Abstract
Background: Longitudinal growth associations with genetic variants identified for adult BMI may provide insights into the timing of obesity susceptibility.
Objective: The objective was to explore associations of known BMI loci with measures of body size from birth to adulthood.
Design: A total of 2537 individuals from a longitudinal British birth cohort were genotyped for 11 genetic variants robustly associated with adult BMI (in/near FTO, MC4R, TMEM18, GNPDA2, KCTD15, NEGR1, BDNF, ETV5, SEC16B, SH2B1, and MTCH2). We derived an obesity-risk-allele score, comprising the sum of BMI-increasing alleles in each individual, and examined this for an association with birth weight and repeated measures of weight, height, and BMI SD scores (SDS) at 11 time points between ages 2 and 53 y.
Results: The obesity-risk-allele score showed borderline significant association with birth weight (0.019 SDS/allele; P = 0.05) and was more clearly associated with higher weight and BMI at all time points between ages 2 and 53 y; the strongest associations with weight occurred at ages 11 and 20 y (both 0.056 SDS/allele). In longitudinal analyses, the score was positively associated with weight gain only between birth and 11 y (0.003 SDS/allele per year; 95% CI: 0.001, 0.004; P = 0.001). The risk-allele score was associated with taller height at 7 y (0.031 SDS/allele; P = 0.002) and greater height gains between 2 and 7 y (0.007 SDS/allele per year; P < 0.001), but not with adult height (P = 0.5).
Conclusions: The combined effect of adult obesity susceptibility variants on weight gain was confined to childhood. These variants conferred a faster tempo of height growth that was evident before the pubertal years.
INTRODUCTION
Accumulation of body fat resulting in overweight and obesity is a gradual process that usually occurs over many years (1). It is likely that the tendency to gain weight excessively and its significance for later obesity risk is not constant throughout the life span (2). Whereas the rising level of obesity in recent decades is attributable to lifestyle and environmental changes, genetic differences between individuals may explain why some people gain more weight than others in the same obesogenic setting. Evidence indicates that genetic influences on BMI vary with age; BMI heritability estimates appear to become progressively stronger during childhood (3) but decrease into adulthood (4). In addition, heritability estimates of BMI change appear to be higher in adolescence than in young adulthood (5).
Common genetic variants that are robustly associated with adult BMI were recently described in or near to FTO, MC4R, TMEM18, SH2B1, KCTD15, BDNF, MTCH2, NEGR1, SEC16B, GNPDA2, and ETV5 (6–9). Several of these loci show larger cross-sectional associations with standardized BMI in children and adolescents than in adults (10). Smaller studies with rich phenotype data are useful to further characterize the timing of effects of these polymorphisms, although such studies are often underpowered to replicate the small effects of individual single nucleotide polymorphisms (SNPs)4 detected in larger meta-analyses of genome-wide association studies. Longitudinal associations between weight gain and the 2 BMI loci with the largest effect sizes in adults, at FTO and MC4R, were previously described and show that the effects of these loci on BMI increase over childhood and decrease in adulthood (11, 12). We previously described the association between a genetic predisposition score of adult BMI-increasing alleles across 8 loci and increased childhood weight gain in the contemporary Avon Longitudinal Study of Parents and Children (ALSPAC) population (13). Such multiple allele scores increase statistical power and also may be taken to represent a more generalizable influence of genetic obesity susceptibility compared with analyses of individual variants. However, because measures of adult body size were yet unavailable in ALSPAC, it was unclear whether this obesity-risk-allele score conferred susceptibility to additional weight gain beyond the age of 11 y.
To explore how genetic susceptibility to higher BMI influences changes in weight from birth into adulthood, longitudinal associations between a multiple risk-allele score for adult BMI and measures of growth were tested in the Medical Research Council National Survey of Health and Development (MRC NSHD), also known as the 1946 British Birth Cohort Study. The aim was to identify the periods of life during which individuals with a greater genetic susceptibility to obesity show greater gains in weight and BMI than those with lower genetic susceptibility. Knowledge of these patterns of growth and weight gain could help to inform the mechanisms of action of the genetic susceptibility to obesity.
SUBJECTS AND METHODS
Study sample
The NSHD is a socially stratified birth cohort of 2547 men and 2815 women of white European descent born during one week in March 1946 who have been followed with repeated data collections since then (14). At age 53 y, 1472 men and 1563 women either were interviewed and examined in their own home by trained research nurses (n = 2989) or completed postal questionnaires (n = 46). Contact was not attempted for 1510 individuals who had previously refused to take part, were living abroad, or were untraced since the last contact at 43 y. A total of 469 individuals had already died. The remaining individuals who were interviewed at 53 y were on the whole representative of the native born population of that age (15). Blood samples for DNA extraction were collected at the 53-y measurement from 2756 members. The study received Multi-Centre Research Ethics Committee approval, and informed consent was given by cohort participants.
Anthropometric measures
Birth weight to the nearest 0.25 lb (113 g) was extracted from medical records and converted to kilograms. Heights and weights were measured by using standard protocols at ages 2, 4, 6, 7, 11, 15, 36, 43, and 53 y and self reported at ages 20 and 26 y. Mothers’ weight and height were measured where possible, or sometimes self-reported, when study members were 6 y of age.
Genotyping information
DNA was extracted and purified from whole blood by using the Puregene DNA isolation Kit (Flowgen) according to the manufacturer's protocol. rs1421085 in FTO and rs17782313 near MC4R were genotyped by Source Bioscience PLC with the use of Applied Biosystems SNPlex technology (Foster Cirt), which is based on an Oligonucleotide Ligation Assay combined with multiplex PCR amplification and capillary electrophoresis. rs6548238 (TMEM18), rs8055138 (SH2B1), rs11084753 (KCTD15), rs10838738 (MTCH2), rs2815752 (NEGR1), rs925946 (BDNF), and rs10913469 (SEC16B) were typed by using the Sequenom iPLEX platform (Sequenom), and rs10938397 (GNPDA2) and rs7647305 (ETV5) were genotyped by using custom TaqMan SNP genotyping assays according to the manufacturer's protocol (Applied Biosystems). Call rates for all SNPs were >95%, and allelic distributions were in Hardy-Weinberg equilibrium (P > 0.05) (see Supplementary Table 1 under “Supplemental data” in the online issue).
Statistical methods
BMI at each measurement and maternal BMI were calculated as weight (in kg) divided by height (in m) squared. Measures of BMI, weight, and height were converted into SD scores (SDS) by using internally generated growth charts, which are preferable for historical cohorts and were constructed by using the LMS (L = skewness; M = median; S = coefficient) method (16).
Missing genotypes were imputed in individuals who had successful genotype calls at ≥7 of the 11 loci by assigning the mean number of BMI-increasing alleles at each locus from individuals with successfully called genotypes. This ensured that individuals who were missing genotypes at only a small number of the variants were not excluded from the risk score analyses, thereby maximizing the statistical power to detect associations. The obesity-risk-allele score was then derived by summing the number of genotyped or imputed BMI-increasing alleles across the 11 loci in each individual. Differences in body size, level of education, and maternal characteristics between included and excluded individuals based on availability of the obesity-risk-allele score were tested by using chi-square and t tests.
In initial cross-sectional analyses, the risk-allele score was tested for linear association with birth weight as well as BMI, weight, and height SDS at each time point between the ages of 2 and 53 y, with adjustment for sex, and additional adjustment for the precise age at measurement in childhood when available (at the 4-, 6-, 7-, 11-, and 15-y visits). The association analyses for birth weight were also adjusted for mother's BMI to remove any potential confounding by intrauterine effects related to mother's genotype. Score-by-sex interaction terms were not significant and therefore all associations were examined in men and women combined.
Multilevel modeling was used to test the association between the risk-allele score and changes in weight, BMI, and height SDS with age by using xtmixed in Stata. This approach takes correlations between repeated measures on the same individual into account and allows for missing measurement data assuming that data are missing at random. We initially tested the association of the risk-allele score with linear change in weight SDS between birth and 53 y, BMI between 2 and 53 y, and height between 2 and 20 y (when final adult height was assumed to be attained). Next, we tested quadratic and cubic models by sequentially adding age2 and age3 and the interaction terms risk-allele-score × age2 and risk-allele-score × age3. The significance of each additional term was tested by using Wald test statistics. Predicted models for trajectories of BMI and weight SDS by tertile of obesity-risk-allele score were plotted in Stata based on models that considered a quadratic influence of age, because the cubic models were not significant. Because the effect of the risk-allele score on body size varied with age, for ease of interpretation of longitudinal models we stratified the data set to generate linear β coefficients from multilevel models that represent the effects of the risk-allele score on gains in weight, height, and BMI in separate age periods. Growth periods were divided according to the timing of the observed peak in effect size in cross-sectional analyses; we modeled periods from birth (2 y for BMI) to 11 y and from 11 to 53 y for weight and BMI SDS and from 2 to 7 y and from 7 to 20 y for height SDS.
To verify that the observed associations were not driven solely by the variants in FTO and MC4R, which tend to have the largest effect sizes on adult BMI and were described previously in the NSHD population (11), additional association analyses were performed by using a 9-SNP obesity-risk-allele-score excluding these 2 loci. Second, to allow comparison with the effect sizes on childhood growth reported in the ALSPAC study (13), we repeated the analyses using the same 8-SNP risk-allele score used in that study, which excluded the SH2B1 and MTCH2 variants that had shown no individual association with childhood BMI (10), as well as the SEC16B variant, which was not available in ALSPAC. All analyses were performed by using Stata version 11.0.
RESULTS
The NSHD population
A total of 2117 members of NSHD were successfully genotyped across all 11 BMI loci, whereas a further 420 had genotype data on ≥7 variants. The imputed obesity-risk-allele score was therefore derived in 2537 participants (1272 men and 1265 women) and was normally distributed, with all individuals carrying between 4 and 17 alleles out of the possible range of 0 to 22 alleles (see Supplementary Figure 1 under “Supplemental data” in the online issue). Details of these 2537 participants are shown in Table 1. Compared with NSHD members who were excluded because of missing DNA or genotype data on >4 loci, those included in the current analysis tended to have higher educational qualifications, were slightly taller from age 7 y onward, and had a slightly lower BMI at age 53 y (P < 0.05; see Supplementary Table 2 under “Supplemental data” in the online issue).
TABLE 1.
Summary of growth data by age and sex in 2537 individuals from the National Survey of Health and Development1
| Men |
Women |
|||||
| No. of observations | Mean | SD | No. of observations | Mean | SD | |
| Weight (kg) | ||||||
| Birth | 1268 | 3.5 | 0.5 | 1262 | 3.3 | 0.5 |
| 2 y | 1068 | 13.2 | 1.5 | 1039 | 12.7 | 1.5 |
| 4 y | 1149 | 17.5 | 2.1 | 1133 | 17.1 | 2.1 |
| 6 y | 1082 | 20.8 | 2.6 | 1064 | 20.4 | 2.6 |
| 7 y | 1063 | 23.0 | 3.0 | 1056 | 22.6 | 3.1 |
| 11 y | 1079 | 34.3 | 6.0 | 1054 | 35.2 | 6.8 |
| 15 y | 1005 | 51.8 | 9.4 | 966 | 52.1 | 8.1 |
| 20 y | 1015 | 70.6 | 9.1 | 1041 | 57.9 | 8.3 |
| 26 y | 1091 | 73.3 | 10.1 | 1102 | 59.2 | 8.8 |
| 36 y | 1140 | 76.0 | 11.0 | 1157 | 62.2 | 10.5 |
| 43 y | 1179 | 78.7 | 11.7 | 1199 | 66.3 | 12.3 |
| 53 y | 1263 | 83.4 | 13.1 | 1255 | 71.6 | 13.9 |
| BMI (kg/m2) | ||||||
| 2 y | 1014 | 18.0 | 2.6 | 964 | 17.6 | 2.4 |
| 4 y | 1103 | 16.3 | 1.6 | 1079 | 16.1 | 1.6 |
| 6 y | 1036 | 15.9 | 1.3 | 1015 | 15.7 | 1.4 |
| 7 y | 1059 | 15.9 | 1.4 | 1050 | 15.7 | 1.5 |
| 11 y | 1069 | 17.3 | 2.2 | 1051 | 17.5 | 2.5 |
| 15 y | 990 | 19.6 | 2.5 | 956 | 20.6 | 2.8 |
| 20 y | 996 | 22.6 | 2.4 | 1026 | 21.9 | 2.9 |
| 26 y | 1091 | 23.4 | 2.8 | 1102 | 22.4 | 3.1 |
| 36 y | 1136 | 24.7 | 3.2 | 1154 | 23.5 | 3.8 |
| 43 y | 1179 | 25.6 | 3.4 | 1191 | 25.1 | 4.6 |
| 53 y | 1263 | 27.3 | 3.9 | 1252 | 27.4 | 5.3 |
| Height (cm) | ||||||
| 2 y | 1054 | 85.8 | 5.2 | 1008 | 84.8 | 4.5 |
| 4 y | 1121 | 103.4 | 5.0 | 1107 | 103.0 | 5.0 |
| 6 y | 1084 | 114.4 | 5.2 | 1059 | 113.9 | 5.3 |
| 7 y | 1101 | 120.4 | 5.7 | 1096 | 119.9 | 5.5 |
| 11 y | 1085 | 140.6 | 6.7 | 1063 | 141.4 | 6.9 |
| 15 y | 1002 | 162.1 | 8.9 | 968 | 158.9 | 6.2 |
| 20 y | 1018 | 177.0 | 6.7 | 1039 | 162.7 | 6.1 |
| 26 y | 1092 | 177.0 | 6.5 | 1104 | 162.6 | 6.3 |
| 36 y | 1139 | 175.3 | 6.5 | 1158 | 162.6 | 5.8 |
| 43 y | 1179 | 175.1 | 6.6 | 1194 | 162.6 | 6.0 |
| 53 y | 1264 | 174.5 | 6.5 | 1260 | 161.8 | 5.9 |
Data are from individuals measured at the 53-y visit with complete or imputed genotype information.
Cross-sectional associations
Weight
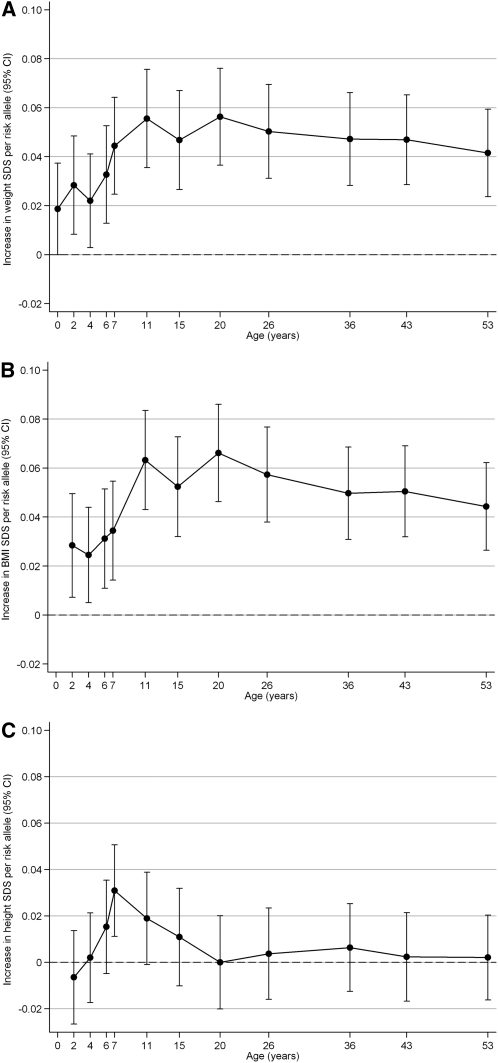
The obesity-risk-allele score showed a nominal positive association with birth weight (0.019 SDS/allele; 95% CI: 0.00, 0.04; P = 0.05) and showed more clearly positive cross-sectional associations with all repeated measures of weight SDS between 2 and 53 y (all P ≤ 0.03; Figure 1A; see Supplementary Table 3 under “Supplemental data” in the online issue). The largest effect sizes were seen at ages 11 y (0.056 SDS/allele; 95% CI: 0.036, 0.076; P = 6.25 × 10−8) and 20 y (0.056 SDS/allele; 95% CI: 0.036, 0.076; P = 2.58 × 10−8). At age 11 y, the risk-allele score explained 1.6% of the variance in weight SDS.
FIGURE 1.
Associations between the obesity-risk-allele score and weight SDS (A), BMI SDS (B), and length/height SDS (C) between birth and 53 y in the National Survey of Health and Development. Regression coefficients (±95% CIs) from linear regression models are shown [adjusted for sex, precise age at measurement (up to the visit at age 15 y), and mother's BMI (for birth weight only)]. SDS, SD scores.
BMI
A similar pattern was observed for associations with BMI SDS, which also peaked at ages 11 y (0.063 SDS/allele; 95% CI: 0.043, 0.083; P = 1.06 × 10−9) and 20 y (0.066 SDS/allele; 95% CI: 0.046, 0.086; P = 8.58 × 10−11) (Figure 1B; see Supplementary Table 3 under “Supplemental data” in the online issue).
Height
The risk-allele score was positively associated with height SDS at 7 y (0.031 SDS/allele; 95% CI: 0.011, 0.051; P = 0.007) (Figure 1C; see Supplementary Table 3 under “Supplemental data” in the online issue) but showed no association with adult height (at age 36 y: 0.006 SDS/allele; 95% CI: −0.012, 0.025; P = 0.5).
Comparison of extremes of the obesity-risk-allele score
Individuals with ≥14 obesity risk alleles (n = 189) were, on average, 1.56 kg/m2 and 3.58 kg heavier at age 11 y and 1.6 cm taller at 7 y than were those with ≤7 alleles (n = 161).
Longitudinal associations
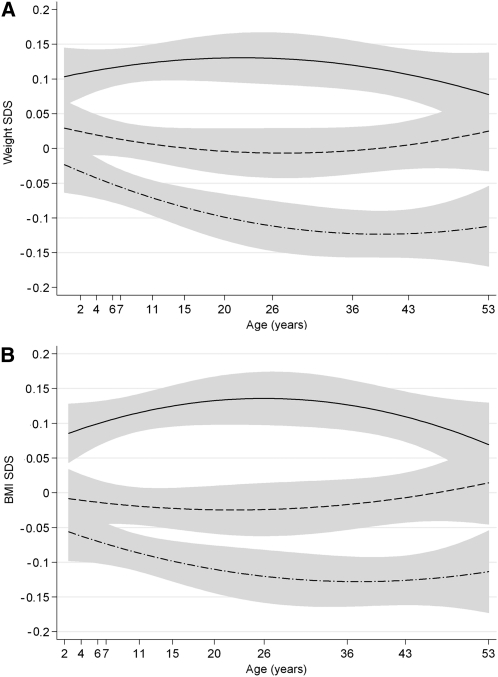
The associations between the risk-allele score and BMI, weight, and height SDS appeared to vary with age in a nonlinear manner (Figure 1, A, B, and C). This was confirmed in quadratic multilevel models in which the interaction term risk-allele-score × age2 was highly significant for BMI and weight SDS to age 53 y (both P < 0.001). Cubic age and risk-allele score × age3 terms were not significant (both P > 0.14). Quadratic prediction models for BMI and weight SDS by risk-allele-score tertiles between birth and 53 y illustrate that growth trajectories diverge during childhood and converge during later adult life according to level of genetic predisposition (Figure 2, A and B). In multilevel models stratified by age period, the risk-allele score was associated with greater weight gain between birth and 11 y (0.003 SDS/allele per year; 95% CI: 0.001, 0.004; P = 0.001) and greater gains in BMI between 2 and 11 y (0.005 SDS/allele per year; 95% CI: 0.002, 0.007; P < 0.001) (Table 2). The risk-allele score association with weight SDS subsequently declined up to age 53 by −0.0003 SDS/allele per year (P = 0.02), and a similar decline was seen in the association with BMI SDS by −0.0005 SDS/allele per year (P = 0.001).
FIGURE 2.
Predicted weight and BMI by tertiles of the obesity-risk-allele score. From prediction models of weight (A) and BMI (B) SDS by risk-allele-score tertiles with fitted quadratic age interaction terms. The solid line represents the mean SDS in those in the highest tertile of the risk-allele score, the dashed line the middle tertile, and the dash-dot line the lowest tertile. Shaded areas indicate 95% CIs. SDS, SD scores.
TABLE 2.
Longitudinal multilevel modeling of the associations between the risk-allele score with changes in weight, BMI, and height SDS1
| N | Effect size per risk allele per year2 | 95% CI | P3 | |
| Change in weight SDS | ||||
| 0–11 y | 2537 | 0.0027 | (0.0011, 0.0042) | 0.001 |
| 11–53 y | 2537 | −0.0003 | (−0.0006, 0.0000) | 0.023 |
| Change in BMI SDS | ||||
| 2–11 y | 2465 | 0.0049 | (0.0024, 0.0074) | <0.001 |
| 11–53 y | 2537 | −0.0005 | (−0.0009, −0.0002) | 0.001 |
| Change in height SDS | ||||
| 2–7 y | 2461 | 0.0065 | (0.0029, 0.0100) | <0.001 |
| 7–20 y | 2414 | −0.0014 | (−0.0024, 0.0004) | 0.008 |
SDS, SD scores.
Regression coefficients from the interaction term “risk-allele score × age” represent the association between risk-allele score and changes in weight, BMI, or height SDS per risk allele per year.
Statistical tests were performed by using multilevel modeling.
For height SDS, in view of a different pattern of cross-sectional associations over time and the limited period for growth, we divided the data into 2 periods, 2–7 and 7–20 y, which broadly represented height growth before and after the onset of puberty given the data available. The obesity-risk-allele score was associated with greater height growth between 2 and 7 y (0.007 SDS/allele per year; 95% CI: 0.003, 0.010; P < 0.001) and subsequently with slower height growth (−0.002 SDS/allele per year; P = 0.007) between 7 and 20 y (Table 2).
Sensitivity analyses
When the FTO and MC4R variants were removed from the risk allele-score, the cross-sectional associations between the resulting 9-SNP risk-allele score and weight or BMI SDS between birth and 53 y were only slightly lower than the original 11-SNP score (see Supplementary Table 4 under “Supplemental data” in the online issue). Associations remained significant (P < 0.05) at all time points except age 4 y. As with the original risk-allele score, this smaller 9-SNP risk-allele score showed similar patterns of association with body size and weight gain, with the strongest associations for BMI SDS at ages 11 and 20 y, and a positive association with height at 7 y (P = 0.02) (see Supplementary Table 3 under “Supplemental data” in the online issue). Longitudinal associations of the risk-allele score persisted with greater childhood gains in BMI and height SDS. However, the 9-SNP risk-allele-score associations with adulthood weight and BMI SDS did not show significant attenuation with increasing age (P > 0.2). Further sensitivity analyses using the same 8-SNP risk-allele score as that used in the ALSPAC study (13) resulted in slightly larger per-allele effect sizes compared with the 11-SNP score. For example, the association of the 8-SNP risk-allele score with BMI SDS at weight gain between birth and 11 y was 0.004 SDS/allele per year (95% CI: 0.003, 0.006). This remains slightly lower than the effect size of this 8-SNP risk-allele score on weight gain during the same age period in the more contemporary ALSPAC study (0.005 SDS/allele per year; 95% CI: 0.004, 0.006).
DISCUSSION
The results from this study show that genetic variants that predispose to higher adult BMI are in combination associated with greater gains in weight up to age 11 y and with gains in height up to age 7 y. After age 11 y, individuals with more obesity risk alleles remain heavier but do not continue to gain weight more rapidly during adulthood than do those who carry fewer risk alleles. In contrast with the associations with weight, the positive association between these obesity susceptibility variants and childhood height did not persist into adulthood. This pattern of faster early childhood gains in height followed by subsequent slower height growth and unchanged adult height indicates that obesity risk alleles may confer a more rapid tempo of growth rather than altered long-term height potential.
Associations between a faster tempo of childhood growth and adult obesity risk have been described previously in nongenetic studies. In the 1958 British Birth cohort, individuals who achieved a greater proportion of their adult height by age 7 y had an increased risk of obesity at age 33 y (17). Similarly, a US study recently reported that overweight or obese adults were taller as children but had relatively less growth during their teenage years (18). Notably, the acceleration in childhood height becomes apparent before the age of pubertal onset and so it is not simply a marker of an earlier pubertal growth spurt. Our observed association between the obesity-risk-allele score and height SDS suggests that being taller at age 7 y relative to one's adult height potential may indicate genetic susceptibility to a rapid growth trajectory that is associated with later obesity risk. This prepubertal indicator may occur at an age at which children are more conducive to interventions to prevent excessive weight gain than during adolescence. The challenge is to accurately and safely differentiate these taller young children, who will experience earlier cessation of growth because of earlier maturation from those on a healthy trajectory to becoming taller adults.
This NSHD cohort is unique in being able to analyze longitudinal data on weight and BMI over such a long period. The positive influence of adult obesity susceptibility variants on weight gain was attained largely in childhood, which is consistent with previous cross-sectional findings showing that these loci have similar effect sizes on BMI SDS in children and adults (10). Furthermore, the difference in childhood BMI of ∼0.5 SDS between the highest and lowest groups of the obesity-risk-allele score in the NSHD is similar to that observed in other adult populations (13). This provides further evidence that greater gains in weight associated with these loci are already achieved in childhood and is further supported by recent findings of a large-scale adult study showing that the BMI-increasing allele at the FTO locus does not confer greater weight gain in adulthood (19).
Whereas combining established genetic variants for BMI into a risk-allele score maximized statistical power, this approach masks possible heterogeneity in effects of individual variants. The FTO and MC4R loci were the first to be identified by genome-wide association studies for adult BMI as these variants are common and have the largest effect sizes (6, 7). We previously described in the NSHD cohort that the FTO and MC4R variants showed comparable associations with BMI and weight, with a biphasic pattern over the life course; the associations with both loci strengthened during childhood and adolescence, peaked at age 20 y, and weakened into adulthood (11). Sensitivity analyses were therefore performed excluding these loci, and confirmed that this biphasic pattern of association with BMI SDS was also seen with the remaining 9 SNPs in combination, which suggested that this timing of association may be generic across multiple BMI loci. The current study is underpowered to distinguish between individual genotype associations on patterns of growth over the life course, and larger cross-study approaches would be required for this. Information on the association of individual SNPs with childhood weight gain to inform such future efforts is shown elsewhere (see Supplementary Table 5 under “Supplemental data” in the online issue).
The NSHD has been shown to be broadly representative of the nonmigrant British population of a similar age (15). Their mean BMI values at age 53 y (Table 1) were in the middle of the overweight range (BMI: 25–30), which is consistent with other studies of this age group. It is important to recognize that it is not possible to disentangle age and period effects in the NSHD, because all study members were born within the same week in 1946. NSHD members experienced postwar food rationing from birth to age 8 y, and the prevalence of childhood obesity was very low, which could lead to different penetrance of the influence of these genetic variants on weight gain compared with populations with unrestricted calorific intake (20). It is possible that standardization of diet achieved by food rationing could contribute to stronger observed genetic influences on childhood versus later weight gain. Comparison between different birth cohorts measured across the same time periods is necessary to clarify whether life-course differences in weight gain associated with obesity-susceptibility loci are truly due to age or whether they may also be modified by environmental changes over time.
The per-allele effect size of an identical risk-allele score on rate of weight gain between birth and 11 y was slightly lower in the NSHD (0.003 SDS/allele per year) than that reported in the ALSPAC population—a cohort born in 1991–1992 (0.005 SDS/allele per year) (13). In ALSPAC, a significant association of the risk-allele score with length was observed as early as age 6 wk and persisted throughout childhood. Whereas the NSHD does not have data on infant length before the age of 2 y, a trend toward increased height attributable to BMI-increasing alleles was not observed until much later, at 6 y of age. It is possible that these differences could reflect an increase in the penetrance of genetic obesity susceptibility from the 1940s to the 1990s. This interpretation is consistent with findings from a multigenerational study, which demonstrated that loss-of-function MC4R mutations had higher penetrance in younger generations (21). It is possible that “obesogenic” conditions allow genetic susceptibility to greater adiposity and weight gain to become more visible.
Another limitation of this study was that the variants used in the risk-allele score explained only a small fraction of the variance and heritability of BMI. A recent expanded genome-wide association study yielded further loci associated with adult BMI (22); addition of these variants will permit more powerful longitudinal analyses of the growth patterns associated with obesity susceptibility. A greater understanding of the role of these obesity risk alleles will provide a more complete picture of the mechanisms of obesity susceptibility. Whereas this study provides insights into the timing of weight changes associated with genetic obesity risk, it does not explain the molecular or physiologic processes that bring about these changes. Given that the effects of these variants on weight gain are only apparent in childhood, it is likely that the actions of these genes may become less important with age; therefore, we suggest that future mechanistic studies should include these key age periods.
We found a borderline significant association between the obesity-risk-allele score and increased birth weight. A recent large meta-analysis of 24,274 individuals found no association between a similar composite obesity susceptibility score and birth weight (23). Although that study largely relied on studies of self-reported birth weight data, other studies with more accurate birth weight records also found no associations with obesity susceptibility risk scores (13, 24). Maternal genotype may confound the association between fetal genotype and birth weight, because mother-offspring genotypes are correlated and birth weight is influenced by the in utero environment. However, in this study, the association remained even after adjustment for mother's BMI, and there is no clear explanation for these contrasting findings.
In conclusion, the combined influence of adult obesity-susceptibility variants on faster rates of weight gain appears to be confined to the first 11 y of life rather than continuing into later adolescence and adulthood. However, differences in BMIs between groups with higher or lower obesity-risk-allele scores continue to track throughout adult life, which suggests an active persistence of their effects on energy homeostasis. This persistence might be modifiable by adult life environmental factors (25). Finally, the association between genetic obesity-risk-allele scores and a faster tempo of height growth suggests that this growth trajectory may contribute to the identification of young children with high long-term obesity susceptibility.
Acknowledgments
We thank the NSHD study members and the staff involved in data collection for this cohort over the past 65 y and the staff of the MRC Epidemiology Unit technical team for laboratory work.
The authors’ responsibilities were as follows—CEE, RH, and KKO: conceived and designed the study; CEE, RH, RJFL, AKW, AW, and KKO: analyzed and interpreted the data; DK and NJW: oversaw the study; and CEE and KKO: wrote the first draft of the manuscript and had primary responsibility for the final content. All authors revised and contributed to the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: ALSPAC, Avon Longitudinal Study of Parents and Children; MRC, Medical Research Council; NSHD, National Survey of Health and Development; SDS, SD scores; SNP, single nucleotide polymorphism.
REFERENCES
- 1.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–5 [DOI] [PubMed] [Google Scholar]
- 2.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr 1994;59:955–9 [DOI] [PubMed] [Google Scholar]
- 3.Haworth CM, Carnell S, Meaburn EL, Davis OS, Plomin R, Wardle J. Increasing heritability of BMI and stronger associations with the FTO gene over childhood. Obesity (Silver Spring) 2008;16:2663–8 [DOI] [PubMed] [Google Scholar]
- 4.Korkeila M, Kaprio J, Rissanen A, Koskenvuo M. Effects of gender and age on the heritability of body mass index. Int J Obes 1991;15:647–54 [PubMed] [Google Scholar]
- 5.North KE, Graff M, Adair LS, Lange EM, Lange LA, Guo G, Gordon-Larsen P. Genetic epidemiology of BMI and body mass change from adolescence to young adulthood. Obesity (Silver Spring) 2010;18:1474–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 2008;40:768–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009;41:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 2009;41:18–24 [DOI] [PubMed] [Google Scholar]
- 10.den Hoed M, Ekelund U, Brage S, Grontved A, Zhao JH, Sharp SJ, Ong KK, Wareham NJ, Loos RJ. Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes 2010;59:2980–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy R, Wills AK, Wong A, Elks CE, Wareham NJ, Loos RJ, Kuh D, Ong KK. Life course variations in the associations between FTO and MC4R gene variants and body size. Hum Mol Genet 2010;19:545–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sovio U, Mook-Kanamori DO, Warrington NM, Lawrence R, Briollais L, Palmer CN, Cecil J, Sandling JK, Syvanen AC, Kaakinen M, et al. Association between common variation at the FTO locus and changes in body mass index from infancy to late childhood: the complex nature of genetic association through growth and development. PLoS Genet 2011;7:e1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elks CE, Loos RJ, Sharp SJ, Langenberg C, Ring SM, Timpson NJ, Ness AR, Davey Smith G, Dunger DB, Wareham NJ, et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med 2010;7:e1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadsworth M, Kuh D, Richards M, Hardy R. Cohort profile: the 1946 National Birth Cohort (MRC National Survey of Health and Development). Int J Epidemiol 2006;35:49–54 [DOI] [PubMed] [Google Scholar]
- 15.Wadsworth ME, Butterworth SL, Hardy RJ, Kuh DJ, Richards M, Langenberg C, Hilder WS, Connor M. The life course prospective design: an example of benefits and problems associated with study longevity. Soc Sci Med 2003;57:2193–205 [DOI] [PubMed] [Google Scholar]
- 16.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992;11:1305–19 [DOI] [PubMed] [Google Scholar]
- 17.Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ 2001;323:1331–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stovitz SD, Demerath EW, Hannan PJ, Lytle LA, Himes JH. Growing into obesity: patterns of height growth in those who become normal weight, overweight, or obese as young adults. Am J Hum Biol 2011;23(5):635–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertel JK, Johansson S, Sonestedt E, Jonsson A, Lie RT, Platou CG, Nilsson PM, Rukh G, Midthjell K, Hveem K, et al. FTO, type 2 diabetes, and weight gain throughout adult life: a meta-analysis of 41,504 subjects from the Scandinavian HUNT, MDC, and MPP studies. Diabetes 2011;60:1637–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Hardy R, Kuh D, Lo Conte R, Power C. Child-to-adult body mass index and height trajectories: a comparison of 2 British birth cohorts. Am J Epidemiol 2008;168:1008–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O'Rahilly S, et al. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 2008;57:2511–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan J, Magi R, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilpeläinen TO, den Hoed M, Ong KK, Grontved A, Brage S, Jameson K, Cooper C, Khaw KT, Ekelund U, Wareham NJ, et al. Obesity-susceptibility loci have a limited influence on birth weight: a meta-analysis of up to 28,219 individuals. Am J Clin Nutr 2011;93:851–60 [DOI] [PubMed] [Google Scholar]
- 24.Andersson EA, Pilgaard K, Pisinger C, Harder MN, Grarup N, Faerch K, Sandholt C, Poulsen P, Witte DR, Jorgensen T, et al. Do gene variants influencing adult adiposity affect birth weight? A population-based study of 24 loci in 4,744 Danish individuals. PLoS ONE 2010;5:e14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vimaleswaran KS, Li S, Zhao JH, Luan J, Bingham SA, Khaw KT, Ekelund U, Wareham NJ, Loos RJ. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am J Clin Nutr 2009;90:425–8 [DOI] [PubMed] [Google Scholar]