Abstract
Background: Consumption of sugar-sweetened soda has been associated with an increased risk of cardiometabolic disease. The relation with cerebrovascular disease has not yet been closely examined.
Objective: Our objective was to examine patterns of soda consumption and substitution of alternative beverages for soda in relation to stroke risk.
Design: The Nurses’ Health Study, a prospective cohort study of 84,085 women followed for 28 y (1980–2008), and the Health Professionals Follow-Up Study, a prospective cohort study of 43,371 men followed for 22 y (1986–2008), provided data on soda consumption and incident stroke.
Results: We documented 1416 strokes in men during 841,770 person-years of follow-up and 2938 strokes in women during 2,188,230 person-years of follow-up. The pooled RR of total stroke for ≥1 serving of sugar-sweetened soda/d, compared with none, was 1.16 (95% CI: 1.00, 1.34). The pooled RR of total stroke for ≥1 serving of low-calorie soda/d, compared with none, was 1.16 (95% CI: 1.05, 1.28). Compared with 1 serving of sugar-sweetened soda/d, 1 serving of decaffeinated coffee/d was associated with a 10% (95% CI: 1%, 19%) lower risk of stroke and 1 serving of caffeinated coffee/d with a 9% (95% CI: 0%, 17%) lower risk. Similar estimated reductions in risk were seen for substitution of caffeinated or decaffeinated coffee for low-calorie soda.
Conclusions: Greater consumption of sugar-sweetened and low-calorie sodas was associated with a significantly higher risk of stroke. This risk may be reduced by substituting alternative beverages for soda.
INTRODUCTION
Consumption of sugar-sweetened beverages, including sodas, vitamin water, and energy drinks, has increased in the United States over the past 3 decades. Per capita consumption in 2009 was estimated at 45 gallons/y, or nearly half of the total beverage intake (1). Sodas, or carbonated soft drinks, are consumed more than any other sugar-sweetened beverage and, despite a recent report that intake of sugar-sweetened soda in the United States may be declining, soda remains the largest source of added sugar in the diet (2).
Sugar-sweetened beverages have been linked with weight gain, diabetes, hypertension, hyperlipidemia, gout, and coronary artery disease (CAD)4 (3–7). The associations with cardiometabolic disease appear to be independent of BMI and energy intake, which suggests that other mechanisms, such as hyperglycemia, dyslipidemia, inflammation, or endothelial dysfunction, underlie the association (6). Low-calorie sodas are less-well studied than are sugar-sweetened sodas. Although intake of these beverages was recently linked with the metabolic syndrome, reverse causality or residual confounding could explain the association (8), and a recent prospective study found no association between low-calorie soda intake and diabetes risk (9). Intake of low-calorie soda has been associated with progression of kidney disease (10).
Given the associations between soda consumption, cardiometabolic risk factors, and CAD, we sought to evaluate the relation between sodas and stroke risk. This relation has not been closely examined, yet stroke remains a significant cause of morbidity, mortality, and health care expenditures in the United States (11, 12) and shares many of the same risk factors as CAD (13). We therefore examined patterns of soda consumption and the substitution of alternative beverages for soda in relation to stroke risk in 2 large prospective cohorts of men and women.
SUBJECTS AND METHODS
Study populations
The Nurses’ Health Study (NHS) began in 1976, when 121,700 female registered nurses aged 30–55 y residing in the United States provided information on their medical history and lifestyle. The Health Professionals Follow-Up Study (HPFS) began in 1986, when 51,529 male dentists, pharmacists, optometrists, osteopaths, podiatrists, and veterinarians aged 40–75 y and residing in the United States provided information on their medical history and lifestyle. Every 2 y, follow-up questionnaires have been sent to both populations to update their information. In 1980, a 61-item food-frequency questionnaire (FFQ) was included to assess intake of specific foods in the NHS. Expanded questionnaires updated dietary intake in 1986, 1990, 1994, 1998, 2002, and 2006. In 1986, a 131-item FFQ assessed intake of specific foods in the HPFS, and similar questionnaires were used to update dietary intake in 1990, 1994, 1998, 2002, and 2006. As in our previous analyses, we excluded participants who left excessive items blank on their baseline FFQ, those who reported implausibly low or high energy intakes, and those with previously diagnosed cancer, diabetes, angina, myocardial infarction, stroke, or other cardiovascular disease [including a history of percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)] (14–18). The final 1980 baseline population consisted of 84,085 women, and the final 1986 baseline population consisted of 43,371 men. This study was approved by the Committee on the use of Human Subjects in Research at Brigham and Women's Hospital. Return of a questionnaire was considered to imply consent.
Assessment of soda intake
The types of sodas on the FFQs included low-calorie cola with caffeine (eg, Diet Coke, Tab with caffeine), low-calorie cola without caffeine (eg, Pepsi Free), other low-calorie carbonated beverages (eg, Diet 7-Up, Fresca, Diet Mountain Dew, diet ginger ale), sugar-sweetened cola with caffeine (eg, Coke, Pepsi), sugar-sweetened cola without caffeine (eg, caffeine-free Coke, caffeine-free Pepsi), and other carbonated beverages with sugar (eg, 7-Up, Mountain Dew, Surge, Dr Pepper). We categorized soda as sugar-sweetened or low-calorie (diet or artificially sweetened). Colas are a type of soda, or carbonated soft drink, originally made with kola nuts to add caffeine. To calculate each participant's soda intake, the participant was asked how often on average during the previous year he or she had consumed one glass, bottle, or can. Nine responses were possible, ranging from “never or almost never” to “more than 6 times per day.” The reproducibility and validity of the FFQs in measuring beverage intake were previously described (19–23): the Pearson correlation coefficient, corrected for within-person variation, between the FFQ and dietary records was previously reported as 0.84 for cola and 0.36–0.40 for noncola soda (21).
Ascertainment of incident stroke cases
The primary endpoint for this study was incident stroke occurring after the return of the 1980 FFQ but before 1 June 2008 in the NHS and after the return of the 1986 FFQ but before 31 January 2008 in the HPFS. After report of a stroke, permission to obtain medical records was requested, and these were reviewed by study physicians with no knowledge of the subjects’ self-reported risk factor status. Stroke was classified as ischemic (thrombotic, embolic, or nonhemorrhagic), hemorrhagic (intraparenchymal hemorrhage or subarachnoid hemorrhage), or of unknown type, as per criteria in the National Survey of Stroke (24). Nonfatal strokes for which confirmatory information was obtained by interview or letter but no medical records and no neuro-imaging (computerized tomography or magnetic resonance imaging) were available, were designated as probable [225 of 1111 (or 20%) of cases in men and 737 of 2379 (or 31%) of cases in women]. Deaths were identified from state vital records or the National Death Index or were reported by next of kin or the postal system. Follow-up for deaths was shown to be >98% complete (25). Stroke was confirmed as fatal only if confirmed by medical records or autopsy report. Fatal stroke was designated as probable if stroke was reported on the death certificate or reported by next of kin, but no medical records were available. These cases constituted 98 of 305 (or 32%) cases in men and 236 of 560 (or 42%) cases in women. We included all confirmed and probable cases in our report because results were similar after probable cases were excluded.
Statistical analysis
We first assessed whether a report of intermediate outcomes (diabetes, hypertension, hypercholesterolemia, angina, or CABG/PCI), myocardial infarction, or cancer were associated with a subsequent change in sugar-sweetened or low-calorie soda intake (see online supplementary material under “Supplemental data” in the online issue). These associations were weak; thus, these diagnoses could not be important time-dependent confounders. Therefore, to avoid misclassification of participants’ long-term soda intake, we continued updating each participant's intake throughout follow-up using data from all repeated FFQs.
To reduce within-person variation and best represent long-term diet, participants were divided into categories of cumulative average intake of sugar-sweetened and low-calorie soda consumption. The categories included none, up to once per week, once per week up to once per day, and once per day or more. Cumulative averages were calculated by taking the mean of all reported FFQ intakes up to the beginning of a follow-up interval. The cumulative average intake was then associated with stroke incidence from the time of the last returned questionnaire until the next follow-up cycle. If dietary data from a particular FFQ was missing, then that cycle's cumulative average was the last cumulative average carried forward.
We evaluated the associations between sugar-sweetened and low-calorie soda consumption and total stroke incidence (ischemic, intraparenchymal hemorrhage, subarachnoid hemorrhage, and unknown type) and the incidence of hemorrhagic and ischemic strokes. Because the American Heart Association and American Stroke Association have developed guidelines for the primary prevention of stroke (inclusive of both ischemic and hemorrhagic subtypes), and because of the recognized overlap of risk factors and prevention strategies, we evaluated the association between soda consumption and total stroke (26). In the NHS, person-years of follow-up were calculated from the return of the 1980 FFQ to the date of the first stroke event, death, or 1 June 2008, whichever came first. In the HPFS, person-years of follow-up were calculated from the return of the 1986 FFQ to the date of the first stroke event, death, or 31 January 2008, whichever came first. The RR was computed by using a multivariable Cox proportional hazards regression model, with the incidence rate in a specific category of cumulative average soda intake divided by that in the lowest category (none). All models were mutually adjusted for the other beverage type; ie, the sugar-sweetened soda analysis adjusted for low-calorie soda and vice versa. The first multivariate model was stratified on age (mo) and calendar time (2-y time intervals) and included red meat, poultry, fish, whole-fat dairy products, low-fat dairy products, and nuts (all in servings/d) and simultaneously controlled for intakes of cereal fiber (g/d), alcohol (g/d), fruit and vegetable intake (servings/d), and trans unsaturated fatty acids (g/d) and other potential nondietary confounding variables. These variables were updated biennially and included physical exercise (<3, 3 to <9, 9 to <18, 18 to <27, or ≥≥27 metabolic equivalent tasks/wk), cigarette smoking [never, past, or current (1–14, 15–24, or ≥25 cigarettes/d)], menopausal status in women (premenopausal, postmenopausal with no history of hormone replacement, postmenopausal with a history of hormone replacement, or postmenopausal with current hormone replacement), parental history of early myocardial infarction (before age 60 y), years of multivitamin use, vitamin E supplement use (yes or no), and aspirin use at least once per week (yes or no). The last value was carried forward for one 2-y cycle to replace missing values. If participants left excessive items blank on a follow-up questionnaire, or reported implausibly high or low energy intakes, they were considered to be missing data from that particular questionnaire, and their most recent value (of physical exercise, for instance) was carried forward. If the last value was missing, a missing value indicator was created. The median value for each category of intake was used to test for a linear trend across categories. Neither a diagnosis of diabetes, angina, hypertension, hypercholesterolemia, CABG/PCI, or myocardial infarction nor the use of the medications prescribed to treat these diseases (including statins and β-blockers) was included, because all of these may be considered intermediate outcomes on the causal pathway between diet and stroke. In a second multivariate model, we included the above covariates as well as total energy (kcal) and BMI (in kg/m2; <22, 22–24, 24–25, 25–27, 27–29, 29–30, 30–32, 32–35, 35–40, or >40), both of which may be considered either confounders or intermediates on the causal pathway between soda consumption and stroke. RRs and SEs for each category of intake from each cohort were then pooled in fixed-effects models to arrive at summary estimates as the Q statistic P value for between-study heterogeneity was not statistically significant (null hypothesis is that there is no heterogeneity between the HPFS and the NHS). To estimate the absolute risk of total stroke by category of soda intake, we multiplied the multivariable RRs by the incidence rates in the referent groups (cases/per 100,000 person-years) (27).
In sensitivity analyses, to assess whether metabolic changes related to excess body weight before the onset of cohort follow-up influenced potential low-calorie soda–stroke associations, we adjusted our multivariate models for weight change (increase or decrease) in the 5 y before 1986 for men and in the 4 y before 1980 for women. We evaluated the relation between soda intake and subarachnoid hemorrhage in a multivariate model. To evaluate whether a soda-stroke association was mediated or confounded by the presence of hypertension or diabetes, we adjusted our multivariate models for these diseases. Separately, we stratified our multivariate model on the presence of diabetes (yes or no) or hypertension (yes or no) to assess for effect modification of soda intake by one of these diagnoses. We tested the significance of the interaction with a likelihood ratio test by comparing a model with the interaction terms to a model with only the main effects.
We also estimated the RR of stroke associated with substituting caffeinated coffee, decaffeinated coffee, water, tea, skim milk, or orange juice for either sugar-sweetened or low-calorie soda. To do so, we fit multivariable Cox proportional hazards models, which included the covariates from our multivariable model with energy intake and BMI, plus the above beverages entered as continuous variables rather than as categorical variables (cumulative average servings/d). The cumulative average intakes of caffeinated coffee, decaffeinated coffee, water, tea, skim milk, or orange juice were calculated in the same way as for soda. Decaffeinated coffee was first asked about in the NHS in 1984, and water was first asked about in 1986. The difference in the coefficients of 2 beverages, plus their covariance, was used to estimate the RR and variance for the substitution (28, 29). RRs and variances for each substitution from each cohort were then pooled in fixed-effects models to arrive at a summary estimate of the effect of substituting one beverage for another in relation to total stroke risk. All P values are 2-sided, with P values <0.05 considered statistically significant.
RESULTS
Cohort characteristics
During 2,188,230 person-years of follow-up from 1980 through 2008 in the NHS and during 841,770 person-years of follow-up from 1986 through 2008 in the HPFS, we documented 2938 strokes in women (1513 ischemic, 267 intraparenchymal hemorrhages, 252 subarachnoid hemorrhages, and 906 unspecified) and 1416 strokes in men (843 ischemic, 163 intraparenchymal hemorrhages, 54 subarachnoid hemorrhages, and 356 unspecified). Our follow-up rate through 2006 (defined as person-years contributed by study participants until their last returned FFQ or death divided by person-years contributed by study participants until death) was 96% in men and 97% in women. Characteristics of the study participants during follow-up, averaged according to the proportion of person-time in each category of soda intake, are shown in Tables 1 and 2. Compared with men and women who did not consume sugar-sweetened soda, those who consumed ≥1 serving/d had higher rates of hypertension and hypercholesterolemia and lower physical activity. More frequent consumption of sugar-sweetened soda was associated with more frequent consumption of red meat and whole-fat dairy products. A higher consumption of low-calorie soda consumption was also associated with higher rates of chronic disease and a higher BMI.
TABLE 1.
Age-standardized characteristics of 84,085 women in the Nurses’ Health Study, 1980–2008
| Low-calorie soda consumption |
Sugar-sweetened soda consumption |
|||||||
| None | None up to once per week | Once per week up to once per day | Once per day or more | None | None up to once per week | Once per week up to once per day | Once per day or more | |
| Age (y) | 58 ± 111 | 59 ± 11 | 58 ± 10 | 58 ± 10 | 58 ± 10 | 59 ± 10 | 58 ± 10 | 58 ± 10 |
| BMI (kg/m2) | 24 ± 3 | 25 ± 5 | 26 ± 5 | 28 ± 6 | 26 ± 5 | 26 ± 5 | 26 ± 5 | 26 ± 6 |
| Current smoker (%) | 22 | 15 | 12 | 13 | 16 | 13 | 15 | 23 |
| Family history of early coronary artery disease (%) | 17 | 18 | 19 | 21 | 19 | 18 | 19 | 19 |
| History of high blood pressure (%) | 30 | 33 | 37 | 42 | 34 | 35 | 36 | 39 |
| History of high cholesterol (%) | 30 | 39 | 41 | 42 | 35 | 40 | 40 | 39 |
| Weight change before onset of follow-up, 1976–1980 (kg) | 0.75 ± 3.7 | 0.88 ± 3.9 | 1.02 ± 4.1 | 1.11 ± 4.8 | 0.80 ± 4.2 | 0.88 ± 3.8 | 1.06 ± 4.1 | 1.27 ± 4.6 |
| Physical activity (METs2/wk) | 17 ± 23 | 18 ± 22 | 18 ± 22 | 17 ± 23 | 19 ± 24 | 18 ± 21 | 16 ± 21 | 14 ± 21 |
| Aspirin use at least once per week (%) | 27 | 31 | 32 | 31 | 30 | 30 | 31 | 27 |
| Vitamin E use (%) | 20 | 25 | 25 | 22 | 24 | 25 | 21 | 16 |
| Current hormone use (%) | 20 | 26 | 27 | 25 | 24 | 27 | 24 | 20 |
| Dietary intake (servings/d) | ||||||||
| Red meat | 1.16 ± 0.69 | 1.04 ± 0.58 | 1.05 ± 0.57 | 1.10 ± 0.61 | 0.98 ± 0.62 | 1.03 ± 0.56 | 1.20 ± 0.62 | 1.36 ± 0.71 |
| Poultry | 0.27 ± 0.19 | 0.30 ± 0.17 | 0.33 ± 0.19 | 0.36 ± 0.22 | 0.33 ± 0.21 | 0.32 ± 0.18 | 0.30 ± 0.19 | 0.29 ± 0.20 |
| Fish | 0.19 ± 0.15 | 0.22 ± 0.16 | 0.25 ± 0.18 | 0.27 ± 0.20 | 0.26 ± 0.20 | 0.24 ± 0.17 | 0.21 ± 0.15 | 0.18 ± 0.15 |
| Whole-fat dairy | 1.56 ± 1.32 | 1.29 ± 1.03 | 1.21 ± 0.96 | 1.23 ± 1.01 | 1.18 ± 1.05 | 1.28 ± 1.03 | 1.47 ± 1.15 | 1.67 ± 1.37 |
| Low-fat dairy | 0.85 ± 0.87 | 1.01 ± 0.82 | 1.04 ± 0.81 | 1.00 ± 0.82 | 1.06 ± 0.88 | 1.03 ± 0.81 | 0.88 ± 0.79 | 0.67 ± 0.76 |
| Nuts | 0.14 ± 0.24 | 0.13 ± 0.19 | 0.13 ± 0.18 | 0.13 ± 0.21 | 0.14 ± 0.24 | 0.13 ± 0.19 | 0.13 ± 0.19 | 0.13 ± 0.20 |
| Fruit and vegetables | 4.53 ± 2.19 | 4.78 ± 2.07 | 4.85 ± 2.04 | 4.79 ± 2.15 | 4.89 ± 2.19 | 4.84 ± 2.08 | 4.60 ± 2.06 | 4.10 ± 2.08 |
| Alcohol (g/d) | 6.0 ± 10.5 | 5.3 ± 9.6 | 5.7 ± 9.8 | 6.0 ± 10.5 | 7.0 ± 11.2 | 5.5 ± 9.6 | 5.1 ± 9.3 | 4.9 ± 10.1 |
| Energy intake (kcal/d) | 1691 ± 467 | 1673 ± 432 | 1661 ± 428 | 1662 ± 452 | 1541 ± 421 | 1658 ± 412 | 1772 ± 447 | 1907 ± 496 |
| Cereal fiber intake (g/d)3 | 3.6 ± 1.9 | 4.2 ± 2.0 | 4.0 ± 1.9 | 3.7 ± 1.9 | 3.9 ± 2.2 | 4.2 ± 1.9 | 3.7 ± 1.7 | 2.9 ± 1.6 |
| trans Fatty acid intake (g/d)3 | 3.5 ± 1.2 | 3.3 ± 1.0 | 3.3 ± 0.96 | 3.3 ± 1.01 | 3.3 ± 1.1 | 3.3 ± 1.0 | 3.5 ± 1.0 | 3.4 ± 1.0 |
Values are means ± SDs and are standardized to the age distribution of the study population.
METs, metabolic equivalent tasks.
Cereal fiber and trans fatty acid intakes were calorie-adjusted by the residual method.
TABLE 2.
Age-standardized characteristics of 43,371 men in Health Professionals Follow-Up Study, 1986–2008
| Low-calorie soda consumption |
Sugar-sweetened soda consumption |
|||||||
| None | None up to once per week | Once per week up to once per day | Once per day or more | None | None up to once per week | Once per week up to once per day | Once per day or more | |
| Age (y) | 62 ± 111 | 62 ± 11 | 62 ± 11 | 61 ± 10 | 62 ± 11 | 62 ± 11 | 62 ± 11 | 61 ± 10 |
| BMI (kg/m2) | 25 ± 3 | 25 ± 3 | 26 ± 3 | 27 ± 4 | 26 ± 4 | 26 ± 3 | 26 ± 3 | 26 ± 4 |
| Current smoker (%) | 6 | 4 | 4 | 4 | 4 | 4 | 5 | 8 |
| Family history of early coronary artery disease (%) | 11 | 11 | 13 | 14 | 13 | 11 | 11 | 13 |
| History of high blood pressure (%) | 27 | 33 | 36 | 42 | 33 | 35 | 33 | 35 |
| History of high cholesterol (%) | 31 | 41 | 43 | 45 | 36 | 42 | 39 | 39 |
| Gain of >5 lb (2.27 kg) before onset of follow-up, 1981–1986 (%) | 30 | 31 | 34 | 40 | 31 | 32 | 34 | 37 |
| Physical activity (METs2/wk) | 33 ± 40 | 36 ± 40 | 36 ± 40 | 36 ± 42 | 35 ± 41 | 36 ± 40 | 34 ± 40 | 33 ± 41 |
| Aspirin use at least once per week (%) | 30 | 37 | 38 | 39 | 33 | 38 | 36 | 33 |
| Vitamin E use (%) | 18 | 21 | 20 | 19 | 21 | 21 | 18 | 14 |
| Dietary intake (servings/d) | ||||||||
| Red meat | 1.28 ± 0.98 | 1.12 ± 0.79 | 1.15 ± 0.82 | 1.23 ± 0.88 | 1.00 ± 0.85 | 1.08 ± 0.77 | 1.35 ± 0.88 | 1.73 ± 1.11 |
| Poultry | 0.36 ± 0.26 | 0.40 ± 0.24 | 0.42 ± 0.26 | 0.47 ± 0.30 | 0.41 ± 0.28 | 0.41 ± 0.25 | 0.41 ± 0.26 | 0.41 ± 0.30 |
| Fish | 0.30 ± 0.26 | 0.31 ± 0.24 | 0.34 ± 0.26 | 0.37 ± 0.29 | 0.37 ± 0.29 | 0.32 ± 0.244 | 0.31 ± 0.25 | 0.28 ± 0.25 |
| Whole-fat dairy | 1.39 ± 1.38 | 1.17 ± 1.08 | 1.08 ± 0.99 | 1.12 ± 0.99 | 1.02 ± 1.08 | 1.13 ± 1.05 | 1.33 ± 1.19 | 1.59 ± 1.35 |
| Low-fat dairy | 1.13 ± 1.23 | 1.25 ± 1.13 | 1.18 ± 1.08 | 1.15 ± 1.09 | 1.16 ± 1.16 | 1.20 ± 1.06 | 1.18 ± 1.16 | 1.05 ± 1.23 |
| Nuts | 0.25 ± 0.37 | 0.25 ± 0.33 | 0.24 ± 0.33 | 0.26 ± 0.37 | 0.25 ± 0.38 | 0.24 ± 0.33 | 0.25 ± 0.33 | 0.26 ± 0.35 |
| Fruit and vegetables | 5.46 ± 2.74 | 5.52 ± 2.63 | 5.52 ± 2.59 | 5.56 ± 2.73 | 5.76 ± 2.81 | 5.56 ± 2.63 | 5.38 ± 2.56 | 4.90 ± 2.60 |
| Alcohol (g/d) | 11.5 ± 15.6 | 10.9 ± 14.4 | 11.8 ± 14.8 | 11.6 ± 15.7 | 12.4 ± 16.0 | 11.8 ± 15.0 | 10.8 ± 14.3 | 10.0 ± 15.3 |
| Energy intake (kcal/d) | 2027 ± 580 | 1989 ± 546 | 1942 ± 543 | 1982 ± 568 | 1814 ± 519 | 1919 ± 509 | 2090 ± 555 | 2396 ± 616 |
| Cereal fiber intake (g/d)3 | 6.1 ± 3.4 | 6.7 ± 3.3 | 6.6 ± 3.4 | 6.4 ± 3.6 | 7.0 ± 4.1 | 6.9 ± 3.3 | 6.0 ± 2.9 | 4.8 ± 2.3 |
| trans Fatty acid intake (g/d)3 | 2.9 ± 1.2 | 3.0 ± 1.1 | 3.0 ± 1.1 | 3.0 ± 1.1 | 2.7 ± 1.2 | 3.0 ± 1.1 | 3.1 ± 1.1 | 3.1 ± 1.0 |
Values are means ± SDs and are standardized to the age distribution of the study population.
METs, metabolic equivalent tasks.
Cereal fiber and trans fatty acid intakes were calorie-adjusted by the residual method.
Soda intake and total incident stroke
In multivariable analyses adjusted for dietary and nondietary cardiovascular disease risk factors, higher intakes of both sugar-sweetened and low-calorie soda were associated with a higher risk of overall stroke (Table 3). In men, ≥1 serving sugar-sweetened soda/d, compared with none, was associated with a statistically nonsignificant RR of total stroke of 1.08 (95% CI: 0.82, 1.41); in women, the statistically significant RR was 1.19 (95% CI: 1.00, 1.42). These risks corresponded to absolute risk differences of 14 and 24 cases/100,000 person-years. In men, ≥1 serving low-calorie soda/d, compared with none, was associated with an RR of total stroke of 1.10 (95% CI: 0.92, 1.32); in women, it was 1.18 (95% CI: 1.05, 1.33). These RRs corresponded to absolute risk differences of 17 and 21 cases/100,000 person-years. Pooled multivariable risk of stroke among men and women was 1.12 (95% CI: 1.02, 1.24) for ≥1 serving sugar-sweetened soda/d and 1.09 (95% CI: 1.04, 1.15) for ≥1 serving low-calorie soda. Risk of stroke with consumption of either sugar-sweetened or low-calorie soda was greater in women than in men, but the results of formal tests of heterogeneity comparing the 2 study populations were not significant. Adjustment for BMI, energy intake, and weight change before the beginning of cohort follow-up slightly attenuated the associations between low-calorie soda consumption and stroke risk in men, but did not materially change the associations in women or with sugar-sweetened beverages.
TABLE 3.
RRs (and 95% CIs) for stroke associated with soda consumption among 43,371 men in the Health Professionals Follow-Up Study and 84,085 women in the Nurses’ Health Study1
| Servings |
||||||
| None | None up to once per week | Once per week up to once per day | Once per day or more | P-trend2 | RR for 1 serving/d | |
| Sugar-sweetened soda | ||||||
| Men | ||||||
| Cases | 464 | 381 | 499 | 72 | ||
| Person-years | 259,630 | 204,418 | 323,569 | 54,153 | ||
| Age-adjusted | 1.00 | 0.94 (0.82, 1.09) | 1.02 (0.89, 1.16) | 1.18 (0.92, 1.53) | 0.11 | 1.16 (0.97, 1.40) |
| Multivariate3 | 1.00 | 0.93 (0.80, 1.07) | 0.99 (0.86, 1.13) | 1.07 (0.82, 1.40) | 0.43 | 1.08 (0.89, 1.31) |
| Multivariate4 | 1.00 | 0.93 (0.80, 1.08) | 0.99 (0.86, 1.14) | 1.08 (0.82, 1.41) | 0.43 | 1.08 (0.89, 1.32) |
| Women | ||||||
| Cases | 918 | 950 | 896 | 174 | ||
| Person-years | 717,209 | 632,223 | 693,974 | 144,825 | ||
| Age-adjusted | 1.00 | 0.99 (0.90, 1.09) | 1.17 (1.07, 1.29) | 1.47 (1.25, 1.74) | <0.0001 | 1.34 (1.21, 1.49) |
| Multivariate3 | 1.00 | 1.00 (0.91, 1.11) | 1.12 (1.02, 1.24) | 1.25 (1.05, 1.48) | 0.004 | 1.17 (1.05, 1.30) |
| Multivariate4 | 1.00 | 1.00 (0.91, 1.10) | 1.11 (1.00, 1.22) | 1.19 (1.00, 1.42) | 0.02 | 1.14 (1.02, 1.27) |
| Pooled5 | ||||||
| Multivariate4 | 1.00 | 0.98 (0.90, 1.06) | 1.07 (0.98, 1.16) | 1.16 (1.00, 1.34) | 0.02 | 1.12 (1.02, 1.24) |
| Low-calorie soda | ||||||
| Men | ||||||
| Cases | 459 | 246 | 509 | 202 | ||
| Person-years | 271,527 | 130,815 | 294,173 | 145,255 | ||
| Age-adjusted | 1.00 | 0.90 (0.77, 1.06) | 1.02 (0.89, 1.16) | 1.14 (0.96, 1.35) | 0.04 | 1.11 (1.00, 1.23) |
| Multivariate3 | 1.00 | 0.93 (0.79, 1.10) | 1.05 (0.92, 1.20) | 1.16 (0.97, 1.38) | 0.03 | 1.12 (1.01, 1.24) |
| Multivariate4 | 1.00 | 0.93 (0.79, 1.09) | 1.03 (0.90, 1.18) | 1.10 (0.92, 1.32) | 0.13 | 1.09 (0.98, 1.21) |
| Multivariate6 | 1.00 | 0.92 (0.78, 1.09) | 1.02 (0.89, 1.17) | 1.08 (0.91, 1.30) | 0.18 | 1.07 (0.97, 1.19) |
| Women | ||||||
| Cases | 797 | 504 | 1069 | 568 | ||
| Person-years | 655,418 | 328,868 | 758,680 | 445,264 | ||
| Age-adjusted | 1.00 | 0.91 (0.81, 1.02) | 1.01 (0.91, 1.11) | 1.15 (1.03, 1.28) | <0.001 | 1.11 (1.04, 1.17) |
| Multivariate3 | 1.00 | 1.00 (0.89, 1.12) | 1.11 (1.01, 1.23) | 1.21 (1.08, 1.35) | <0.001 | 1.11 (1.04, 1.18) |
| Multivariate4 | 1.00 | 1.00 (0.89, 1.12) | 1.10 (1.00, 1.22) | 1.18 (1.05, 1.33) | 0.003 | 1.10 (1.03, 1.17) |
| Multivariate6 | 1.00 | 1.00 (0.89, 1.12) | 1.09 (0.99, 1.21) | 1.17 (1.04, 1.31) | 0.007 | 1.09 (1.02, 1.16) |
| Pooled5 | ||||||
| Multivariate4 | 1.00 | 0.97 (0.88, 1.07) | 1.08 (0.99, 1.17) | 1.16 (1.05,1.28) | <0.0001 | 1.09 (1.04,1.15) |
The Cox proportional hazards multivariate regression model is stratified on age (mo) and calendar time (11 time periods for the Health Professionals Follow-up Study; 13 for the Nurses’ Health Study) and includes intakes of red meat, poultry, fish, nuts, whole- and low-fat dairy products, and fruit and vegetables (all in quintiles of servings/d); cereal fiber (quintiles of g/d); alcohol intake (quintiles of g/d); trans fat intake (quintiles of g/d); cigarette smoking [never, past, or current (1–14, 15–24, or ≥25 cigarettes/d)]; parental history of early myocardial infarction (before age 60 y); multivitamin use (quintiles of years); aspirin use at least once per week (yes or no); vitamin E supplement use (yes or no); menopausal status in women (premenopausal, postmenopausal with no history of hormone replacement, postmenopausal with a history of hormone replacement, or postmenopausal with current hormone replacement); and physical exercise (<3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent tasks/wk); both sugar-sweetened and low-calorie sodas are included in the model.
Calculated by assigning median values to each quartile and treating them as a continuous variable.
Multivariate model without adjustment for BMI or energy intake.
Multivariate model with BMI (10 categories) and energy intake (quintiles of kcal/d).
Results from the multivariate model4 were combined with the use of a fixed-effects model.
Multivariate model with BMI and energy intake and weight change before 1986 (pounds gained or lost during 5 y before 1986 in men or 4 y before 1980 in women).
Soda intake and ischemic and hemorrhagic stroke
In women, greater consumption of sugar-sweetened soda was associated with a higher risk of ischemic stroke (RR per ≥1 serving/d: 1.19; 95% CI: 1.01, 1.39), whereas greater consumption of low-calorie soda was associated with a greater risk of hemorrhagic stroke (RR per ≥1 serving/d: 1.31; 95% CI: 1.15, 1.51) (Table 4). There were only 8 cases of hemorrhagic stroke among men with a cumulative average intake of sugar-sweetened soda of once per day or more (Table 4). The association between low-calorie soda and hemorrhagic stroke risk remained when results for men and women were pooled (RR per ≥1 serving/d: 1.27; 95% CI: 1.12, 1.43). Given the potential for unstable estimates from the few cases in men, we did not evaluate the relation between soda intake and subarachnoid hemorrhage in men. In women, when we fit a multivariate model including BMI and energy intake, we observed that a greater intake of low-calorie soda was associated with a significantly higher risk of subarachnoid hemorrhage (RR per ≥1 serving/d: 1.37; 95% CI: 1.13, 1.65), whereas sugar-sweetened soda was not (RR per ≥1 serving/d: 0.89; 95% CI: 0.62, 1.89).
TABLE 4.
RRs (and 95% CIs) for ischemic and hemorrhagic stroke associated with soda consumption among 43,371 men in the Health Professionals Follow-Up Study and 84,085 women in the Nurses’ Health Study1
| Servings |
||||||
| None | None up to once per week | Once per week up to once per day | Once per day or more | P-trend2 | RR for 1 serving/d | |
| Sugar-sweetened soda | ||||||
| Ischemic stroke | ||||||
| Men | 1.00 | 0.90 (0.75, 1.08) | 0.89 (0.74, 1.06) | 1.02 (0.72, 1.45) | 0.98 | 1.00 (0.77, 1.30) |
| Women | 1.00 | 1.05 (0.92, 1.20) | 1.18 (1.02, 1.35) | 1.28 (0.99, 1.65) | 0.04 | 1.19 (1.01, 1.39) |
| Pooled3 | 1.00 | 1.00 (0.89, 1.11) | 1.06 (0.95, 1.18) | 1.19 (0.97, 1.46) | 0.07 | 1.13 (0.99, 1.30) |
| Hemorrhagic stroke | ||||||
| Men | 1.00 | 0.75 (0.51, 1.11) | 1.21 (0.86, 1.71) | 0.82 (0.38, 1.77) | 0.72 | 1.10 (0.66, 0.1.81) |
| Women | 1.00 | 0.95 (0.75, 1.19) | 1.00 (0.79, 1.26) | 0.85 (0.56, 1.29) | 0.54 | 0.92 (0.71, 1.20) |
| Pooled3 | 1.00 | 0.90 (0.73, 1.09) | 1.06 (0.88, 1.29) | 0.85 (0.59, 1.22) | 0.71 | 0.96 (0.76, 1.21) |
| Low-calorie soda | ||||||
| Ischemic stroke | ||||||
| Men | 1.00 | 0.95 (0.77, 1.17) | 0.98 (0.82, 1.17) | 1.10 (0.87, 1.38) | 0.24 | 1.08 (0.95, 1.24) |
| Women | 1.00 | 1.05 (0.90, 1.23) | 1.08 (0.94, 1.24) | 1.15 (0.97, 1.35) | 0.17 | 1.06 (0.97, 1.16) |
| Pooled3 | 1.00 | 1.01 (0.89, 1.15) | 1.04 (0.93, 1.16) | 1.13 (0.99, 1.29) | 0.07 | 1.07 (0.99, 1.15) |
| Hemorrhagic stroke | ||||||
| Men | 1.00 | 0.77 (0.50, 1.20) | 1.00 (0.72, 1.41) | 1.05 (0.66, 1.67) | 0.57 | 1.08 (0.82, 1.43) |
| Women | 1.00 | 0.96 (0.72, 1.28) | 1.08 (0.85, 1.37) | 1.55 (1.20, 2.00) | <0.0001 | 1.31 (1.15, 1.51) |
| Pooled3 | 1.00 | 0.90 (0.71, 1.15) | 1.06 (0.87, 1.28) | 1.42 (1.14, 1.77) | <0.001 | 1.27 (1.12, 1.43) |
The Cox proportional hazards multivariate regression model is stratified on age (mo) and calendar time (11 time periods for the Health Professionals Follow-up Study; 13 for the Nurses’ Health Study) and includes intakes of red meat, poultry, fish, nuts, whole- and low-fat dairy products, and fruit and vegetables (all in quintiles of servings/d); cereal fiber (quintiles of g/d); alcohol intake (quintiles of g/d); trans fat intake (quintiles of g/d); cigarette smoking [never, past, or current (1–14, 15–24, or ≥25 cigarettes/d)]; parental history of early myocardial infarction (before age 60 y); multivitamin use (quintiles of years); aspirin use at least once per week (yes or no); vitamin E supplement use (yes or no); menopausal status in women (premenopausal, postmenopausal with no history of hormone replacement, postmenopausal with a history of hormone replacement, or postmenopausal with current hormone replacement); physical exercise (<3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent tasks/wk); and BMI (10 categories) and energy intake (quintiles of kcal); both sugar-sweetened and low-calorie sodas are included in the model. Number of cases of hemorrhagic stroke across quartiles of sugar-sweetened soda: 71, 46, 92, and 8 in men and 181, 152, 156, and 30 in women. Number of cases of ischemic stroke across quartiles of sugar-sweetened soda: 288, 231, 281, and 43 in men and 462, 508, 463, and 80 in women. Number of cases of hemorrhagic stroke across quartiles of low-calorie soda: 78, 31, 80, and 28 in men and 158, 75, 162, and 124 in women. Number of cases of ischemic stroke across quartiles of low-calorie soda: 272, 150, 292, and 129 in men and 387, 283, 562, and 281 in women.
Calculated by assigning median values to each quartile and treating them as a continuous variable.
Results from the multivariate model without adjustment for BMI or energy intake were combined with the use of a fixed-effects model.
Risk modification by diabetes and hypertension
Controlling for hypertension and diabetes during follow-up in multivariate models each attenuated our results. The associations were further attenuated when controlled for both: the RRs of stroke in women across quartiles of sugar-sweetened soda intake were 1.00, 1.00, 1.09, and 1.14 (P-trend = 0.08) and across quartiles of low-calorie soda intake were 1.00, 0.99, 1.06, and 1.11 (P-trend = 0.07); the RRs in men across quartiles of sugar-sweetened soda intake were 1.00, 0.93, 0.99, and 1.05 (P-trend = 0.52) and across quartiles of low-calorie soda intake were 1.00, 0.91, 0.99, and 1.03 (P-trend = 0.42) (see supplementary Table 1 under “Supplemental data” in the online issue). We did not observe a significant interaction between sugar-sweetened or low-calorie soda intake and a history of either diabetes or hypertension (see supplementary Tables 2 and 3 under “Supplemental data” in the online issue).
Substitution of alternative beverages for soda with stroke risk
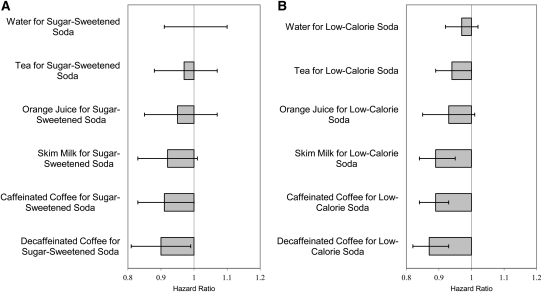
Compared with 1 serving of sugar-sweetened or low-calorie soda per day, 1 serving of either caffeinated or decaffeinated coffee per day was associated with a significantly lower risk of stroke (Figure 1, A and B). When compared with 1 serving sugar-sweetened soda/d, 1 serving decaffeinated coffee/d was associated with a 10% (95% CI: 1%, 19%) lower risk of stroke and 1 serving caffeinated coffee/d with a 9% (95% CI: 0%, 17%) lower risk. When compared with 1 serving low-calorie soda/d, 1 serving decaffeinated coffee/d was associated with a 13% (7%, 18%) lower risk of stroke and 1 serving caffeinated coffee/d with an 11% (95% CI: 7%, 16%) lower risk. Skim milk was associated with an 11% (95% CI: 5%, 16%) lower risk when substituted for low-calorie soda. When substituted for sugar-sweetened or low-calorie soda, tea and orange juice were associated with a trend toward reduced risk.
FIGURE 1.
Pooled RRs and 95% CIs associated with substitution of alternative beverages for sugar-sweetened soda (A) and low-calorie soda (B) among 43,371 men and 84,085 women (1 serving/d). RRs and variances for each substitution from each cohort were derived from a Cox proportional hazards multivariate model and then pooled in a fixed-effects model to arrive at a summary estimate of the effect of substituting one beverage for another in relation to total stroke risk; the Q statistic P value for between-study heterogeneity (null hypothesis is that there is no heterogeneity between Health Professionals Follow-Up Study and Nurses’ Health Study) for estimate of effect of substitutions is >0.05.
DISCUSSION
In these large prospective cohort studies of men and women, we observed that a higher consumption of sugar-sweetened and low-calorie sodas was associated with a higher risk of stroke. The risks appeared higher in women than in men. The associations were independent of established dietary and nondietary cardiovascular disease risk factors, including BMI and energy intake.
Multiple mechanisms may explain these findings. In sugar-sweetened sodas, the sugar load may lead to rapid increases in blood glucose and insulin, which over time lead to glucose intolerance, insulin resistance, and inflammation; these physiologic changes in turn influence atherosclerosis, plaque stability, and thrombosis—a risk factor for ischemic stroke (3). Fructose can be converted to visceral and hepatic fat (30) and enhances lipogenesis and triglyceride synthesis (31). Fructose can also increase serum uric acid, which can reduce endothelial nitric oxide and increase blood pressure—a risk factor for ischemic and hemorrhagic stroke (32). Because intake of sugar-sweetened beverages is not regulated by the body in the same manner as foods, their consumption may lead to excess caloric intake (33); however, this mechanism is unlikely to explain our findings because control for energy intake in our models did not alter our results. The caramel coloring in sugar-sweetened and low-calorie colas has advanced-glycation end products, which have been linked with inflammation, and which in turn has been linked with the initiation, growth, and destabilization of atherosclerotic lesions (3, 26). Whereas an inverse U-shaped association has been reported between caffeine intake and incident hypertension, a linear association has been reported between increasing soda intake and hypertension, which suggests that constituents in soda other than caffeine affect blood pressure (34).
In comparison with soda, coffee contains chlorogenic acids, lignans, and magnesium, which act as antioxidants and mediators of glucose metabolism and may reduce stroke risk (35). Higher intakes of potassium, magnesium, and calcium have been associated with a reduced stroke risk (36): one or more of these nutrients may help explain the reduction in risk seen when skim milk or orange juice is substituted for soda. Although a modest benefit with substitution of water for soda cannot be excluded because the CI for the substitution includes a modest benefit, the significant substitutions for soda were with beverages that appear to have inherently positive benefits for stroke reduction.
We did not observe a strong association between a diagnosis of hypertension or diabetes and a subsequent change in soda intake. We also did not find a significant interaction between soda intake and hypertension or diabetes. However, when we controlled for these diagnoses in our multivariate model, the strength of the associations between both sugar-sweetened and low-calorie soda intakes and stroke risk was attenuated. These findings suggest that hypertension and diabetes may be intermediates, rather than confounders or effect-modifiers, of the soda-stroke association.
Our work builds on previous research on the relation between sugar-sweetened beverages and incident chronic disease. Other studies have evaluated the relation between soda consumption and metabolic disorders (3, 4, 9, 37–39), yet few have examined soda consumption and incident cardiovascular disease (6, 40, 41). A case-crossover study with only 209 men and 181 women reported no increase in stroke risk in the hour after cola consumption (RR: 1.0; 95% CI: 0.4, 2.4; P = 0.95) (40), and intake was not associated with total mortality or cardiovascular mortality among 1900 men and women followed for an average of 3.8 y after an acute myocardial infarction (41). Of the sugar-sweetened beverages, cola was recently reported to be associated with the greatest risk of CAD (RR per a 2-serving/d increase: 1.35; 95% CI: 1.15, 1.57) (6), whereas low-calorie beverages were not associated with risk (6).
Our analysis had strengths and limitations. The prospective collection of data on soda consumption and lifestyle factors minimized the potential for recall bias or reverse causation. The high rate of follow-up reduced bias due to loss to follow-up. Repeated dietary intake data served to reduce random measurement error, and any residual random error would likely lead to an underestimate of the true effect of exposures with outcome. The ability to measure for known cardiovascular disease risk factors permitted control for possible confounders in multivariable models. Adjustment for the presence of behaviors, such as regular exercise and taking a daily multivitamin, reduces the likelihood that lifestyle characteristics confound the associations observed between substituting alternative beverages for soda. Nevertheless, because of the observational nature of this study, we cannot exclude the possibility of residual and unmeasured confounding. Our finding of an association between low-calorie soda intake and stroke risk should be interpreted with caution, because we previously did not find an association between low-calorie beverages and weight gain (42), diabetes (9), or CAD (6), and there is not a clear biologic mechanism between low-calorie soda consumption and incident stroke. Finally, there were few cases of hemorrhagic stroke among men, especially among men consuming ≥1 sugar-sweetened soda/d; therefore, our analysis of the association between sugar-sweetened soda consumption and hemorrhagic stroke among men must also be interpreted cautiously.
In conclusion, in these large studies of US men and women, we found that greater consumption of sugar-sweetened and low-calorie sodas was associated with a higher risk of stroke. Compared with the same number of servings of soda, consumption of caffeinated or decaffeinated coffee was associated with a lower risk. Stroke burden may therefore be reduced by changing patterns of beverage consumption in the United States.
Acknowledgments
The authors’ responsibilities were as follows—AMB and WCW: designed the research; AMB, LdK, AJF, AJF, KMR, and WCW: analyzed the data and wrote the manuscript; and AMB: conducted the research and had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors declared a conflict of interest. Neither the NIH nor the Harvard Human Nutrition Program had a role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Abbreviations used: CABG, coronary artery bypass grafting; CAD, coronary artery disease; FFQ, food-frequency questionnaire; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; PCI, percutaneous coronary intervention.
REFERENCES
- 1.Andreyeva T, Chaloupka FJ, Brownell KD. Estimating the potential of taxes on sugar-sweetened beverages to reduce consumption and generate revenue. Prev Med 2011;52:413–6 [DOI] [PubMed] [Google Scholar]
- 2.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34 [DOI] [PubMed] [Google Scholar]
- 6.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009;89:1037–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA 2010;304:2270–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 2008;117:754–61 [DOI] [PubMed] [Google Scholar]
- 9.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol 2011;6:160–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 2006;296:2939–46 [DOI] [PubMed] [Google Scholar]
- 12.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke 2006;37:345–50 [DOI] [PubMed] [Google Scholar]
- 13.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002;106:388–91 [DOI] [PubMed] [Google Scholar]
- 14.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med 2006;355:1991–2002 [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr 1999;70:1001–8 [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–9 [DOI] [PubMed] [Google Scholar]
- 17.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000;343:16–22 [DOI] [PubMed] [Google Scholar]
- 18.Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Lack of association between dietary protein intake and risk of stroke among middle-aged men. Am J Clin Nutr 2010;91:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 1991;133:810–7 [DOI] [PubMed] [Google Scholar]
- 21.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67 [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127:188–99 [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 24.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke 1981;12(suppl 1):I13–44 [PubMed] [Google Scholar]
- 25.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the National Death Index. Am J Epidemiol 1984;119:837–9 [DOI] [PubMed] [Google Scholar]
- 26.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517–84 [DOI] [PubMed] [Google Scholar]
- 27.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA 2006;296:193–201 [DOI] [PubMed] [Google Scholar]
- 28.Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and French fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr 2006;83:284–90 [DOI] [PubMed] [Google Scholar]
- 29.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bray GA. How bad is fructose? Am J Clin Nutr 2007;86(4):895–6 [DOI] [PubMed] [Google Scholar]
- 31.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011;123:2292–333 [DOI] [PubMed] [Google Scholar]
- 32.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 2007;86(4):899–906 [DOI] [PubMed] [Google Scholar]
- 33.Mattes RD. Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. Physiol Behav 1996;59(1):179–87 [DOI] [PubMed] [Google Scholar]
- 34.Winkelmayer WC, Stampfer MJ, Willett WC, Curhan GC. Habitual caffeine intake and the risk of hypertension in women. JAMA 2005;294:2330–5 [DOI] [PubMed] [Google Scholar]
- 35.van Dam RM. Coffee and type 2 diabetes: from beans to beta-cells. Nutr Metab Cardiovasc Dis 2006;16:69–77 [DOI] [PubMed] [Google Scholar]
- 36.Ding EL, Mozaffarian D. Optimal dietary habits for the prevention of stroke. Semin Neurol 2006;26:11–23 [DOI] [PubMed] [Google Scholar]
- 37.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav 2010;100:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr 2009;89:438–9 [DOI] [PubMed] [Google Scholar]
- 39.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mostofsky E, Schlaug G, Mukamal KJ, Rosamond WD, Mittleman MA. Coffee and acute ischemic stroke onset: the Stroke Onset Study. Neurology 2010;75:1583–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Caffeinated coffee consumption and mortality after acute myocardial infarction. Am Heart J 2004;147:999–1004 [DOI] [PubMed] [Google Scholar]
- 42.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]