Abstract
Background: Although the accumulation of white adipose tissue (WAT) is a risk factor for disease, brown adipose tissue (BAT) has been suggested to have a protective role against obesity.
Objective: We studied whether changes in BAT were related to changes in the amounts of subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) in children treated for malignancy.
Design: We examined the effect of BAT activity on weight, SAT, and VAT in 32 pediatric patients with cancer whose positron emission tomography–computed tomography (PET-CT) scans at diagnosis showed no BAT activity. Changes in weight, SAT, and VAT from diagnosis to remission for children with metabolically active BAT at disease-free follow-up (BAT+) were compared with those in children without visualized BAT when free of disease (BAT−).
Results: Follow-up PET-CT studies (4.7 ± 2.4 mo later) after successful treatment of the cancer showed BAT+ in 19 patients but no active BAT (BAT−) in 13 patients. BAT+ patients, in comparison with BAT− patients, gained significantly less weight (3.3 ± 6.6% compared with 11.0 ± 11.6%; P = 0.02) and had significantly less SAT (18.2 ± 26.5% compared with 67.4 ± 71.7%; P = 0.01) and VAT (22.6 ± 33.5% compared with 131.6 ± 171.8%; P = 0.01) during treatment. Multiple regression analysis indicated that the inverse relations between BAT activation and measures of weight, SAT, and VAT persisted even after age, glucocorticoid treatment, and the season when the PET-CT scans were obtained were accounted for.
Conclusion: The activation of BAT in pediatric patients undergoing treatment of malignancy is associated with significantly less adipose accumulation. This trial was registered at clinicaltrials.gov as NCT01517581.
INTRODUCTION
White and brown adipocytes differ in their expression of hormones, cytokines, and inflammatory factors and modulate different biological functions (1–3). Although white adipose tissue (WAT)5 serves as the primary site of energy storage, brown adipose tissue (BAT) instead metabolizes fat to produce heat and regulate body temperature (4–7). Available information from cross-sectional studies in patients undergoing positron emission tomography–computed tomography (PET-CT) examinations suggests that WAT and BAT are inversely related (4, 5, 8–10). Lean subjects exhibit greater BAT activity than do obese subjects, and several studies have observed a negative relation between body mass or body fat and the degree of metabolically active BAT (4, 5, 8–12). Additional support of the link between weight and BAT comes from animal studies that reported that a reduced amount or function of BAT led to obesity, insulin resistance, and dyslipidemia (13–15), whereas an increased amount or function protected against weight gain and its comorbidities (16–18).
Although BAT is likely present in all humans (19), the low prevalence of BAT depiction in adults and in elderly subjects has hindered longitudinal assessments of the relation between BAT activity and WAT. Under typical imaging conditions, BAT is detected more frequently in children and teenagers than in adults with malignancy (20–23). Because most children with cancer have significantly shorter treatment courses and greater survival rates than do adult patients (24), by selecting pediatric patients we have the ability to examine the relation of repeated measures of body composition and BAT. In this study, we longitudinally examined the relations between BAT activity and changes in weight and the amounts of subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), and abdominal muscle in children successfully treated for pediatric cancer.
SUBJECTS AND METHODS
Study subjects
Study subjects were patients seen regularly in the division of Hematology and Oncology at the Children's Hospital Los Angeles from October 2008 to September 2011. This study was compliant with the Health Insurance Portability and Accountability Act, and the investigational protocol was approved by the Institutional Review Board for clinical investigations at the Children's Hospital Los Angeles; informed consent was waived because all imaging was performed for clinical purposes. Eligibility criteria for this study included children ≤18 y of age who 1) had PET-CT scans with evidence of malignant disease but no metabolically active BAT at diagnosis and 2) were disease free within 1 y of diagnosis. With the use of this approach, we studied 32 pediatric patients (6.8–18.5 y of age); 28 patients had lymphoma (20 patients with Hodgkin, 4 patients with B cell, 3 patients with Burkitt, and 1 patient with anaplastic large cell lymphoma), 1 patient had acute lymphoblastic leukemia, 1 patient had melanoma, 1 patient had medullablastoma, and 1 patient had rhabdomyosarcoma. Of the 32 patients, 14 patients (all with lymphoma) were treated with glucocorticoids as part of their treatment regimen.
Outcome measures
Age, height, and weight measures were determined at the time of each PET-CT examination. The BMI-for-age percentile was calculated via the CDC's BMI-for-age growth chart calculator by using age, sex, and height and weight measurements (http://apps.nccd.cdc.gov/dnpabmi/Calculator.aspx). Body surface area (BSA) calculations were made by using the Dubois and Dubois formula as follows:
PET-CT scans were performed with a Gemini system (GXL Release 3.3; Philips Healthcare) in patients after 12 h of fasting. Patients were injected with fluorodeoxyglucose between 2.4 and 13.6 mCi, depending on body weight (0.14 mCi/kg) (22). The radiotracer was administered immediately after the serum glucose concentration was measured. PET scans were not performed if the glucose concentration was >200 mg/dL. All studies were performed after intravenous and oral contrast (meglumine diatrizoate; Gastrografin, Bristol-Myers Squibb) enhancement as set forth by the PET-CT protocol of the imaging department of the Children's Hospital Los Angeles. No muscle relaxants or additional agents were administered, and all patients were indoors at room temperature (22°C) for ≥2 h before the examination. Hospital gowns were worn during the radiologic study as well as for the 2 h immediately before the study. The total acquisition time for each study was 1 h. The axial in-plane spatial resolutions for PET and CT slices were 4 and 1.17 mm, respectively, and slice thicknesses were 4 and 5 mm, respectively.
All PET-CT examinations were reviewed independently by 2 radiologists to determine the presence or absence of metabolically active BAT. A patient was considered to have BAT when both radiologists diagnosed its presence. Studies with discrepant assessments were reevaluated by both radiologists together to arrive at a consensus. Subjects were categorized into 2 groups (BAT+ and BAT−) on the basis of the presence or absence of metabolically active BAT at disease-free follow-up examinations.
Measures of SAT, VAT, and abdominal musculature were obtained from the CT component at baseline and follow-up. Abdominal adipose tissue and muscle volumes were measured semiquantitatively on the basis of CT Hounsfield units (HU) by using an offline computer workstation running SliceOmatic image segmentation software (Tomovision Inc) (25). In brief, measurements of SAT and VAT were obtained by measuring all voxels with negative HU values (with exclusion of air in the bowel) in a 2.5-cm section at the level of the umbilicus (26). For the purposes of this study, SAT was defined as the volume (in cm3) of adipose tissue located between the skin and rectus muscles, the external oblique muscles, lumbar quadrate muscles, and the erector muscles of the spine, whereas VAT was defined as the intraabdominal adipose tissue surrounded by the rectus muscles, the oblique muscles, the lumbar quadrate muscle, the psoas muscles, and the vertebral body at the same site. At the same locations and from the same CT images, the volume of abdominal musculature (rectus muscles, oblique muscles, lumbar quadrate muscles, psoas muscles, and erector muscles of the spine) was determined by measuring all voxels with positive HU values (with exclusion of bone, viscera, vessels, and bowel) (27). CVs for CT measures of SAT, VAT, and abdominal musculature have been reported to be between 1.5% and 3.5% (28).
Statistical analyses
Statistical analyses were performed with Statview software (version 5.0.1; SAS Institute) by using unpaired t tests, chi-square tests, and simple and multiple linear regression analyses. The goodness-of-fit for regression models was evaluated with the postestimation procedures of STATA software (version 8 StataCorp). All models presented passed the following goodness-of-fit criteria: residuals appeared to be random and no strong influence or leverage points were present, on the basis of both a graphical and distribution evaluation. All values are expressed as means ± 1 SD, except when otherwise indicated.
RESULTS
The age, anthropometric characteristics, and CT measures of SAT, VAT, and abdominal musculature of the 32 patients at diagnosis are shown in Table 1. As shown in Table 2, 19 patients (13 boys and 6 girls) had metabolically active BAT when they were free of disease (BAT+), whereas 13 patients (7 boys and 6 girls) remained without visible BAT activity at follow-up (BAT−).
TABLE 1.
Characteristics of 32 pediatric cancer patients at diagnosis1
| All (n = 32) | Males (n = 20) | Females (n = 12) | |
| Age (y) | 13.8 ± 3.6 | 14.6 ± 3.0 | 12.6 ± 4.2 |
| Weight (kg) | 58.7 ± 20.8 | 62.1 ± 18.7 | 53.0 ± 23.6 |
| Height (cm) | 158.5 ± 19.9 | 165.6 ± 17.7 | 146.6 ± 18.0 |
| BMI-for-age percentile | 69.0 ± 29.4 | 63.2 ± 32.1 | 78.7 ± 22.3 |
| BSA (m2) | 1.6 ± 0.4 | 1.7 ± 0.3 | 1.4 ± 0.4 |
| SAT (cm3) | 465 ± 320 | 414 ± 313 | 550 ± 328 |
| VAT (cm3) | 70.9 ± 48.9 | 72.8 ± 49.7 | 67.7 ± 49.5 |
| Muscle (cm3) | 284 ± 93.4 | 315 ± 88.1 | 233 ± 81.4 |
All values are means ± SDs. BSA, body surface area; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
TABLE 2.
Characteristics of BAT+ and BAT− pediatric cancer patients at disease-free follow-up1
| All (n = 32) |
Males (n = 20) |
Females (n = 12) |
|||||||
| BAT+ (n = 19) | BAT− (n = 13) | P | BAT+ (n = 13) | BAT− (n = 7) | P | BAT+ (n = 6) | BAT− (n = 6) | P | |
| Age (y) | 15.0 ± 3.6 | 13.1 ± 3.4 | 0.16 | 15.1 ± 3.5 | 14.6 ± 2.1 | 0.73 | 14.7 ± 4.1 | 11.4 ± 4.0 | 0.20 |
| Weight (kg) | 65.4 ± 21.9 | 56.5 ± 18.6 | 0.24 | 68.0 ± 22.0 | 58.8 ± 10.8 | 0.32 | 59.9 ± 22.6 | 53.8 ± 26.0 | 0.67 |
| Height (cm) | 164.4 ± 19.6 | 151.6 ± 17.9 | 0.07 | 170.5 ± 18.7 | 159.0 ± 12.8 | 0.17 | 151.2 ± 15.3 | 142.9 ± 20.2 | 0.44 |
| BMI-for-age percentile | 70.6 ± 29.1 | 76.9 ± 33.6 | 0.57 | 64.3 ± 32.3 | 68.2 ± 41.1 | 0.82 | 84.3 ± 14.9 | 87.2 ± 20.9 | 0.79 |
| BSA (m2) | 1.71 ± 0.4 | 1.5 ± 0.3 | 0.07 | 1.8 ± 0.4 | 1.6 ± 0.2 | 0.12 | 1.5 ± 0.4 | 1.4 ± 0.4 | 0.53 |
| SAT (cm3) | 547 ± 329 | 596 ± 338 | 0.69 | 473 ± 314 | 521 ± 289 | 0.74 | 709 ± 329 | 684 ± 397 | 0.91 |
| VAT (cm3) | 91.2 ± 57.0 | 89.2 ± 32.0 | 0.92 | 81.3 ± 52.8 | 91.3 ± 31.9 | 0.64 | 112.8 ± 64.9 | 86.8 ± 34.9 | 0.41 |
| Muscle (cm3) | 322 ± 97.3 | 251 ± 80.2 | 0.036 | 349 ± 101 | 284 ± 66.0 | 0.14 | 264 ± 62.8 | 211 ± 82.6 | 0.24 |
All values are means ± SDs. P values were obtained by using unpaired t tests. BAT−, subjects without metabolically active brown adipose tissue at disease-free follow-up; BAT+, subjects with metabolically active brown adipose tissue at disease-free follow-up; BSA, body surface area; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Sex did not influence the incidence of BAT depiction (in 65% of boys compared with 50% of girls; P = 0.70). The time interval between the initial diagnosis and disease-free PET-CT scans was 4.7 ± 2.4 mo and was similar for BAT+ and BAT− patients (4.8 ± 2.6 compared with 4.6 ± 2.1 mo; P = 0.82). There was also no difference in BAT depiction between patients treated with or without glucocorticoids (57% compared with 61%; P = 0.82). In contrast, there was a tendency for more occurrences of BAT+ during the winter months, which, for the purpose of this study, were November, December, and January, than during the remaining months (82% compared with 48%; P = 0.06).
There were no significant differences in the age, anthropometric measures, or CT values of SAT and VAT between BAT+ and BAT− groups at follow-up; this was true regardless of whether all subjects were analyzed together or whether boys and girls were analyzed separately (Table 2). When all children were analyzed together, values for abdominal muscle were greater in BAT+ subjects.
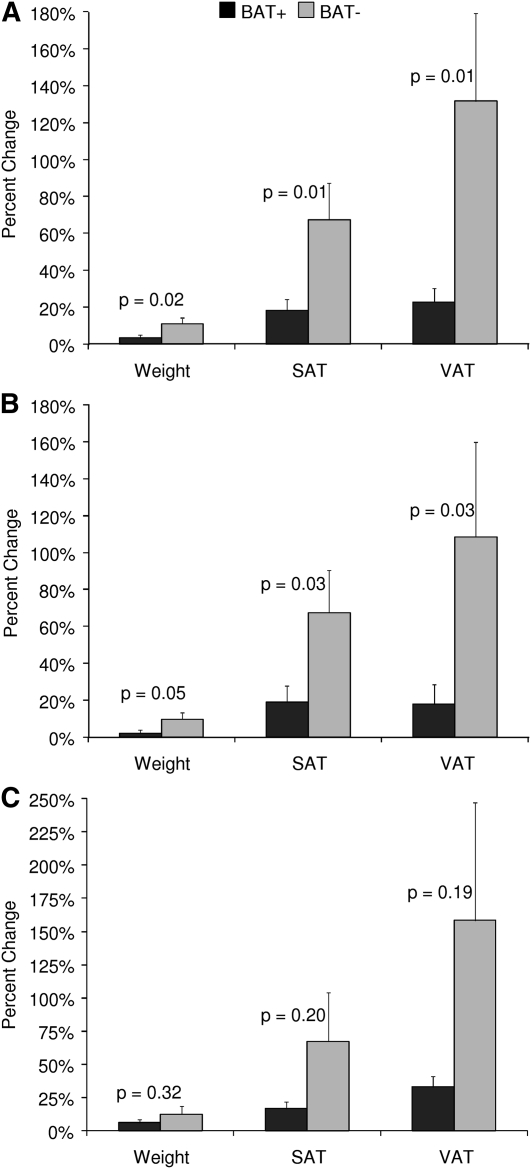
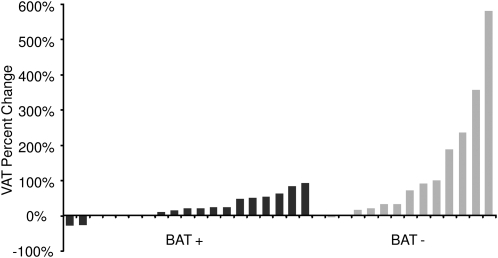
Percentage gains in weight, SAT, and VAT were significantly greater in BAT− subjects than in BAT+ subjects (Figure 1). This was true for all subjects together and when boys were analyzed separately (Figure 1, A and B). Although the percentage gains in weight, SAT, and VAT also were greater in girls without BAT, these changes were not significant (Figure 1C). The greatest difference in adiposity between BAT+ and BAT− subjects was observed in the VAT depot. Changes in visceral adiposity for each patient are shown in Figure 2. There was no difference in the percentage change in abdominal musculature between BAT groups (4.7% in BAT+ patients compared with 3.5% in BAT− patients; P = 0.77).
FIGURE 1.
Mean (±SEM) percentage changes in weight, SAT, and VAT in 32 pediatric cancer patients with respect to BAT depiction at disease-free follow-up (BAT+, n = 19; BAT−, n = 13) (A), 20 male pediatric cancer patients with respect to BAT depiction at disease-free follow-up (BAT+, n = 13; BAT−, n = 7) (B), and 12 female pediatric cancer patients with respect to BAT depiction at disease-free follow-up (BAT+, n = 6; BAT−, n = 6) (C). P values were obtained by using unpaired t tests. BAT, brown adipose tissue; BAT−, subjects without metabolically active brown adipose tissue at disease-free follow-up; BAT+, subjects with metabolically active brown adipose tissue at disease-free follow-up; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
FIGURE 2.
Percentage changes in VAT in 32 pediatric cancer patients with respect to BAT depiction at disease-free follow-up. BAT, brown adipose tissue; BAT−, subjects without metabolically active brown adipose tissue at disease-free follow-up; BAT+, subjects with metabolically active brown adipose tissue at disease-free follow-up; VAT, visceral adipose tissue.
Compared with the 18 patients whose treatment regimen did not include glucocorticoid therapy, the 14 patients treated with glucocorticoids did not have significant gains in weight (8 ± 12.4% compared with 5 ± 6.7%; P = 0.28), SAT (58 ± 66% compared with 23 ± 40%; P = 0.07), or VAT (101 ± 155% compared with 40 ± 85%; P = 0.16). However, in patients treated with glucocorticoids, gains in VAT were significantly greater in BAT− patients than in BAT+ patients (202 ± 200% compared with 26 ± 30%; P = 0.029). Multiple linear regression analyses indicated that BAT depiction had a negative association with the percentage change in weight, SAT, and VAT independent of age, season, and whether or not the patients were treated with glucocorticoids (Table 3).
TABLE 3.
Multiple regression models for the prediction of percentage change in weight, SAT, and VAT in 32 pediatric cancer patients at disease-free follow-up1
| B | σ | β | P | |
| Percentage change in weight | ||||
| BAT+/minus | −0.074 | 0.033 | −0.385 | 0.033 |
| Age | −0.009 | 0.005 | −0.334 | 0.057 |
| Glucocorticoids+/minus | 0.028 | 0.032 | 0.147 | 0.387 |
| Season2 | 0.045 | 0.034 | 0.226 | 0.198 |
| Percentage change in SAT | ||||
| BAT+/minus | −0.527 | 0.189 | −0.480 | 0.010 |
| Age | −0.014 | 0.026 | −0.091 | 0.593 |
| Glucocorticoids+/minus | 0.351 | 0.182 | 0.323 | 0.064 |
| Season2 | 0.233 | 0.195 | 0.205 | 0.243 |
| Percentage change in VAT | ||||
| BAT+/minus | −1.110 | 0.432 | −0.452 | 0.016 |
| Age | −0.056 | 0.059 | −0.163 | 0.353 |
| Glucocorticoids+/minus | 0.566 | 0.417 | 0.233 | 0.186 |
| Season2 | 0.452 | 0.446 | 0.178 | 0.320 |
B, unstandardized coefficient; BAT+/−, presence or absence of metabolically active brown adipose tissue at disease-free follow-up; glucocorticoids+/−, presence or absence of glucocorticoids in treatment regimen; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; β, standardized coefficient; σ, SE of unstandardized coefficient.
Winter (November, December, and January) compared with other seasons.
DISCUSSION
We studied the effect of BAT activation on the weight and measures of adiposity in children successfully treated for malignancy whose PET-CT scan at diagnosis showed no evidence of BAT function. Children and teenagers who had evidence of BAT activity at follow-up PET-CT studies gained significantly less weight and had significantly less subcutaneous and visceral adiposity than did those who remained without BAT activity when disease free. On average, increases in weight and SAT were 3 times greater, and increases in VAT were 6 times greater, in patients who did not show any BAT activity compared with patients who had metabolically active BAT. In accordance with previous investigations, we showed that BAT becomes more metabolically activated during the winter months (21, 29), even in patients living in sunny California (23). Consistent with the need for nonshivering thermogenesis, BAT activity is greater in colder weather (30). This was even seen over narrow temperature ranges, as in previous studies that reported BAT depiction in nearly all PET-CT examinations of young men when obtained at 16°C (31) but in only one-half of young men when obtained at 19°C (32). However, the effect of BAT activity on changes in weight, SAT, or VAT persisted even after age, glucocorticoid treatment, and season were accounted for. Altogether, the findings of this longitudinal study provide strong evidence that the metabolic activation of BAT is inversely related to changes in adiposity.
Approximately one-half of the patients in our cohort were treated with high doses of glucocorticoids as part of the treatment protocol. However, we showed that BAT activity was not influenced by glucocorticoid treatment. Previous data on the influence of glucocorticoids on BAT were primarily based on animal studies and were conflicting. Although some studies suggested that glucocorticoids enhance the metabolic activity of BAT (33), other studies reported an inactivating effect of the glucocorticoid system on BAT, with brown adipocytes that became more lipid filled and white adipocyte-like (34, 35). Other studies showed no effect on BAT mass in rats, despite a marked reduction in visceral WAT after adrenalectomy (36).
Glucocorticoids are known to promote central adiposity (37), and as expected, glucocorticoid-treated patients gained more visceral adiposity than did patients who were not treated with glucocorticoids. It should be noted that in glucocorticoid-treated patients, those who showed no BAT activity gained significantly more visceral adiposity than did those with metabolically active BAT when disease free. Although both glucocorticoid treatment and BAT activity had competing effects on the accumulation of VAT, only BAT function was a significant predictor of gains in VAT. This observation was consistent with available cross-sectional data that suggested that measures of central adiposity, even more than BMI, are inversely correlated with the amount of BAT (5, 38).
Although the basis for the negative relation observed between BAT and WAT has yet to be fully defined, it is likely the reflection of greater energy dissipation and increased energy expenditure associated with BAT activity. BAT contains a unique protein, uncoupling protein 1, which uncouples oxidative phosphorylation and releases energy stored in the mitochondria as heat (7, 8, 39, 40). BAT activity positively correlates with resting metabolic rate (9), and Virtanen et al (40) have estimated that ∼63 g BAT could combust the energy equivalent of 4.1 kg WAT over 1 y. Additional support for the link between BAT and energy balance comes from observations that showed that BAT thermogenesis protected against diet-induced weight gains in animals lacking BAT (41–44). However, it is also possible that WAT suppresses BAT function. WAT is known to produce cytokines and chemokines, such as TNF-α, IL-6, and monocyte chemoattractant protein 1, that induce inflammation (45–48) and could potentially have cytotoxic effects on BAT. Indeed, high concentrations of TNF-α have been shown to induce apoptotic degeneration of brown adipocytes (49). Studies are needed to determine the direction of causality between BAT activity and WAT accumulation.
Regardless of the mechanisms that account for the reciprocal relation between BAT and WAT, these 2 adipose tissues differ in structure, function, and lineage, as evidenced by their differing gene-expression profiles; thus, WAT is akin to macrophages, but BAT resembles muscle (50–52). We recently observed that children with metabolically active BAT had significantly greater muscle volume than did those without identifiable BAT (23), and that large increases in the amount of BAT, such as that of skeletal muscle, occur during puberty (53, 54). These clinical observations were consistent with data from cell cultures that indicated that brown adipocytes and myocytes may derive from a common lineage in the paraxial mesoderm (55, 56). Although, in the current study, we observed that subjects with visualized BAT had greater musculature than did those without visualized BAT, we did not find differences in the percentage of change in muscle between groups. The degree to which our results were confounded by the effect of disease severity, inactivity, and glucocorticoid therapy on muscle mass is unknown.
The factors behind the occurrence of metabolically active BAT after treatment of cancer are unknown, and few studies have previously examined the relation between BAT and malignancy. We recently observed an inverse association between the presence of metabolically active BAT and the presence of tumors in children with lymphoma (57), and the possibility exists that malignancy suppresses BAT function. Patients with malignant lymphomas have high circulating concentrations of TNF-α (30, 49, 58, 59). The apoptotic degeneration of brown adipocytes has been reported to be mediated by the p55 TNF-α receptor subtype (58), and its deletion has been shown to increase thermogenesis with an associated increase of uncoupling protein 1 in BAT (30, 49, 58, 59). However, it is unknown whether disease influences BAT status after treatment, and additional studies are needed to determine the mechanisms that control BAT activation after disease treatment.
To decrease the potential for confounding bias, we purposely chose to study children with visible tumors but no visualization of BAT on the initial PET-CT scans. Shorter treatment periods, higher cure rates, and greater BAT prevalence in pediatric patients when compared with adults strengthened the value of our cohort. The use of sophisticated imaging technology, the evaluation of both white adipose depots, and the longitudinal assessments were additional strengths of the study.
This study had several notable limitations. It was based on the analysis of PET-CT examinations, and BAT depiction was used as the sole measure of BAT. However, the validity of this outcome measure was supported by recent histologic data from Lee et al (19) that indicated that the amount of BAT was related to its activity and showed a greater abundance of brown fat in BAT-positive than in BAT-negative PET studies. Moreover, our data were based on retrospective examinations of children treated for malignancy, and we could not control for the potential effects of the severity of disease, treatment regimen, diet, or physical activity on BAT activity. By selecting patients with disease-free PET-CT scans within 12 mo of diagnosis, the confounders associated with not recruiting healthy subjects were somewhat minimized. Unfortunately, no technique is currently available for safe prospective examinations of BAT in pediatric populations. Radiation exposure from PET-CT imaging precludes such studies in normal, healthy subjects.
In conclusion, the adipose organ is not homogenous but composed of brown and white adipocytes that, paradoxically, have contrasting implications for metabolic health, with WAT serving as a risk factor for disease and with BAT suggested as having a protective role. Previous evidence that suggested an inverse association between BAT and WAT was based on cross-sectional data that showed that BAT activity correlates inversely with BMI in adult and pediatric populations (4, 5, 8–11, 38). Our longitudinal study provides additional evidence in support of the notion that BAT activity prevents weight gain and the accumulation of subcutaneous and, more strikingly, visceral adiposity. Such findings underscore the potential importance of metabolic activity of BAT in the regulation of body weight, which may be harnessed for the treatment of obesity (39, 60, 61).
Acknowledgments
The authors’ responsibilities were as follows—VG and CHF: designed the research; VG and FG: conducted the research; JSC, MLS, and FJD: analyzed the data; VG, JSC, MLS, and HHH: wrote the manuscript; VG: had primary responsibility for the final content of the manuscript; and all authors: participated intellectually and practically in this work, including in the conception, design, and conduct of the experiment, participated in data interpretation, and read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: BAT, brown adipose tissue; BAT−, without metabolically active brown adipose tissue at disease-free follow-up; BAT+, with metabolically active brown adipose tissue at disease-free follow-up; BSA, body surface area; CT, computed tomography; HU, Hounsfield units; PET, positron emission tomography; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; WAT, white adipose tissue.
REFERENCES
- 1.Trayhurn P, Drevon CA, Eckel J. Secreted proteins from adipose tissue and skeletal muscle—adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem 2011;117:47–56 [DOI] [PubMed] [Google Scholar]
- 2.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci 2009;54:1847–56 [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res 2009;104:541–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enerbäck S. Human brown adipose tissue. Cell Metab 2010;11:248–52 [DOI] [PubMed] [Google Scholar]
- 7.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 8.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009;23:3113–20 [DOI] [PubMed] [Google Scholar]
- 9.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–8 [DOI] [PubMed] [Google Scholar]
- 10.Rousseau C, Bourbouloux E, Campion L, Fleury N, Bridji B, Chatal JF, Resche I, Campone M. Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging 2006;33:785–91 [DOI] [PubMed] [Google Scholar]
- 11.Drubach LA, Palmer EL, III, Connolly LP, Baker A, Zurakowski D, Cypess AM. Pediatric brown adipose tissue: detection, epidemiology, and differences from adults. J Pediatr 2011;159:939–44 [DOI] [PubMed] [Google Scholar]
- 12.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2010;299:E601–6 [DOI] [PubMed] [Google Scholar]
- 13.Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993;366:740–2 [DOI] [PubMed] [Google Scholar]
- 14.Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology 1996;137:21–9 [DOI] [PubMed] [Google Scholar]
- 15.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009;9:203–9 [DOI] [PubMed] [Google Scholar]
- 16.Kim JK, Kim HJ, Park SY, Cederberg A, Westergren R, Nilsson D, Higashimori T, Cho YR, Liu ZX, Dong J, et al. Adipocyte-specific overexpression of FOXC2 prevents diet-induced increases in intramuscular fatty acyl CoA and insulin resistance. Diabetes 2005;54:1657–63 [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet 2003;35:49–56 [DOI] [PubMed] [Google Scholar]
- 18.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 2001;106:563–73 [DOI] [PubMed] [Google Scholar]
- 19.Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR, Freund J, Ho KK. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab 2011;96:2450–5 [DOI] [PubMed] [Google Scholar]
- 20.Bar-Sever Z, Keidar Z, Ben-Barak A, Bar-Shalom R, Postovsky S, Guralnik L, Ben Arush MW, Israel O. The incremental value of 18F-FDG PET/CT in paediatric malignancies. Eur J Nucl Med Mol Imaging 2007;34:630–7 [DOI] [PubMed] [Google Scholar]
- 21.Zukotynski KA, Fahey FH, Laffin S, Davis R, Treves ST, Grant FD, Drubach LA. Seasonal variation in the effect of constant ambient temperature of 24 degrees C in reducing FDG uptake by brown adipose tissue in children. Eur J Nucl Med Mol Imaging 2010;37:1854–60 [DOI] [PubMed] [Google Scholar]
- 22.Gelfand MJ, O'Hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol 2005;35:984–90 [DOI] [PubMed] [Google Scholar]
- 23.Gilsanz V, Chung SA, Jackson H, Dorey FJ, Hu HH. Functional brown adipose tissue is related to muscle volume in children and adolescents. J Pediatr 2011;158:722–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizzo PA, Poplack DG, Principles and practice of pediatric oncology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2006 [Google Scholar]
- 25.Bonekamp S, Ghosh P, Crawford S, Solga SF, Horska A, Brancati FL, Diehl AM, Smith S, Clark JM. Quantitative comparison and evaluation of software packages for assessment of abdominal adipose tissue distribution by magnetic resonance imaging. Int J Obes (Lond) 2008;32:100–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–6 [DOI] [PubMed] [Google Scholar]
- 27.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 2000;89:104–10 [DOI] [PubMed] [Google Scholar]
- 28.Arfai K, Pitukcheewanont P, Goran MI, Tavare CJ, Heller L, Gilsanz V. Bone, muscle, and fat: sex-related differences in prepubertal children. Radiology 2002;224:338–44 [DOI] [PubMed] [Google Scholar]
- 29.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med 2003;44:1267–70 [PubMed] [Google Scholar]
- 30.Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, Milanski M, Moraes JC, Bonfleur ML, Degasperi GR, Picardi PK, et al. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem 2009;284:36213–22 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Allen-Auerbach M, Quon A, Weber WA, Obrzut S, Crawford T, Silverman DH, Ratib O, Phelps ME, Czernin J. Comparison between 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography and positron emission tomography/computed tomography hardware fusion for staging of patients with lymphoma. Mol Imaging Biol 2004;6:411–6 [DOI] [PubMed] [Google Scholar]
- 32.Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Häring HU, Claussen CD, Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 2010;59:1789–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mostyn A, Pearce S, Stephenson T, Symonds ME. Hormonal and nutritional regulation of adipose tissue mitochondrial development and function in the newborn. Exp Clin Endocrinol Diabetes 2004;112:2–9 [DOI] [PubMed] [Google Scholar]
- 34.Soumano K, Desbiens S, Rabelo R, Bakopanos E, Camirand A, Silva JE. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol Cell Endocrinol 2000;165:7–15 [DOI] [PubMed] [Google Scholar]
- 35.Nieman LK, Ilias I. Evaluation and treatment of Cushing's syndrome. Am J Med 2005;118:1340–6 [DOI] [PubMed] [Google Scholar]
- 36.Berthiaume M, Sell H, Lalonde J, Gélinas Y, Tchernof A, Richard D, Deshaies Y. Actions of PPARgamma agonism on adipose tissue remodeling, insulin sensitivity, and lipemia in absence of glucocorticoids. Am J Physiol Regul Integr Comp Physiol 2004;287:R1116–23 [DOI] [PubMed] [Google Scholar]
- 37.Stewart PM. The adrenal cortex. : Larsen PR, Kronenberg H, Melmed S, Polonsky K, Williams Textbook of Endocrinology. 11th ed. Philadelphia, PA: WB Saunders Company, 2008:445–503 [Google Scholar]
- 38.Wang Q, Zhang M, Ning G, Gu W, Su T, Xu M, Li B, Wang W. Brown adipose tissue in humans is activated by elevated plasma catecholamines levels and is inversely related to central obesity. PLoS ONE 2011;6:e21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes (Lond) 2008;32(suppl 7):S32–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–25 [DOI] [PubMed] [Google Scholar]
- 41.Hamann A, Flier JS, Lowell BB. Obesity after genetic ablation of brown adipose tissue. Z Ernahrungswiss 1998;37(suppl 1):1–7 [PubMed] [Google Scholar]
- 42.Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell 2005;4:147–55 [DOI] [PubMed] [Google Scholar]
- 43.Hansen JB, Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem J 2006;398:153–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond) 1983;64:19–23 [DOI] [PubMed] [Google Scholar]
- 45.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:367–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cartier A, Lemieux I, Almeras N, Tremblay A, Bergeron J, Despres JP. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab 2008;93:1931–8 [DOI] [PubMed] [Google Scholar]
- 47.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 2007;116:1234–41 [DOI] [PubMed] [Google Scholar]
- 48.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 49.Nisoli E, Briscini L, Tonello C, De Giuli-Morghen C, Carruba MO. Tumor necrosis factor-alpha induces apoptosis in rat brown adipocytes. Cell Death Differ 1997;4:771–8 [DOI] [PubMed] [Google Scholar]
- 50.Charrière G, Cousin B, Arnaud E, André M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem 2003;278:9850–5 [DOI] [PubMed] [Google Scholar]
- 51.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA 2007;104:4401–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephens M, Ludgate M, Rees DA. Brown fat and obesity: the next big thing? Clin Endocrinol (Oxf) 2011;74:661–70 [DOI] [PubMed] [Google Scholar]
- 53.Lowrey GH. Growth and development of children. 8th ed. Chicago, IL: Year Book Medical Publishers Inc, 1986 [Google Scholar]
- 54.Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr (Epub ahead of print 31 October 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol 2004;36:585–97 [DOI] [PubMed] [Google Scholar]
- 56.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol 2009;217:318–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilsanz V, Hu HH, Smith ML, Goodarzian F, Carcich SL, Warburton NM, Malogolowkin M. The depiction of brown adipose tissue is related to disease status in pediatric patients with lymphoma. Am J Roentgenol 2011. (in press) [DOI] [PubMed] [Google Scholar]
- 58.Salles G, Bienvenu J, Bastion Y, Barbier Y, Doche C, Warzocha K, Gutowski MC, Rieux C, Coiffier B. Elevated circulating levels of TNFalpha and its p55 soluble receptor are associated with an adverse prognosis in lymphoma patients. Br J Haematol 1996;93:352–9 [DOI] [PubMed] [Google Scholar]
- 59.Warzocha K, Salles G, Bienvenu J, Bastion Y, Dumontet C, Renard N, Neidhardt-Berard EM, Coiffier B. Tumor necrosis factor ligand-receptor system can predict treatment outcome in lymphoma patients. J Clin Oncol 1997;15:499–508 [DOI] [PubMed] [Google Scholar]
- 60.Cannon B, Nedergaard J. Thermogenesis challenges the adipostat hypothesis for body-weight control. Proc Nutr Soc 2009;68:401–7 [DOI] [PubMed] [Google Scholar]
- 61.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 2010;11:268–72 [DOI] [PubMed] [Google Scholar]