made antibodies targeting zinc transporters (3). Differential expression of these transporter genes during terminal erythroid differentiation was shown by using a primary cell model inducible by erythropoietin treatment in vitro (3). Upregulation of the importers, Zip8 and Zip10, preceded the induction of ZnT1 by erythropoietin. These findings implicate the involvement of zinc transporter activity in the development of optimal zinc balance required for the transactivation of genes by zinc-finger transcription factors (20, 21) and for hemoglobin synthesis during the differentiation process (5). The results from the current study confirmed the expression of these transporter proteins in the plasma membrane of human RBCs.
The enhancement in erythrocyte zinc uptake by low dietary concentrations of zinc has been identified in animal models (22–24). The downregulation of ZnT1 and the upregulation of Zip10 observed in erythrocytes of zinc-deficient mice (3) suggested that these transporters are the factors exerting this effect of zinc deficiency. The differential expression of both ZnT1 and Zip10 in response to zinc, however, with an opposite mode of response, has been shown to be mediated by the zinc-sensing transcription factor metal-regulatory transcription factor 1 (18, 25, 26).
Even though the observations from the mouse model agreed with the mode of metal-regulatory transcription factor 1–meditated regulation of ZnT1 and Zip10 by zinc, there were no changes in these zinc transporter proteins in human erythrocytes by acute dietary zinc depletion. During the differentiation of erythroid progenitors to reticulocytes, enucleation occurs and the capability of these cells to carry out gene regulation is lost. Thus, regulation of the erythrocyte proteome may occur exclusively through posttranscriptional or posttranslational mechanisms on maturation. The estimated life spans of human erythrocytes and mouse RBCs are 120 and 40 d, respectively (27). The dietary zinc regimen of the current human study for zinc depletion was limited to 10 d, which covers <10% of the life span of circulating erythrocytes. Thus, a substantial portion of circulating erythrocytes collected after the zinc depletion phase would be those formed under adequate zinc conditions. The length of the zinc depletion period in mice was ∼50% of the life span of their RBCs (3), which implied that a higher portion of erythrocytes in the bloodstream are produced during zinc deprivation. In addition, as indicated by changes in plasma/serum zinc concentrations observed in the mice and in human subjects (reduction of ∼60% compared with 20%), the severity of zinc deficiency induced by the 21-d dietary zinc deprivation in mice was greater than that produced by the 10-d dietary regimen in the human subjects. This indicates that the murine model of zinc deficiency is close to a moderate or severe deficiency, whereas the human model used here represents conditions of short-term modest dietary zinc deprivation. Consequently, the potential of erythrocyte ZnT1 and ZIP10 amounts to be biomarkers of chronic dietary zinc deficiency in humans needs to be further investigated in longer-term studies.
Dematin, a protein initially identified as protein 4.9 in the membrane of human RBCs (28), functions as an actin-bundling protein located at the junctional complex (ie, spectrin-actin junction) of the erythrocyte membrane skeleton (29). Along with other core constituents of the membrane skeleton, such as spectrin, actin, adducin, and protein 4.1 (30, 31), dematin has been shown to be essential for the maintenance of cellular morphology, motility, and membrane structural integrity (9, 10). In the current study, the amount of erythrocyte dematin in the plasma membrane was shown to be highly sensitive to the host's zinc intake. Its rapid response implicates the presence of a posttranslational regulatory mechanism mediating the effects of zinc on this protein. Two protein kinases, cyclic AMP-dependent protein kinase (PKA) and protein kinase C (PKC), are involved in the regulation of the actin cytoskeleton, and both have been shown to phosphorylate dematin in vitro (32).
Phosphorylation by PKA, whose activity in RBCs is evident (33), mediates an inhibitory effect on the actin-bundling activity of dematin by causing a conformational change in the headpiece domain (32, 34, 35). Zinc has been shown to predominantly inhibit the hydrolysis of cyclic AMP and cyclic guanosine-5′-monophosphate by phosphodiesterase acitivity in vitro (36). In addition, this inhibitory effect on phosphodiesterase activity has been recently suggested as an indirect mechanism for zinc to enhance PKA activity in blood cells (37). Conversely, suboptimal zinc conditions may result in lower PKA activity, and thus lead to less phosphorylation of dematin. The inhibitory effect of phosphorylation on the actin-binding of dematin can be reversed by phosphatase treatment (32). The capability of zinc to inhibit phosphatase activities has been identified in various signaling pathways (17, 38–40). Taken together with the observations of the present study, dietary zinc depletion may increase the translocation of cytosolic dematin to the plasma membrane by disrupting the balance between PKA and phosphatase activities.
Phosphorylation of the component proteins associated with the junctional complex by modulated PKC activity can also lead to the formation of an instable membrane skeleton (41). Zinc can enhance PKC activity through binding to a metal-binding site and induce the translocation of cytosolic PKC to the plasma membrane (42). This mode of regulation agrees with the present observation of increased membrane amounts of a cytoskeletal protein under a zinc-depleted condition, and thus suggests PKC as another possible mediator of the effects of dietary zinc depletion on membrane dematin amounts.
Impaired cellular membrane stability and increased osmotic fragility have been identified in RBCs of zinc-deficient rodents and humans (6, 7). Whether an excess of dematin in the membrane complex produces beneficial or detrimental effects to the structural integrity of normal erythrocytes is unclear. Overexpression of dematin has been shown to alter the cellular phenotype of prostate cancer cells toward that of normal prostate epithelial cells (43). Overexpression also caused numerous phenotypic changes such as cytoplasmic shrinkage and structural defects. Thus, the increase in membrane dematin amounts may contribute to the effect of zinc deficiency on RBC integrity, in particular by causing a dysfunctional assembly of the actin-based erythrocyte cytoskeleton.
Collectively, we have confirmed the expression of the zinc transporters, initially identified and characterized with mouse models, in human erythrocytes. The acute response of dematin in erythrocyte membranes to dietary zinc deprivation supports the presence of a posttranslational mechanism exerting the effects of inadequate zinc intake on erythrocyte membrane fragility as previously suggested (44). Furthermore, the increased erythrocyte dematin amounts in response to zinc depletion in both human and mice models substantiate the potential of this protein to serve as a biomarker of dietary zinc deficiency. Such a protein marker would be amenable to immunologically based assays such as ELISA. Of particular relevance is that the life of the human RBC is such that a historical record of low zinc intake may be imprinted in these cells through increased dematin content. This would decrease the likelihood of measuring only recent zinc intake, which is a criticism of some zinc biomarkers that have been suggested as status assessment tools.
Acknowledgments
We acknowledge the contributions of Bobbi Langkamp-Henken, Wendy Dahl, and Meena N Shankar to the dietary protocol and the valuable advice of Shou-Mei Chang, Louis A Lichten, and Liang Guo, which was provided throughout this study. We also thank Desmond Shatz and the staff of the University of Florida Clinical Research Center and the Proteomics Core at the University of Florida Interdisciplinary Center for Biotechnology Research.
The authors’ responsibilities were as follows—M-SR, GJG, and RJC: designed the research; M-SR and ABM: conducted the research; M-SR: supervised the dietary phase of the project; TBA: prepared and characterized the human ZIP8 antibody; and M-SR and RJC: analyzed the data and wrote the manuscript. All authors read and approved the final manuscript. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: LC-MS/MS, liquid chromatography–tandem mass spectrometry; PKA, cyclic AMP-dependent protein kinase; PKC, protein kinase C; RBC, red blood cell; ZIP, Zrt/Irt-like protein; ZnT, zinc transporter.
REFERENCES
- 1.Ohno H, Doi R, Yamamura K, Yamashita K, Iizuka S, Taniguchi N. A study of zinc distribution in erythrocytes of normal humans. Blut 1985;50:113–6 [DOI] [PubMed] [Google Scholar]
- 2.Grider A, Bailey LB, Cousins RJ. Erythrocyte metallothionein as an index of zinc status in humans. Proc Natl Acad Sci USA 1990;87:1259–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryu MS, Lichten LA, Liuzzi JP, Cousins RJ. Zinc transporters ZnT1 (Slc30a1), Zip8 (Slc39a8), and Zip10 (Slc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J Nutr 2008;138:2076–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas EA, Bailey LB, Kauwell GA, Lee DY, Cousins RJ. Erythrocyte metallothionein response to dietary zinc in humans. J Nutr 1992;122:2408–14 [DOI] [PubMed] [Google Scholar]
- 5.Bloomer JR, Reuter RJ, Morton KO, Wehner JM. Enzymatic formation of zinc-protoporphyrin by rat liver and its potential effect on hepatic heme metabolism. Gastroenterology 1983;85:663–8 [PubMed] [Google Scholar]
- 6.O'Dell BL, Browning JD, Reeves PG. Zinc deficiency increases the osmotic fragility of rat erythrocytes. J Nutr 1987;117:1883–9 [DOI] [PubMed] [Google Scholar]
- 7.Woodhouse LR, Lederer LJ, Lowe NM, King JC. The effects of zinc status on the osmotic fragility of human erythrocytes. : Fischer PWF, Abbe MR, Cockell KA, Gibson RS, Trace elements in man and animals–9. Proceedings of the Ninth International Symposium on Trace Elements on Man and Animals. Ottawa, Canada: NRC Research Press, 1997:636–8 [Google Scholar]
- 8.King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr 2011;94(suppl):679S–84S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna R, Chang SH, Andrabi S, Azam M, Kim A, Rivera A, Brugnara C, Low PS, Liu SC, Chishti AH. Headpiece domain of dematin is required for the stability of the erythrocyte membrane. Proc Natl Acad Sci USA 2002;99:6637–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Khan AA, Liu F, Gilligan DM, Peters LL, Messick J, Haschek-Hock WM, Li X, Ostafin AE, Chishti AH. Combined deletion of mouse dematin-headpiece and beta-adducin exerts a novel effect on the spectrin-actin junctions leading to erythrocyte fragility and hemolytic anemia. J Biol Chem 2007;282:4124–35 [DOI] [PubMed] [Google Scholar]
- 11.Hedera P, Peltier A, Fink JK, Wilcock S, London Z, Brewer GJ. Myelopolyneuropathy and pancytopenia due to copper deficiency and high zinc levels of unknown origin II. The denture cream is a primary source of excessive zinc. Neurotoxicology 2009;30:996–9 [DOI] [PubMed] [Google Scholar]
- 12.Gordon PR, Woodruff CW, Anderson HL, O'Dell BL. Effect of acute zinc deprivation on plasma zinc and platelet aggregation in adult males. Am J Clin Nutr 1982;35:113–9 [DOI] [PubMed] [Google Scholar]
- 13.Ryu MS, Langkamp-Henken B, Chang SM, Shankar MN, Cousins RJ. Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis. Proc Natl Acad Sci USA 2011;108:20970–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King JC, Shames DM, Lowe NM, Woodhouse LR, Sutherland B, Abrams SA, Turnlund JR, Jackson MJ. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am J Clin Nutr 2001;74:116–24 [DOI] [PubMed] [Google Scholar]
- 15.Bonafoux B, Commes T. Serial analysis of gene expression adapted for downsized extracts (SAGE/SADE) analysis in reticulocytes. Methods Mol Biol 2009;496:299–311 [DOI] [PubMed] [Google Scholar]
- 16.McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci USA 1998;95:4841–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aydemir TB, Liuzzi JP, McClellan S, Cousins RJ. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J Leukoc Biol 2009;86:337–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichten LA, Ryu MS, Guo L, Embury J, Cousins RJ. MTF-1-mediated repression of the zinc transporter Zip10 is alleviated by zinc restriction. PLoS ONE 2011;6:e21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydemir TB, Blanchard RK, Cousins RJ. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci USA 2006;103:1699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira R, Ohneda K, Yamamoto M, Philipsen S. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol 2005;25:1215–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodge D, Coghill E, Keys J, Maguire T, Hartmann B, McDowall A, Weiss M, Grimmond S, Perkins A. A global role for EKLF in definitive and primitive erythropoiesis. Blood 2006;107:3359–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naber TH, van den Hamer CJ, van den Broek WJ, van Tongeren JH. Zinc uptake by blood cells of rats in zinc deficiency and inflammation. Biol Trace Elem Res 1992;35:137–52 [DOI] [PubMed] [Google Scholar]
- 23.Van Wouwe JP, Veldhuizen M, De Goeij JJ, Van den Hamer CJ. Laboratory assessment of early dietary, subclinical zinc deficiency: a model study on weaning rats. Pediatr Res 1991;29:391–5 [DOI] [PubMed] [Google Scholar]
- 24.Chesters JK, Will M. The assessment of zinc status of an animal from the uptake of 65Zn by the cells of whole blood in vitro. Br J Nutr 1978;39:297–306 [DOI] [PubMed] [Google Scholar]
- 25.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem 2000;275:34803–9 [DOI] [PubMed] [Google Scholar]
- 26.Zheng D, Feeney GP, Kille P, Hogstrand C. Regulation of ZIP and ZnT zinc transporters in zebrafish gill: zinc repression of ZIP10 transcription by an intronic MRE cluster. Physiol Genomics 2008;34:205–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glader B. Destruction of erythrocytes. : Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Wintrobe's clinical hematology. Philadelphia, PA: Lippincott Williams & Wilkins, 2008:156–69 [Google Scholar]
- 28.Siegel DL, Branton D. Partial purification and characterization of an actin-bundling protein, band 4.9, from human erythrocytes. J Cell Biol 1985;100:775–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rana AP, Ruff P, Maalouf GJ, Speicher DW, Chishti AH. Cloning of human erythroid dematin reveals another member of the villin family. Proc Natl Acad Sci USA 1993;90:6651–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salomao M, Zhang X, Yang Y, Lee S, Hartwig JH, Chasis JA, Mohandas N, An X. Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc Natl Acad Sci USA 2008;105:8026–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anong WA, Franco T, Chu H, Weis TL, Devlin EE, Bodine DM, An X, Mohandas N, Low PS. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood 2009;114:1904–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Husain-Chishti A, Levin A, Branton D. Abolition of actin-bundling by phosphorylation of human erythrocyte protein 4.9. Nature 1988;334:718–21 [DOI] [PubMed] [Google Scholar]
- 33.Tsukamoto T, Suyama K, Germann P, Sonenberg M. Adenosine cyclic 3′,5′-monophosphate uptake and regulation of membrane protein kinase in intact human erythrocytes. Biochemistry 1980;19:918–24 [DOI] [PubMed] [Google Scholar]
- 34.Husain-Chishti A, Faquin W, Wu CC, Branton D. Purification of erythrocyte dematin (protein 4.9) reveals an endogenous protein kinase that modulates actin-bundling activity. J Biol Chem 1989;264:8985–91 [PubMed] [Google Scholar]
- 35.Frank BS, Vardar D, Chishti AH, McKnight CJ. The NMR structure of dematin headpiece reveals a dynamic loop that is conformationally altered upon phosphorylation at a distal site. J Biol Chem 2004;279:7909–16 [DOI] [PubMed] [Google Scholar]
- 36.Donnelly TE., Jr Effects of zinc chloride on the hydrolysis of cyclic GMP and cyclic AMP by the activator-dependent cyclic nucleotide phosphodiesterase from bovine heart. Biochim Biophys Acta 1978;522:151–60 [DOI] [PubMed] [Google Scholar]
- 37.von Bülow V, Dubben S, Engelhardt G, Hebel S, Plumakers B, Heine H, Rink L, Haase H. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J Immunol 2007;179:4180–6 [DOI] [PubMed] [Google Scholar]
- 38.Brautigan DL, Bornstein P, Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem 1981;256:6519–22 [PubMed] [Google Scholar]
- 39.Ho Y, Samarasinghe R, Knoch ME, Lewis M, Aizenman E, DeFranco DB. Selective inhibition of mitogen-activated protein kinase phosphatases by zinc accounts for extracellular signal-regulated kinase 1/2-dependent oxidative neuronal cell death. Mol Pharmacol 2008;74:1141–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, et al. Zinc is a novel intracellular second messenger. J Cell Biol 2007;177:637–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal 2006;18:276–84 [DOI] [PubMed] [Google Scholar]
- 42.Csermely P, Szamel M, Resch K, Somogyi J. Zinc can increase the activity of protein kinase C and contributes to its binding to plasma membranes in T lymphocytes. J Biol Chem 1988;263:6487–90 [PubMed] [Google Scholar]
- 43.Lutchman M, Pack S, Kim AC, Azim A, Emmert-Buck M, van Huffel C, Zhuang Z, Chishti AH. Loss of heterozygosity on 8p in prostate cancer implicates a role for dematin in tumor progression. Cancer Genet Cytogenet 1999;115:65–9 [DOI] [PubMed] [Google Scholar]
- 44.O'Dell BL. Role of zinc in plasma membrane function. J Nutr 2000;130(suppl):1432S–6S [DOI] [PubMed] [Google Scholar]