Abstract
Background: Maternal calcium intake and vitamin D status may affect fetal bone development.
Objective: This study was designed to examine relations between maternal calcium intake, 25-hydroxyvitamin D [25(OH)D] status, and fetal bone growth across pregnancy.
Design: This was a prospective longitudinal design. Maternal 25(OH)D, parathyroid hormone, and 1,25-dihydroxyvitamin D [1,25(OH)2D] were determined at midgestation (∼26 wk) and at delivery in 171 adolescents (≤18 y). Dietary recalls and fetal sonograms were performed up to 3 times across gestation, and fetal femur and humerus z scores were generated.
Results: Fetal femur and humerus z scores and neonatal birth length were significantly greater (P < 0.03) in adolescents consuming ≥1050 mg than in those consuming <1050 mg Ca/d. Maternal 25(OH)D >50 nmol/L was significantly positively associated with fetal femur and humerus z scores (P < 0.01). When maternal smoking, height, race, weight gain, and gestational age were controlled for, these relations remained significant. Interactions between calcium intake and 25(OH)D were evident. Calcium intake was associated with fetal femur z scores and birth length only when maternal 25(OH)D was ≤50 nmol/L (P < 0.05). Similarly, maternal 25(OH)D was associated with fetal femur and humerus z scores only when maternal calcium intake was <1050 mg/d (P < 0.03).
Conclusions: Optimal calcium intake and adequate maternal vitamin D status are both needed to maximize fetal bone growth. Interactions between these nutrients were evident when either calcium or vitamin D status was limited. Improving maternal calcium intake and/or vitamin D status during pregnancy may have a positive effect on fetal skeletal development in pregnant adolescents.
INTRODUCTION
Vitamin D deficiency, defined as a 25-hydroxyvitamin D [25(OH)D]5 concentration ≤25 nmol/L), in pregnant women residing at northern latitudes has been reported to range between 21% and 50% (1–3), and an even larger number of women are vitamin D insufficient [25(OH)D ≤50 nmol/L] (3). This high prevalence is of concern given the number of reports linking insufficient vitamin D status during pregnancy with detrimental effects on fetal growth and development and subsequent risk of chronic diseases (4). Both vitamin D and calcium are needed to fully mineralize the developing skeleton. Thus, low calcium intake, especially in combination with insufficient vitamin D status, may limit fetal bone growth and mineralization (5).
To date, maternal 25(OH)D status during pregnancy has been linked to infant birth weight (6) and adverse neonatal bone outcomes, including impaired fetal femoral development (7), reduced bone mineral content (BMC), and reduced neonatal bone density at birth (8, 9). Bone alterations in utero may affect subsequent pediatric bone mineralization, because children whose mothers had low 25(OH)D status during pregnancy have low BMC at 9 y of age (10).
Maternal calcium intake also affects fetal skeletal development. Supplemental calcium provided to women with a low calcium intake (<600 mg/d) resulted in increased neonatal bone density and total-body BMC (11, 12). In studies of pregnant adolescents, maternal dairy product intake was positively associated with increased fetal femur length (13), and milk intake was associated with infant total-body calcium (14). In studies of adults, maternal milk and vitamin D intake were significantly associated with infant birth weight (15). Maternal milk intake during pregnancy has also been linked to the subsequent spinal bone mineral density (BMD) of offspring at 16 y of age (16). In food-based calcium supplementation studies (ie, dairy products), it is difficult to distinguish whether it is the calcium and/or the vitamin D or other components of dairy products that are affecting neonatal bone outcomes. Similarly, many studies fail to control for possible interactions between maternal calcium intake and vitamin D status when investigating how the maternal status of these nutrients affects neonatal outcomes. These interactions are biologically plausible given that the active form of vitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D], is generated from 25(OH)D and this hormone stimulates active intestinal calcium absorption, particularly when calcium intakes are low. Currently, few studies addressing the effects of maternal calcium intake and vitamin D status on fetal development have also measured circulating 1,25(OH)2D. This is of concern because there is a growing awareness that local paracrine/autocrine effects of 1,25(OH)2D are influenced by circulating 25(OH)D (17).
Pregnancy during adolescence may result in a competition for nutrients between the mother and her developing fetus, placing both at risk of adverse outcomes (18). To address possible associations and interactions between maternal calcium intake and 25(OH)D status with fetal bone growth, we followed a large cohort of pregnant adolescents across gestation, longitudinally monitoring fetal skeletal development and calcitropic hormones.
SUBJECTS AND METHODS
Participants and study procedures
A cohort of 171 pregnant adolescents (≤18 y) was recruited to participate in a prospective longitudinal study designed to characterize changes in maternal bone quality and fetal bone growth across gestation. Thirteen participants were recruited from the Maternity Center East Clinic in Baltimore, MD, beginning in July 2005, and were followed until delivery at Johns Hopkins Hospital (Baltimore, MD). The remainder of the adolescents (n = 158) were recruited from the Rochester Adolescent Maternity Program in Rochester, NY, beginning in June 2007. Pregnant adolescents were eligible to participate if they were between 12 and 30 wk of gestation at entry into the study, were otherwise healthy, and were carrying a single fetus. Exclusion criteria included known medical complications, including HIV infection, diabetes, diagnosed eating disorders, or malabsorption diseases. Informed written consent was obtained from all participants, and the study procedures were approved by the Institutional Review Boards of the University of Rochester, Cornell University, and Johns Hopkins University. Maternal prepregnancy weight and smoking history were self-reported. Smoking status was self-reported as never, previously, or currently smoking, and information on the number of cigarettes smoked per day was obtained from those who were currently smoking. Maternal ethnicity (Hispanic or non-Hispanic) and race (African American, white, or other) were self-reported. Neonatal race was classified as African American if the mother reported both herself and the father as African American, as white if the mother reported both herself and the father as white, or biracial if maternal and paternal race differed. Infant ethnicity was similarly classified as Hispanic, non-Hispanic, or multiethnic. Data on markers of bone turnover and concentrations of osteoprotegerin and maternal calcitropic hormone concentrations were published previously (19, 20).
Each adolescent attended up to 3 study visits across pregnancy, roughly timed to coincide with early, mid-, and late gestation. At each visit, maternal anthropometric measures were recorded, and a 24-h dietary recall was administered by study personnel using food models to help estimate portion sizes. All 24-h dietary recalls were analyzed by a registered dietitian using the Nutrition Data System for Research (University of Minnesota, Minneapolis, MN; versions 2006, 2008, and 2009) at the University of Rochester Clinical and Translational Research Center. Tertiles of dietary calcium intake were defined based on the reported calcium intakes in this cohort.
Up to 3 times across pregnancy, both standard fetal biometry measures (femur length, biparietal diameter, abdominal circumference, and head circumference) and humerus length were recorded by certified sonographers. Fetal femur length z scores were generated from equations previously published by our laboratory derived from a large cohort (n = 929) of African American pregnant adolescents in Baltimore, MD (13). The curves generated for fetal femur growth in these pregnant adolescents are comparable with similar curve fits generated from data obtained in adult women (13, 21). Fetal humerus length z scores were calculated from published curves generated by Chitty and Altman (21) in adult women, because no published normative fetal humerus curves from adolescent pregnancies exist at present. At birth, infant weight, length, and head circumference were recorded by clinical staff.
Biochemical analyses
Maternal blood (10 mL) was obtained at midgestation (∼26 wk) and again at delivery. All blood samples were allowed to clot at room temperature, before serum was separated by centrifugation. An aliquot of serum was immediately sent to Quest Laboratories for assessment of 25(OH)D with the use of Diasorin RIA (Diasorin Inc). This laboratory participates in the vitamin D External Quality Assessment Scheme as a means of quality assurance. Serum collected for other biochemical assessments was stored at −80°C until analyzed. The season of each blood collection was classified as winter (November–February), spring (March–April), summer (May–August), or autumn (September–October) by using seasonal classifications for the northeast United States (22). Vitamin D insufficiency was defined as 25(OH)D ≤50 nmol/L in accordance with recent 2010 Institute of Medicine guidelines (23). After a high prevalence of vitamin D insufficiency was observed in the first 37 study participants, all subsequent participants found to be vitamin D insufficient at midgestation were provided with an additional 400 IU vitamin D3 at their next prenatal visit and were instructed to take one pill daily over the remainder of gestation. Compliance with all prenatal supplements provided was queried by self-report at each study visit. Calcitriol [1,25(OH)2D] was analyzed at Boston University in the laboratory of Michael Holick (Boston, MA) by using an in-house thymus receptor binding assay as previously described (24). Because of the lack of standardized reference ranges for calcitriol concentrations across pregnancy, adolescents were classified as exhibiting 1,25(OH)2D concentrations above or below the mean 1,25(OH)2D value at midgestation and at delivery. Intact parathyroid hormone (PTH) was analyzed by using a commercially available ELISA (DSL Laboratories). Elevated PTH was defined as a concentration ≥4.83 pmol/L (25).
Statistical analyses
Analyses were performed by using SAS 9.2 and JMP 8.0 (SAS Institute Inc). Results are reported as means ± SDs unless stated otherwise. Paired t tests or nonparametric tests were used to assess changes in hormones across gestation within subjects. Independent t tests or ANOVA were used to determine whether normally distributed variables differed by race, season, or categories of vitamin D status and calcium intake; Wilcoxon's rank-sum test was used for nonparametric data.
Simple linear regression was used to explore relations between calcium intake, 25(OH)D, and 1,25(OH)2D and fetal skeletal growth. Multiple linear regression was used to control for covariates and interactions between variables and to model statistical predictors of measures of fetal skeletal growth. Previous research had identified maternal height, prepregnancy BMI, and dairy product intake as predictors of fetal femur length in a group of 350 African American adolescents (13). As such, we initially considered maternal height and prepregnancy BMI as well as infant sex, maternal race, weight gain, smoking status, chronologic age, and gynecologic age as potential predictors of fetal femur and humerus z scores and neonatal birth length. With the use of simple linear regression, maternal smoking status, race, height, weight gain, and gestational age at delivery (for birth length) were identified as potential statistical predictors (P < 0.20) of fetal femur and humerus z scores and/or neonatal birth length. These variables were controlled for as covariates in the generated models of fetal bone z scores and birth length.
Interactions between maternal calcium intake and vitamin D status on fetal and neonatal bone outcomes were assessed statistically. Relations between maternal calcium intake and bone outcomes were also analyzed stratified by categories of vitamin D status to describe the nature of interactions between calcium and vitamin D status (≤50 or >50 nmol/L) on fetal and neonatal bone outcomes. Similarly, relations between maternal vitamin D status and fetal bone outcomes were analyzed when stratified by categories of calcium intake (both as tertiles of calcium intake and as intakes <1050 compared with those ≥1050 mg/d and <1100 compared with ≥1100 mg/d), to assess potential interactions between the 2 nutrients.
Using published means and SDs for fetal femur length reported in pregnant adolescents (13), we determined that a sample size of 146 participants would provide us with sufficient power (0.85) to characterize the mean fetal femur lengths in this population and to obtain a minimal detectable difference of 0.25 z scores from expected fetal bone measures, with an α level of 0.05. We overrecruited by 14% to allow for missing data and possible subject loss to follow-up because of the known increased risk of fetal death in utero and preterm birth in this age group. Variables were tested for normality by using the Shapiro-Wilks test. Nonnormally distributed variables were log transformed as necessary to ensure normality of the residuals. P values <0.05 were considered significant, and P values between 0.05 and 0.10 were considered trends.
RESULTS
Subject characteristics
Characteristics of the adolescents are presented in Table 1. Birth data were missing or unavailable in 9 adolescents: 3 (1.8%) of whom suffered a fetal death in utero and 6 of whom dropped out of the study before delivery or delivered at another hospital, so birth data were not obtained. Among neonates assessed at delivery, birth length was recorded in the medial chart of 153 (94.4%) infants. In 163 adolescents (95.3%), at least one dietary assessment was obtained during pregnancy, and 25(OH)D was assessed at least once across gestation in 168 (98.2%) of study participants. Gestational age at delivery ranged from 20.7 to 43.0 wk; 8.5% of births were premature (<37 wk gestation). Two of the 171 adolescents self-identified their race as American Indian. None of the study results differed if these 2 adolescents were excluded from analyses or combined with either the African American or white cohort. To avoid eliminating data from these 2 adolescents, they were grouped with the African American cohort to collapse maternal race into a bivariate variable.
TABLE 1.
Characteristics of pregnant adolescents enrolled1
| Maternal characteristics | Value |
| Total subjects recruited (n) | 171 |
| Age at enrollment (y) | 17.1 ± 1.12 (170) |
| Racial group (%) | |
| African American3 | 66.7 (114) |
| White | 33.3 (57) |
| Ethnicity (%) | |
| Hispanic | 23.7 (39) |
| Non-Hispanic | 76.2 (125) |
| Parity ≥1 (%) | 8.2 (14/170) |
| Smoking at entry into study (%) | 10.0 (17/170) |
| Prepregnancy BMI (kg/m2) | 24.7 ± 5.5 (165) |
| Weight gain (kg) | 16.8 ± 0.7 (150) |
| Dietary calcium intake (mg/d)4 | 917 ± 416 (163) |
| Dietary vitamin D intake (IU/d)4 | 216 ± 135 (163) |
| Gestational age at delivery (wk) | 39.2 ± 2.9 (165) |
| Maternal 25(OH)D at delivery (nmol/L) | 54.7 ± 27.5 (168) |
| Maternal 1,25(OH)2D at delivery (pmol/L) | 276.1 ± 79.6 (96) |
| Maternal PTH at delivery (pmol/L) | 4.74 ± 3.09 (80) |
n in parentheses. PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Mean ± SD (all such values).
Two adolescents who self-identified as Native American were included in the African American cohort.
Mean intakes were calculated from the mean of all 24-h dietary recalls administered (≤3 for each participant).
In adolescents with multiple 24-h dietary recall data, reported calcium, vitamin D, and caloric intake did not significantly differ between study visits, so the mean of all visits for each participant was used to calculate the cohort mean; this mean value was used for all subsequent analyses. The dietary calcium intake ranged from 257 to 3220 mg (Table 1), with only 29.4% of adolescents meeting the Estimated Average Requirement (EAR; 1100 mg/d) (23), and 15.3% of adolescents meeting the Recommended Dietary Allowance (1300 mg/d). Maternal vitamin D intake was highly correlated with calcium intake (P < 0.0001, R2 = 0.40). Because maternal 25(OH)D concentrations at midgestation did not significantly differ from concentrations at delivery (P = 0.64), when 25(OH)D was not assessed at delivery (n = 31 of 168), the 25(OH)D concentration obtained at midgestation was substituted for this measure to have a measure of vitamin D status from each participant (Table 1). Maternal 25(OH)D was correlated with both maternal vitamin D (P = 0.002, R2 = 0.06) and calcium (P = 0.004, R2 = 0.05) intake (n = 162). Vitamin D insufficiency [25(OH)D ≤50 nmol/L] was present in 47.6% of adolescents. Supplemental vitamin D3 (400 IU/d) was provided to 56% of teens whose midgestation 25(OH)D concentration was ≤50 nmol/L. Those who were vitamin D insufficient and did not receive supplements either joined the study before the supplements were provided (n = 19), failed to return to the clinic to receive the supplements (n = 15), or refused to accept them (n = 4). Vitamin D–insufficient adolescents who received supplements began supplementation on average at 30.3 ± 3.5 wk gestation. Self-reported data indicated that 35.8% of these adolescents consumed the vitamin D supplement ≥2 times/wk, and only 26.4% of adolescents self-reported that they consumed these supplements daily. The change in 25(OH)D concentrations from midgestation to delivery observed in those who received supplements (8.0 nmol/L, n = 45) was significantly different from the change observed in those who were not supplemented (−5.6 nmol/L; n = 90; P = 0.0002). Despite this increase in 25(OH)D, adolescents who received vitamin D supplements still had significantly lower 25(OH)D concentrations at delivery (44.6 ± 18.2 nmol/L compared with 57.6 ± 32.4 nmol/L, P = 0.001) and a higher prevalence of vitamin D insufficiency (63.3% compared with 44.4%, P = 0.03) at delivery than did adolescents who did not receive supplements (n = 117). When considered as a potential covariate, maternal receipt of vitamin D supplements was not significant in any models of fetal or neonatal skeletal outcomes.
Birth weight and length did not differ by maternal or infant race, infant sex, season of delivery, or receipt of vitamin D supplements. Data from at least one sonogram were available in all but 2 adolescents. The femur and humerus lengths from the last sonogram measure obtained (at 33.8 ± 4.0 wk gestation) were used to generate gestational age-specific fetal bone z scores. Humerus measures were not obtained from 2 adolescents because of the position of the fetus at the time of measurement. Fetal femur and humerus length z scores were both significantly below zero [P < 0.0001 (n = 169) and P = 0.009 (n = 167), respectively] and did not differ by maternal race (Table 2), infant race, infant sex, season of delivery, or receipt of vitamin D supplements, although there was a trend for higher fetal femur z scores in African American than in white adolescents (P = 0.054). Fetal femur length z scores were significantly more positive, by 0.424 SD, in nonsmoking adolescents than in adolescents who were smoking (P = 0.030).
TABLE 2.
Fetal and neonatal measures as a function of maternal race1
| Neonatal characteristics | All adolescents | African American | White | P2 |
| Birth weight (g) | 3238 ± 586 (162) | 3210 ± 599 (108) | 3293 ± 561 (54) | 0.300 |
| Birth length (cm) | 51.0 ± 2.6 (153) | 51.0 ± 2.6 (102) | 51.2 ± 2.2 (51) | 0.932 |
| Fetal femur length z score | −0.540 ± 0.918 (169) | −0.446 ± 0.917 (114) | −0.734 ± 0.897 (55) | 0.054 |
| Fetal humerus length z score | −0.201 ± 0.971 (167) | −0.197 ± 1.024 (112) | −0.207 ± 0.865 (55) | 0.950 |
All values are means ± SDs; n in parentheses.
Reflects the comparison between neonates born to African Americans and white adolescents (t test).
Maternal 1,25(OH)2D and PTH concentrations at midgestation and delivery were not significantly related to fetal femur or humerus length z scores or neonatal birth length. Furthermore, fetal femur and humerus z scores and neonatal birth length did not differ between adolescents with elevated PTH and those with PTH <4.83 pmol/L at either time point or between adolescents with 1,25(OH)2D concentrations above or below the mean concentration observed at midgestation (303.9 pmol/L) or delivery (276.1 pmol/L).
Maternal calcium intake and fetal bone outcomes
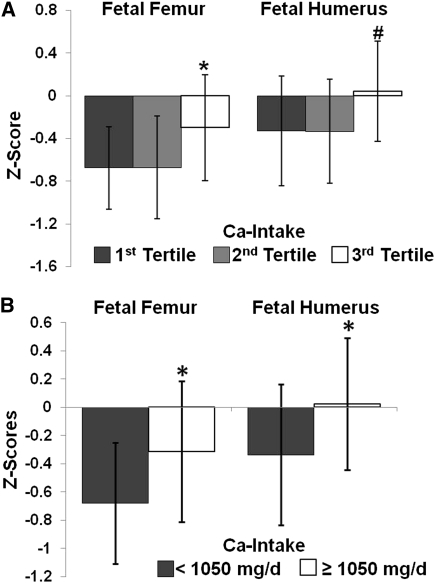
Maternal calcium intake was not linearly related to fetal femur or humerus z scores. However, when the cohort was divided into tertiles of observed calcium intake (<653 and >653 and <1066 and ≥1066 mg/d), those in the highest tertile had a significantly more positive fetal femur length z scores (P < 0.040) than did those in the lower 2 tertiles (Figure 1A). The same trend was present for fetal humerus length z scores, but this difference was not significant (P < 0.070). To further define the minimum calcium intake between the second and third tertiles (>653 and <1066 mg/d) at which significant increases in fetal bone z scores became evident, possible differences in fetal bone z scores were evaluated at 50-mg intervals across the calcium intake range of 653 to 1066 mg/d. With this approach, a calcium intake of ≥1050 mg/d was identified as the intake at which differences in both fetal femur length z scores (−0.319 ± 0.994 compared with −0.680 ± 0.860; P = 0.020, n = 161) and fetal humerus length z scores (0.003 ± 0.938 compared with −0.337 ± 0.999; P = 0.032, n = 159) became evident (Figure 1B). This calcium intake is very close to the EAR for this group of 1100 mg/d, so we also tested for differences in mean fetal femur and humerus z scores when the cohort was categorized by calcium intakes ≥1100 or <1100 mg/d; we detected similar but not significantly different trends. Adolescents who consumed ≥1100 mg/d had a trend for longer fetal femur (−0.32 ± 0.94 compared with −0.63 ± 0.91; P = 0.057) and humerus length [0.02 ± 0.88 (n = 47) compared with −0.30 ± 1.02 (n = 112); P = 0.050] z scores. This lack of significance was likely due to the fact that even fewer adolescents consumed ≥1100 mg Ca/d (27%) than consumed ≥1050 mg Ca/d (38%), which resulted in skewed sample sizes for comparison.
FIGURE 1.
Fetal femur and humerus lengths were evaluated by sonogram at 33.8 ± 4.0 wk gestation in 169 pregnant adolescents, and z scores were calculated for each measure by using published equations (13, 21). Calcium intake was categorized into tertiles for fetal femur and humerus z scores, respectively: 1 <653 mg/d (n = 52, 50), 2 = 653–1066 mg/d (n = 52, 52), and 3 >1066 mg/d (n = 57, 57). A: Adolescents with a calcium intake within tertile 3 had better fetal femur length z scores (ANOVA; *P < 0.039) and tended to have better fetal humerus length z scores (ANOVA, #P < 0.070) than did those in calcium intake tertiles 1 and 2. B: Adolescents consuming ≥1050 mg Ca/d (n = 61) had significantly higher fetal femur (P = 0.020) and humerus (P = 0.032) z scores than did those consuming <1050 mg Ca/d (n = 100; 98 for fetal femur and humerus z scores, respectively).
The raw differences in those consuming <1050 mg/d was equivalent to a 0.98-mm shorter femur at 33.8 wk, which represents 1.5% of the mean predicted femur length at that week of gestation. The raw difference in fetal humerus z scores observed in those consuming <1050 mg/d was equivalent to a 0.92-mm shorter humerus at 33.8 wk, which represents 1.6% of the predicted humerus length at that week of gestation.
Multiple regression models of fetal femur and humerus z scores were generated to test the main effect of maternal calcium intake after control for the identified covariates: maternal smoking status, race, height, and weight gain. After control for these maternal characteristics, a maternal calcium intake ≥1050 mg/d remained a significant predictor of fetal femur z score (parameter estimate = 0.009) and was associated with an increase in fetal femur z score of 0.196. A maternal calcium intake ≥1050 mg/d explained an additional 4% of the variation in femur z score (ie, increased the R2 of the model from 0.031 to 0.068). Similarly, when the identified covariates were controlled for, maternal calcium intake ≥1050 mg/d also remained a significant predictor of fetal humerus length z scores (parameter estimate = 0.026). In stepwise regression, all other covariates dropped out of the model of fetal humerus z score, and a maternal calcium intake ≥1050 mg/d was associated with an increase in fetal humerus z score of 0.180 and predicted 3.2% of the variation in this measure.
Multiple regression models of fetal femur and humerus z scores were also generated to test the main effect of maternal calcium intake greater than or equal to the EAR (1100 mg/d). After maternal smoking status, race, height, and weight gain were controlled for, maternal calcium intake ≥1100 mg/d remained a significant predictor of fetal femur z score (parameter estimate = 0.031). In the model of fetal humerus z score, a maternal calcium intake ≥1100 mg/d was nearly significant and was retained in the model as a nonsignificant predictor of fetal humerus z score (parameter estimate = 0.050).
Maternal 25(OH)D status and fetal bone outcomes
When analyzed as a continuous variable, maternal dietary vitamin D intake was significantly positively associated with fetal humerus z score (P = 0.015, R2 = 0.037; n = 158), but not with any other fetal or neonatal outcomes. Maternal 25(OH)D is a biomarker for vitamin D status, and, when analyzed as a categorical variable, pregnant adolescents with sufficient 25(OH)D status (>50 nmol/L) had significantly higher fetal femur and fetal humerus length z scores than did measures obtained in vitamin D–insufficient adolescents [−0.321± 0.919 compared with −0.746 ± 0.878 (P = 0.003; n = 166) and 0.012 ± 0.893 compared with −0.403 ± 1.019 (P = 0.006; n = 164)]. After maternal smoking status, race, height, and weight gain were controlled for, maternal 25(OH)D sufficiency remained a significant predictor of fetal femur length z score (parameter estimate = 0.001) and was associated with an increase in fetal femur z score of 0.225. Maternal 25(OH)D >50 nmol/L explained an additional 6% of the variation in femur z score (ie, increased the R2 of the model from 0.031 to 0.089). Maternal 25(OH)D sufficiency also remained a significant predictor of fetal humerus length z scores when the identified covariates were controlled for (parameter estimate = 0.006). In stepwise regression, all other covariates dropped out of the model of fetal humerus z score, and maternal 25(OH)D sufficiency was associated with an increase in fetal humerus z score of 0.208 and predicted 4.5% of the variation in this measure.
Interaction between maternal calcium intake and 25(OH)D status on fetal bone outcomes
Because both a maternal calcium intake ≥1050 mg/d and a maternal 25(OH)D concentration >50 nmol/L were individually associated with fetal femur and humerus z scores, both of these variables along with an interaction term were entered into a model to test for possible interactions between the effects of maternal 25(OH)D >50 nmol/L and maternal calcium intake ≥1050 mg/d on fetal bone length. In this combined model of fetal femur length z score, the main effect of calcium ≥1050 mg/d, the main effect of 25(OH)D sufficiency, and the interaction term between calcium intake and 25(OH)D all remained significant (P < 0.05) (Table 3). In the best model identified for humerus length z score, the main effect of maternal calcium intake ≥1050 was of borderline significance (P < 0.10), the main effect of 25(OH)D sufficiency remained significant, and the interaction term was not significant. These models are summarized in Table 3. If this modeling was repeated by using maternal calcium intake partitioned by the EAR (1100 mg/d), the interaction term was not significant in either the model of fetal femur or humerus z score.
TABLE 3.
Determinants of fetal femur and humerus z scores in pregnant adolescents1
| Fetal femur z score |
Fetal humerus z score |
|||
| Covariate2 | β ± SE | P | β ± SE | P |
| 25(OH)D <50 nmol/L | −0.1462 ± 0.0738 | 0.049 | −0.1753 ± 0.0784 | 0.027 |
| Calcium intake <1050 mg/d | −0.1655 ± 0.0738 | 0.026 | −0.1341 ± 0.0808 | 0.099 |
| 25(OH)D × calcium intake3 | −0.1628 ± 0.0739 | 0.029 | — | — |
| Maternal race, African American | 0.1744 ± 0.0741 | 0.020 | — | — |
| Maternal height (m) | 0.0203 ± 0.0100 | 0.042 | 0.0166 ± 0.0109 | 0.130 |
| P | <0.0001 | — | 0.007 | — |
| R2 | 0.125 | — | 0.057 | — |
| n | 160 | — | 158 | — |
Stepwise multiple linear regression was used to model the determinants of fetal femur and humerus length z scores and to test for an interaction between calcium intake and 25(OH)D status after control for maternal race, height, weight gain, and smoking status. 25(OH)D, 25-hydroxyvitamin D.
In stepwise regression, maternal weight gain and smoking status were not significant in models of either bone z scores.
Interaction term between maternal calcium intake and 25(OH)D status.
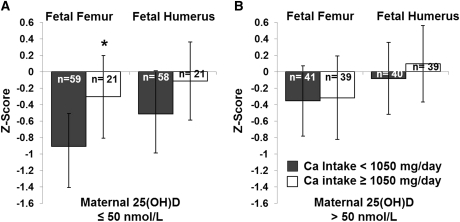
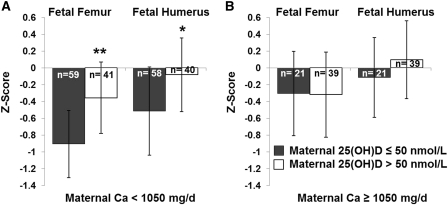
To further investigate the nature of the interaction between maternal calcium intake ≥1050 mg/d and 25(OH)D sufficiency, the initial models of fetal femur and humerus length z scores that included calcium intake as a bivariate variable ≥1050 mg/d were run separately in adolescents with a maternal 25(OH)D concentration ≤50 nmol/L and in adolescents with a 25(OH)D concentration >50 nmol/L. In both the models of fetal femur and humerus z score, calcium intake remained a significant covariate only in adolescents with 25(OH)D ≤50 nmol/L. The effect of a maternal calcium intake ≥1050 mg/d on fetal femur and humerus z scores separately in adolescents who were 25(OH)D-sufficient and 25(OH)D-insufficient is shown in Figure 2. Similarly, when the initial models of fetal femur and humerus length z scores that included 25(OH)D sufficiency were run separately in adolescents consuming ≥1050 and <1050 mg Ca/d, 25(OH)D sufficiency remained a significant predictor only in adolescents consuming <1050 mg Ca/d. The effect of maternal 25(OH)D sufficiency on fetal bone z scores separately in adolescents consuming calcium intakes ≥1050 and <1050 mg/d is shown in Figure 3.
FIGURE 2.
Third-trimester (33.8 ± 4.0 wk gestation) fetal femur and humerus lengths were measured and converted into z scores. Maternal dietary calcium intake and 25(OH)D status interacted to affect bone length outcomes. In adolescents with 25(OH)D ≤ 50 nmol/L, those who consumed ≥1050 mg Ca/d had significantly higher fetal femur z scores (*P = 0.019) and tended to have higher fetal humerus z scores (P = 0.116). Among adolescents who were 25(OH)D sufficient, no significant difference in fetal z scores was found between adolescents who consumed >1050 or <1050 mg Ca/d. 25(OH)D, 25-hydroxyvitamin D.
FIGURE 3.
An interaction between maternal 25(OH)D and maternal calcium intake was observed. Adolescents consuming <1050 mg Ca/d and those with 25(OH)D >50 nmol/L had significantly higher fetal femur (**P = 0.001) and humerus (*P = 0.030) z scores. Among adolescents who consumed ≥1050 mg Ca/d, no significant differences in fetal long bone z scores were found between adolescents who were 25(OH)D sufficient and those who were insufficient. 25(OH)D, 25-hydroxyvitamin D.
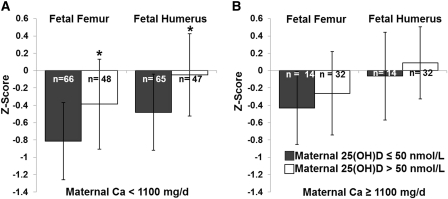
Because the EAR represents a clinically relevant diagnostic cutoff, we also modeled the effect of maternal 25(OH)D sufficiency on fetal bone z scores, separately in adolescents consuming above compared with below the EAR (1100 mg Ca/d), even though the statistical interaction term was not significant in the combined model. In this partitioned analyses, the same interaction between maternal 25(OH)D status and calcium intake was evident. Maternal 25(OH)D sufficiency remained a significant predictor of fetal femur and humerus length z scores only in adolescents consuming <1100 mg Ca/d (Figure 4).
FIGURE 4.
An analogous interaction between maternal 25(OH)D and maternal calcium intake was also observed when calcium intake was partitioned by the Estimated Average Requirement for this age group (1100 mg/d). In adolescents consuming <1100 mg Ca/d, those with 25(OH)D >50 nmol/L had significantly higher fetal femur (*P = 0.019) and humerus (*P = 0.025) z scores, Among adolescents who consumed ≥1100 mg Ca/d, no significant differences in fetal long-bone z scores were found between adolescents who were 25(OH)D sufficient and those who were insufficient. 25(OH)D, 25-hydroxyvitamin D.
The combined effect of 25(OH)D status and calcium intake on fetal bone outcomes
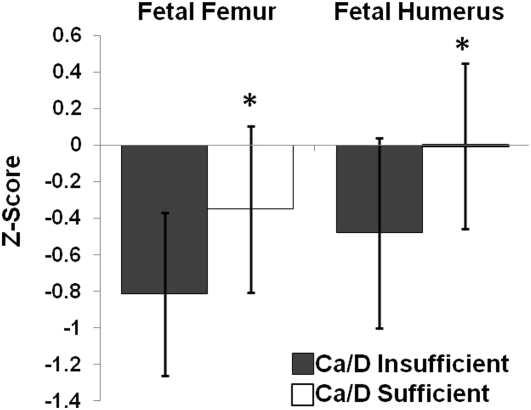
The above interactions suggested that both adequate maternal calcium intake (≥1100 mg/d or ≥1050 mg/d) or sufficient 25(OH)D status were capable of compensating when intake or status of the other nutrient was limited. To further explore this possibility, we classified teens into 2 groupings according to calcium intake and vitamin D status as “Ca/D insufficient” if both maternal 25(OH)D ≤50 nmol/L and calcium intake <1100 mg/d (n = 66), and all others were classified as “Ca/D sufficient” (n = 94). Adolescents classified as Ca/D sufficient had significantly more positive fetal femur (P < 0.002, n = 160) and humerus (P = 0.003, n = 158) z scores in comparison to measures obtained in the Ca/D insufficient group (Figure 5). When controlling for maternal smoking, height, race, and weight gain, Ca/D sufficiency remained a significant determinant of both fetal femur (parameter estimate P < 0.001) and humerus (parameter estimate P = 0.002) z scores and explained an additional 6.5% of the variation in fetal femur z score and an additional 4.6% of the variation in fetal humerus z score. The improvement associated with moving from the Ca/D insufficient to Ca/D sufficient group, by improving either calcium intake or 25(OH)D status, was 0.249 SD in fetal femur z score and 0.240 SD in fetal humerus z score.
FIGURE 5.
Adolescents consuming calcium intakes <1100 mg/d who also had a 25(OH)D concentration ≤50 nmol/L (Ca/D insufficient) were compared with adolescents with a calcium intake ≥1100 mg/d and/or a 25(OH)D concentration >50 nmol/L (Ca/D sufficient). Adolescents within the Ca/D sufficient category (n = 94 and 93, respectively) had higher fetal femur and humerus z scores (*P = 0.002 and P = 0.003, respectively) than did those in the Ca/D insufficient category (n = 66 and 65, respectively). 25(OH)D, 25-hydroxyvitamin D.
The combined effect of 25(OH)D status and calcium intake on neonatal birth length
The observed effects of maternal calcium intake on fetal bone length in utero were also evident at birth by using recorded measures of neonatal birth length. There was a trend for a linear relation between maternal calcium intake and birth length (P = 0.082, R2 = 0.021; n = 147). Similarly, adolescents consuming ≥1100 mg Ca/d delivered neonates that were 1.24 cm longer at birth than were infants delivered to adolescents consuming <1100 mg Ca/d (P = 0.007). In a multiple regression model of neonatal birth length (that controlled for gestational age at delivery, maternal smoking status, race, height and weight gain), maternal calcium intake (as a linear variable) remained a significant predictor of neonatal birth length (parameter estimate P = 0.041) and explained an additional 3% of the variation in birth length. In this model, every 100-mg increase in calcium intake was associated with a 0.12-cm (95% CI: 0.02, 0.21 cm) increase in birth length.
In contrast with the significant associations found between neonatal birth length and maternal calcium intake, maternal 25(OH)D status was not significantly correlated with birth length. Whereas a main effect of maternal 25(OH)D status was not evident, an interaction between maternal calcium intake and maternal 25(OH)D sufficiency was observed. When the initial model of birth length that included calcium intake as a linear variable was run separately in adolescents with maternal 25(OH)D ≤50 nmol/L and in adolescents with 25(OH)D >50 nmol/L, calcium intake remained a significant covariate only in adolescents with 25(OH)D ≤50 nmol/L. Furthermore, when maternal calcium intake and 25(OH)D status were combined and grouped as Ca/D insufficient or Ca/D sufficient (as described above), this Ca/D sufficiency variable remained of borderline significance (parameter estimate P = 0.074) in the model of birth length, increased the R2 of the model from 0.035 to 0.038, and was associated with a 0.34 cm increase in birth length.
DISCUSSION
In these pregnant adolescents, both maternal 25(OH)D status and dietary calcium intake influenced fetal bone growth. A significant interaction was evident between these 2 nutrients, with higher calcium intakes compensating for suboptimal vitamin D status and vice versa. These interactions are biologically feasible given the known role of vitamin D on active calcium absorption. The relations and interactions detected in utero remained evident at delivery, as evidenced by significant differences in neonatal birth length.
In multivariate models, adolescents consuming calcium intakes above or below the EAR had higher fetal femur and humerus z scores. The exact intake at which these significant differences in bone outcomes became evident (1050 mg/d) is similar to the 2010 EAR (1100 mg/d) set for this age group by the Institute of Medicine (23). The raw difference in fetal femur z scores observed in those consuming <1100 mg/d was 0.31 z scores. Other in vivo studies have found that maternal dairy intake is significantly related to fetal femur length in pregnant adolescents (13). Similar nutrient-driven gains in fetal femur length (0.24 z scores at 38 wk) have also been documented in disadvantaged Peruvian women provided daily 25-mg Zn supplements (26). Biological plausibility for a role of calcium in long-bone elongation is supported by several in vitro studies in animal models that have established that extracellular Ca2+ concentrations and Ca2+-sensing receptor activation in developing fetal bone induces type X collagen synthesis, increases rates of development and differentiation of growth-plate chondrocytes, and increases the height of the growth-plate hypertrophic zone (27–29).
Similar to the findings evident for calcium intake, pregnant adolescents with sufficient 25(OH)D exhibited more positive fetal femur and humerus z scores (by 0.42 SD). These results substantiate findings in adult pregnant women that low maternal 25(OH)D is associated with adverse fetal and neonatal bone outcomes (7, 9, 30, 31). Whereas many mechanistic studies have focused on the role of 1,25(OH)2D on regulation of endochondral bone development (32), it is interesting to note that we found no significant relations between maternal 1,25(OH)2D and fetal bone z scores. Increasing attention has been paid to the importance of local production and action of 1,25(OH)2D on bone growth (33), where availability of the 25(OH)D substrate may affect rates of 1,25(OH)2D synthesis. The high prevalence of vitamin D insufficiency observed in these adolescents (48%) is similar to that reported among pregnant adult women in the United States (34).
A significant interaction between dietary calcium intake and 25(OH)D status was evident in partitioned analyses, such that maternal calcium intakes ≥1100 mg/d and maternal 25(OH)D concentrations ≤50 nmol/L were only significantly associated with fetal femur and humerus z scores when the other nutrient was inadequate. This suggests that increases in dietary calcium intake and/or improvements in maternal vitamin D status can offset deficits when the other nutrient is limited. Similar interactions have been described in nonpregnant adolescent females (age 11–16 y, n = 211) in whom reduced areal BMD z scores were observed in girls that had both low dietary calcium intake (<600 mg/d) and low 25(OH)D status (≤40 nmol/L) when compared with girls who exhibited either higher dietary calcium intakes and/or vitamin D status (35). Sufficient 25(OH)D concentrations may be necessary to support renal and extrarenal production of 1,25(OH)2D, which regulates efficiency of intestinal calcium absorption (36). Additionally, increased systemic concentrations or local production of 1,25(OH)2D in the placenta may result in increased placental flux of calcium to the fetus (37, 38). The clinical relevance of the observed increase in fetal long-bone z scores with achievement of either 25(OH)D >50 nmol/L or a calcium intake ≥1050 mg/d is underscored by the number of adolescents (37%) who failed to meet either criteria.
In our adolescent population, the observed differences in long-bone growth in utero were associated with similar differences in birth length as a function of calcium intake. In our models of birth length, increasing maternal calcium intake by 500 mg/d was associated with a 0.60-cm increase in birth length. Data from calcium replete, skeletally mature populations may differ from adolescents, and prior data linking maternal calcium intake with infant birth length has been inconsistent. A study of 222 white women aged >30 y and consuming ∼1300 mg Ca/d on average found no relation between maternal calcium intake during pregnancy and infant birth length (39), but a randomized control trial in 256 adult women showed that supplementation with 2 g Ca/d from <22 wk of gestation to term resulted in a nonsignificant 0.5-cm increase in birth length (11).
In these adolescents the effect of maternal calcium intake on birth length was mediated by maternal vitamin D status and only remained significant when maternal 25(OH)D was suboptimal (≤50 nmol/L). A study of women in Iran reported that infants born to women who consumed adequate amounts of both calcium (>1000 mg/d) and vitamin D (>200 IU/d) were significantly longer at birth by 0.87 cm (40) compared with the 0.4-cm longer birth length we detected in our Ca/D-sufficient pregnant adolescents. In our study, birth length was measured by clinical staff using standard hospital practices. Our lack of standardization of neonatal birth length measures likely only diluted our ability to detect significant associations.
A strength of our study design was the biochemical assessment of vitamin D status combined with dietary assessment of calcium intake, because no analogous biomarker for calcium exists. In this population, vitamin D intake only explained 6% of the variation in 25(OH)D, emphasizing the additional benefits of using a biomarker of vitamin D status. As expected, dietary intakes of vitamin D and calcium in this group were highly correlated, which indicates that effects attributed to calcium intake be interpreted with caution. The collinearity of calcium and vitamin D intakes is likely due to consumption of dairy products and vitamin D–fortified foods; thus, counseling pregnant adolescents to increase their consumption of calcium- and vitamin D–rich foods would be appropriate to ensure the gains in fetal skeletal outcomes attributed to calcium in these models. The wide range in calcium intakes and 25(OH)D concentrations observed and the skeletal immaturity of this age group may have increased our ability to detect the associations noted. We assessed the effect of maternal nutritional status on fetal long bones in both the upper and lower limbs. It is presumed that both of these bones are subject to the same regulatory processes in utero (41), yet possible racial differences in these bone lengths may not be analogous based on data showing that fetal femur, but not humerus length, differ by maternal race (42, 43). A similar trend for racial differences in long bone length was nearly significant in our data. Currently, fetal biometry standard curves are not race-specific, but maternal race was controlled for in our models of fetal bone length to account for potential differences. Note that, whereas physiologic differences between the femur and humerus may exist, the nature of the interactions observed, and size of the effect of maternal calcium intake and vitamin D status on each of these 2 bones was similar.
The interactive relation between maternal calcium intake and 25(OH)D status that we observed in these adolescents suggested that consuming either an adequate calcium intake or achieving sufficient 25(OH)D status may partially attenuate the deficits in fetal long-bone growth and neonatal birth length observed in pregnant adolescents who were Ca/D insufficient. Whether or not the findings in this group of adolescents are generalizable to skeletally mature women remain unknown, the possibility merits investigation because nearly 50% of American women of childbearing age consume <1050 mg Ca/d (23), and vitamin D insufficiency is prevalent in this population.
Acknowledgments
We thank Tera Kent for general laboratory assistance. We are very grateful to the midwives of the Strong Midwifery Group and the adolescents and their infants, who made this research possible.
The authors’ responsibilities were as follows—FW, ZLH, and KOO: designed the research; BEY, AWM, EMC, TJM, and KOO: conducted the research; BEY: analyzed the data; BEY and KOO: wrote the manuscript; and KOO: had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors disclosed a conflict of interest.
Footnotes
Abbreviations used: BMC, bone mineral content; BMD, bone mineral density; EAR, Estimated Average Requirement; PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46:42–4 [DOI] [PubMed] [Google Scholar]
- 2.O'Riordan MN, Kiely M, Higgins JR, Cashman KD. Prevalence of suboptimal vitamin D status during pregnancy. Ir Med J 2008;101:240, 242–3 [PubMed] [Google Scholar]
- 3.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 2007;137:447–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas RM, Ponsonby AL, Pasco JA, Morley R. Future health implications of prenatal and early-life vitamin D status. Nutr Rev 2008;66:710–20 [DOI] [PubMed] [Google Scholar]
- 5.Prentice A. Micronutrients and the bone mineral content of the mother, fetus and newborn. J Nutr 2003;133:1693S–9S [DOI] [PubMed] [Google Scholar]
- 6.Scholl TO, Chen X. Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Hum Dev 2009;85:231–4 [DOI] [PubMed] [Google Scholar]
- 7.Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, Cooper C, Godfrey K; SWS Study Group Low maternal vitamin d status and fetal bone development: cohort study. J Bone Miner Res 2010;25:14–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Namgung R, Tsang RC. Factors affecting newborn bone mineral content: in utero effects on newborn bone mineralization. Proc Nutr Soc 2000;59:55–63 [DOI] [PubMed] [Google Scholar]
- 9.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 2006;91:906–12 [DOI] [PubMed] [Google Scholar]
- 10.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C; Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 2006;367:36–43 [DOI] [PubMed] [Google Scholar]
- 11.Koo WW, Walters JC, Esterlitz J, Levine RJ, Bush AJ, Sibai B. Maternal calcium supplementation and fetal bone mineralization. Obstet Gynecol 1999;94:577–82 [DOI] [PubMed] [Google Scholar]
- 12.Raman L, Rajalakshmi K, Krishnamachari KA, Sastry JG. Effect of calcium supplementation to undernourished mothers during pregnancy on the bone density of the bone density of the neonates. Am J Clin Nutr 1978;31:466–9 [DOI] [PubMed] [Google Scholar]
- 13.Chang SC, O'brien KO, Nathanson MS, Caulfield LE, Mancini J, Witter FR. Fetal femur length is influenced by maternal dairy intake in pregnant African American adolescents. Am J Clin Nutr 2003;77:1248–54 [DOI] [PubMed] [Google Scholar]
- 14.Chan GM, McElligott K, McNaught T, Gill G. Effects of dietary calcium intervention on adolescent mothers and newborns: a randomized controlled trial. Obstet Gynecol 2006;108:565–71 [DOI] [PubMed] [Google Scholar]
- 15.Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ 2006;174:1273–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin J, Dwyer T, Riley M, Cochrane J, Jones G. The association between maternal diet during pregnancy and bone mass of the children at age 16. Eur J Clin Nutr 2010;64:131–7 [DOI] [PubMed] [Google Scholar]
- 17.Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod 2009;80:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenders CM, McElrath TF, Scholl TO. Nutrition in adolescent pregnancy. Curr Opin Pediatr 2000;12:291–6 [DOI] [PubMed] [Google Scholar]
- 19.Young BE, McNanley T, Cooper E, McIntyre AW, Witter F, Harris ZL, O'Brien KO. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with PTH and calcitriol in pregnant adolescents. J Bone Miner Res [Epub ahead of print 28 September 2011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Essley B, McNanley T, Cooper E, McIntyre AW, Witter F, Harris ZL, O'Brien KO. Osteoprotegerin (OPG) differs by race and is related to infant birth weight z-score in pregnant adolescents. J Dev Orig Health Dis 2011;2:272–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chitty LS, Altman DG. Charts of fetal size: limb bones. BJOG 2002;109:919–29 [DOI] [PubMed] [Google Scholar]
- 22.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr 1995;61:638S–45S [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine Dietary Reference Intakes for calcium and vitamin D. Washington, DC: National Academy of Sciences Press, 2011 [Google Scholar]
- 24.Chen TC, Turner AK, Holick MF. A method for the determination of the circulating concentration of 1,25-dihydroxyvitamin D. J Nutr Biochem 1990;1:320–7 [DOI] [PubMed] [Google Scholar]
- 25.Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME, Vitamin D. PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol (Oxf) 2009;70:372–7 [DOI] [PubMed] [Google Scholar]
- 26.Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Costigan KA, Dominici F, Dipietro JA. Randomized controlled trial of prenatal zinc supplementation and fetal bone growth. Am J Clin Nutr 2004;79:826–30 [DOI] [PubMed] [Google Scholar]
- 27.Bonen DK, Schmid TM. Elevated extracellular calcium concentrations induce type X collagen synthesis in chondrocyte cultures. J Cell Biol 1991;115:1171–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez L, Cheng Z, Chen TH, Tu C, Chang W. Extracellular calcium and parathyroid hormone-related peptide signaling modulate the pace of growth plate chondrocyte differentiation. Endocrinology 2005;146:4597–608 [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Palese T, Mishra OP, Delivoria-Papadopoulos M, De Luca F. Effects of Ca2+ sensing receptor activation in the growth plate. FASEB J 2004;18:143–5 [DOI] [PubMed] [Google Scholar]
- 30.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, Andersson S, Laitinen K, Lamberg-Allardt C. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab 2010;95:1749–57 [DOI] [PubMed] [Google Scholar]
- 31.Viljakainen HT, Korhonen T, Hytinantti T, Laitinen EK, Andersson S, Makjtie O, Lamberg-Allardt C. Maternal vitamin D status affects bone growth in early childhood – a prospective cohort study. Osteoporosis Int 2011;22(3):883–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St-Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys 2008;473:225–30 [DOI] [PubMed] [Google Scholar]
- 33.Naja RP, Dardenne O, Arabian A, St Arnaud R. Chondrocyte-specific modulation of Cyp27b1 expression supports a role for local synthesis of 1,25-dihydroxyvitamin D3 in growth plate development. Endocrinology 2009;150:4024–32 [DOI] [PubMed] [Google Scholar]
- 34.Johnson DD, Wagner CL, Hulsey TC, Mcneil RB, Ebeling M, Hollis BW, Vitamin D. Deficiency and insufficiency is common during pregnancy. Am J Perinatol 2011;28:7–12 [DOI] [PubMed] [Google Scholar]
- 35.Esterle L, Nguyen M, Walrant-Debray O, Sabatier JP, Garabedian M. Adverse interaction of low-calcium diet and low 25(OH)D levels on lumbar spine mineralization in late-pubertal girls. J Bone Miner Res 2010;25:2392–8 [DOI] [PubMed] [Google Scholar]
- 36.Sheikh MS, Ramirez A, Emmett M, Santa AC, Schiller LR, Fordtran JS. Role of vitamin D-dependent and vitamin D-independent mechanisms in absorption of food calcium. J Clin Invest 1988;81:126–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lafond J, Simoneau L. Calcium homeostasis in human placenta: role of calcium-handling proteins. Int Rev Cytol 2006;250:109–74 [DOI] [PubMed] [Google Scholar]
- 38.Belkacemi L, Bedard I, Simoneau L, Lafond J. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium 2005;37:1–8 [DOI] [PubMed] [Google Scholar]
- 39.Lagiou P, Mucci L, Tamimi R, Kuper H, Lagiou A, Hsieh CC, Trichopoulos D. Micronutrient intake during pregnancy in relation to birth size. Eur J Nutr 2005;44:52–9 [DOI] [PubMed] [Google Scholar]
- 40.Sabour H, Hossein-Nezhad A, Maghbooli Z, Madani F, Mir E, Larijani B. Relationship between pregnancy outcomes and maternal vitamin D and calcium intake: a cross-sectional study. Gynecol Endocrinol 2006;22:585–9 [DOI] [PubMed] [Google Scholar]
- 41.Scheuer L, Black S. The juvenile skeleton. San Diego, CA: Elsevier, 2004 [Google Scholar]
- 42.Shipp TD, Bromley B, Mascola M, Benacerraf B. Variation in fetal femur length with respect to maternal race. J Ultrasound Med 2001;20:141–4 [DOI] [PubMed] [Google Scholar]
- 43.Zelop CM, Borgida AF, Egan JF. Variation of fetal humeral length in second-trimester fetuses according to race and ethnicity. J Ultrasound Med 2003;22:691–3 [DOI] [PubMed] [Google Scholar]