Abstract
Protein phosphatases, together with protein kinases, regulate protein phosphorylation and dephosphorylation, and play critical roles in plant growth and biotic stress responses. However, little is known about the biological functions of plant protein tyrosine dual-specificity phosphatase (PFA-DSP) in biotic stresses. Here, we found that OsPFA-DSP2 was mainly expressed in calli, seedlings, roots, and young panicles, and localized in cytoplasm and nucleus. Ectopic overexpression of OsPFA-DSP2 in rice increased sensitivity to Magnaporthe grisea (M. grisea Z1 strain), inhibited the accumulation of hydrogen peroxide (H2O2) and suppressed the expression of pathogenesis-related (PR) genes after fungal infection. Interestingly, transgenic Arabidopsis plants overexpressing AtPFA-DSP4, which is homologous to OsPFA-DSP2, also exhibited sensitivity to Pseudomonas syringae pv. tomato DC3000 (Pst DC3000), reduced accumulation of H2O2 and decreased photosynthesic capacity after infection compared with Col-0. These results indicate that OsPFA-DSP2 and AtPFA-DSP4 act as negative regulators of the pathogen response in transgenic plants.
Introduction
Plants face a variety of biotic stresses in nature, including bacteria and fungi, which can strongly affect growth and production [1]. In response to these biotic stresses, plants have evolved many defense mechanisms [2]. In one such defense mechanism, reactive oxygen species (ROS) (H2O2, especially) play a central role in plants by controlling many biological processes, including gene expression, activation of transcription factors, redox balance, programmed cell death (PCD) and regulation of the mitogen-activated protein kinase (MAPK) pathway [3], [4], [5], [6]. Several studies have reported that high levels of ROS lead to the oxidative destruction of cells, but moderate levels can act as signaling molecules to regulate plant growth and the biotic stress response [7]. H2O2 is an important component of ROS; endogenous and exogenous H2O2 directly or indirectly killed pathogen cells or inhibited their growth, penetration and proliferation at the infection site by inducing PCD [3], [8], [9].
Reversible protein phosphorylation, mediated through the MAPK signalling cascade, plays a key role in determining the response to many external stimuli in plants, including biotic stresses [10], [11]. The process of reversible phosphorylation is controlled by a balance between the activities of protein kinases and protein phosphatases in vivo [12]. Plant MAPKs are phosphorylated (and activated) by a series of substrate-specific kinases (e.g., MAPKK, MAPKK). MAPKs are dephosphorylated, and hence deactivated, by dual-specificity MAP kinase phosphatases (DSPs) [12], [13]. The study by Asai et al. [14] reported the identification of the components in a MAPK signalling pathway in Arabidopsis. These authors demonstrated that in response to the flagellin-derived peptide flg22, AtMEKK1 (a MAPKKKs) activates AtMKK4 and AtMKK5 (two MAPKKs), which in turn activate the functionally redundant MAPKs AtMPK3 and AtMPK6. This cascade results in transcription of defense-related genes, and was shown to play an important role in resistance to both bacterial and fungal pathogen [14]. More recent studies have shown that a transcription factor in Nicotiana benthamiana, WRKY8, is phosphylated by several MAPKs (SIPK, NT4 and WIPK), which in turn results in transcription of defense-related genes, and also that phosphorylation of pathogen-inducible WRKY33 by MPK3/MPK6 in Arabidopsis is important for activating genes involved in phytoalexin biosynthesis [15], [16]. Phosphatases as well as protein kinases play critical roles in plant biotic stress. For example, the rice protein phosphatase XB15 (belonging to the PP2C subfamily), phosphorylated and inactivated protein kinase XA21, negatively regulating XA21-mediated innate immunity [17]. AtMKP2, a MAPK phosphatase, interacts differentially with AtMPK3 and AtMPK6 in the negative regulation of specific defense responses [18], [19]. Similarly, knockout mutants in AtMKP1 displayed elevated resistance to Pst DC3000 by regulating AtMPK6 activity, again suggesting that phosphatases play key roles in the biotic stresses response [20].
Based on substrate specificity, protein tyrosine phosphatases (PTPs) can be divided into many groups, such as those that utilize phosphoproteins, lipids, deoxyribonucleic acids and carbohydrates [21]. In land plants, the lipid phosphatases are classified into three groups: tumor suppressor phosphatase and tension homologue deleted in chromosome 10 (PTEN), myotubular myopathy related protein (MTMR) and plant and fungal atypical dual-specificity phosphatases (PFA-DSP) [22]. The PFA-DSP subfamily is found in land plants and fungi, and uses phosphatidylinositol as substrate [22]. We previously demonstrated that OsPFA-DSP1 is an active tyrosine-specific phosphatase, and that it acts as a negative regulator in the abiotic stress response [23]. In addition, the AtPFA-DSP1 protein from Arabidopsis thaliana has been shown to possess phosphatase activity [24]. However, the biological function of PFA-DSPs in the biotic stress response is unknown. Here, we used overexpression and RNA-interference knockdown of OsPFA-DSP2 and AtPFA-DSP4 in transgenic rice and Arabidopsis plants to analyse their biological function during biotic stress. We also assayed the expression patterns and subcellular localization of OsPFA-DSP2, and used genetic techniques to study its biological functions. Our results indicate that OsPFA-DSP2 and AtPFA-DSP4 negatively regulate the host response to a fungal (M. grisea) and a bacterial (Pst DC3000) pathogen.
Materials and Methods
Generation of transgenic plants
The overexpression construct for OsPFA-DSP2 (Loc_Os02g53160) from Oryza sativa ssp. japonica cv. Nipponbare was created by inserting a complete cDNA sequence into the vector pCXUN-flag, which contained a maize (Zea mays) ubiquitin gene promoter [25]. OsPFA-DSP2 primer sequences are as follows: (sense primer) 5′-ATGCAGCTGGAGATTTCG-3′ and (antisense primer) 5′-TTAACACTGTGAGGCCGTC-3′. The OsPFA-DSP2 interference construct was created by inserting a genomic sequence fragment (containing 125 bp of the 3′UTR and 125 bp from the translation stop codon) into the binary vector pCAMBIA1301,which carries the cauliflower mosaic virus (CaMV) 35S promoter. The recombinant plasmids were introduced into Agrobacterium tumefaciens strain EHA105 by electroporation. Agrobacterium tumefaciens mediated transformation were performing using calli derived from mature embryos of japonica line Nipponbare, according to the published protocol [26]. The primer sequences for the interference vector were as follows: sense fragment, 5′-GGTAAGCTTCGAATGTTTTTTCATATCCGGTC-3′ and 5′-GCACCATGGGATGGATACTTTTATGAGGATAA-3′, with HindIII and NcoI sites (underlined); antisense fragment, 5′-CGCACTAGTTGTATTTTGATGGATACTTTTATGAG-3′ and 5′-ATTAGGCCTCCGAACCGAATGTTTTTTCAT-3′, with SpeI and StuI sites (underlined), respectively. Transgenic rice seedlings were screened on 1/2 MS solid medium containing 50 µg/mL hygromycin.
Using gene-specific primers, the full-length cDNA sequence of AtPFA-DSP4 (At4g03960) from Col-0 was amplified and cloned into vector pEN1A between the SalI and EcoRV sites with primers 5′-AGTCGACATGACGTTAGAGAGTTAC-3′ and 5′-CGGATATCTCAGTAATCAATAGTATT-3′. The resulting vector was used to transfer the AtPFA-DSP4 gene to pEarlyGate100 via the Gateway LR reaction [27]. The resulting plasmid 35S::AtPFA-DSP4 was introduced into Agrobacterium tumefaciens strain EHA105 by electroporation. Transformation mediated by A. tumefaciens was performed as described [28]. The transgenic plants were selected by spraying 1-week-old seedlings with 0.01% Glufosinate (Basta).
Examination of transgenic plants and knockout mutants
Transgenic rice plants generated with the overexpression and interference recombinant vectors were examined by RT-PCR using two OsPFA-DSP2 gene-specific primers, 5′-ATGCAGCTGGAGATTTCG-3′ and 5′-TTAACACTGTGAGGCCGTC-3′. The expression level of the OsPFA-DSP2 gene was assayed by quantitative reverse transcription polymerase chain reaction (qRT-PCR) using the OsPFA-DSP2 sequence-specific primers, 5′-CCAGTTCGGTATTGACGG-3′ and 5′-TGAGTGCTTCTCGGATTT-3′. The expression level of the rice β-actin gene was assayed with actin-specific primers and used to standardize the RNA sample for each qRT-PCR. β-actin primers were 5′-GGTATTGTTAGCAACTGGGATG-3′ and 5′-GATGAAAGAGGGCTGGAAGA-3′, respectively. The OsPFA-DSP2 mutant was identified from a rice mutant collection (International Rice Functional Genomic Consontium; http://irfg.irri.org) harboring a Tos17 insertion into the 2nd intron of Os02g53160 (seed stock number NG8341) by PCR with the gene-specific primers, 5′-ATTGTTAGGTTGCAAGTTAGTTAAGA-3′ and 5′-GCATTTTGCTCAAACAGGGT-3′, and the Tos17-specific primer TAIL13, 5′-GAGAGCATCATCGGTTACATCTTCTC-3′. The atpfa-dsp4 mutant which obtained from the Arabidopsis Biological Resource Center(ABRC)at Ohio State University (stock name: salk_016876, named as atpfa-dsp4 mutant) was identified by PCR using the primers: 5′-ATTCCCCAAAACTTCTGA-3′ and 5′-TCTACAACCATCCGATCC-3′. The overexpression in transgenic Arabidopsis was quantified using RT-PCR with the primer pair 5′-AGTCGACATGACGTTAGAGAGTTAC-3′ and 5′-CGGATATCTCAGTAATCAATAGTATT-3′. The expression profile of AtPFA-DSP4 was detected by qRT-PCR using two sequence-specific primers, 5′-TGAGGCATATCCAGAGGT-3′ and 5′-CTACAAGACATCCCGTCC-3′. The expression of the arabidopsis ubiquitin 4 (UBQ4) gene was detected with UBQ-specific primers (5′- GCTTGGAGTCCTGCTTGGACG -3′ and 5′- CGCAGTTAAGAGGACTGTCCGGC -3′) and was used to standardize the sample for each qRT-PCR reaction.
Pathogen culture and plant infection
Magnaporthe oryzae strain Z1 was cultured on PDA medium for 5 days, then transferred to spore production medium and cultured for 24 h at 24°C with 16 h light/8 h dark. Conidia were suspended in sterilized distilled-water at a concentration of ∼3–5×105 conidial/mL. Seedlings at the three-to five-leaf stage were infected with M. grisea by the spraying and immersed infection method [17], [29]. Transgenic rice plants after inoculation were initially cultured for 24 h in the dark at 25°C with humidity >95%, followed by the same conditions for 6 days with 16 h light/8 h dark, after which they were observed for disease reaction phenotype. Disease was scored by measuring the lesion area at 7 days after infection. For all the disease evaluations, the mock-inoculated control was treated under identical conditions.
Arabidopsis plants were grown in a chamber with a 12 h light/12 h dark photoperiod at 22°C for 24 days before bacterial inoculation. Bacterial infections and growth assays with Pst DC3000 were described previously [30]. Pst DC3000 was cultured overnight at 28°C in King's B medium supplemented with 50 µg rifampicin/mL. The cells were pelleted, washed, resuspended, and diluted in H2O to a concentration of 105 cfu/mL. Arabidopsis leaves were syringe-inoculated. Plant leaves were harvested at the indicated time for ROS detection, Fv/Fm measure and bacterial counting. Bacterial growth was assessed by plating a dilution series of leaf discs ground in H2O on King's B plates containing 50 µg rifampicin/mL. The colony-forming units (cfu) were counted after incubation at 28°C for 2 days. Each data point is shown as triplicates.
Gene expression analysis
To examined the influence of rice blast infection on resistance gene (OsPR1a and OsPR5) expression, RNA was extracted from leaves with TRIzol reagent (Invitrogen, USA), and cDNA was synthesized by reverse transcription using Prime Script RTase (Takara, Japan). For qRT-PCR assays, SYBR Green I (Takara, Japan) was added to the reaction system and run on a LC480 real-time PCR detection system according the manufacturer's instruction (Roche, Switzerland). Data were analyzed using optical monitor software (Roche). The expression level of the rice β-actin gene was used to standardize the RNA (20 ng) sample for each qRT-PCR. The assays were repeated at least three times, with each repetion having three replications; similar results were obtained in repeated experiments. The SD was calculated for each data point. The sequence-specific primers were as follows: OsPR1a, 5′-CGTCTTCATCACCTGCAACTACTC-3′ and 5′-CATGCATAAACACGTAGCATAGCA-3′; OsPR5, 5′-CGCTGCCCCGACGCTTAC-3′ and 5′-ACGACTTGGTAGTTCTGTTGC-3′; β-Actin, 5′-GGTATTGTTAGCAACTGGGATG-3′ and 5′-GATGAAAGAGGGCTGGAAGA-3′.
Expression pattern analysis
Total RNA was extracted from different tissues of wild-type rice using TRIzol reagent (Invitrogen, USA). The cDNA was synthesized using Prime Script RTase (Takara, Japan) according to the manufacturer's protocol, and the expression of OsPFA-DSP2 was detected using SYBR Premix Ex Taq (Takara, Japan) with LC480 (Roche, Switzerland). The transcript levels of OsPFA-DSP2 were normalized to the transcript levels of β-actin. Each data point had three replicates, and the experiments were repeated twice. The results from the two experiments were consistent, and those from one set of experiments are shown.
The promoter of the OsPFA-DSP2 gene (1,750 bp DNA fragment upstream of the translation start site) was predicted by Osiris (http://www.bioinformatics2.wsu.edu/cgi-bin/Osiris/cgi/home.pl) and amplified with primers 5′-GGGTCTGCTGCACTATACTGG-3′ and 5′-AAAATCCTCCTCTTGGGCG-3′ from genomic DNA of wild type rice, and cloned into the vector pCXGUS-P, which carried the reporter gene GUS (β-glucuronidase) [40]. Rice tissues at different growth stages were stained and observed using a light microscope (Leica, Germany); histochemical staining was performed according to a published method [31].
Subcellular localization of OsPFA-DSP2
The full-length cDNA sequence of OsPFA-DSP2 was amplified from wild type rice with the primers 5′-GGGTCTGCTGCACTATACTGG-3′ and 5′-AAAATCCTCCTCTTGGGCG-3′, and inserted into pUC-GFP vector. The recombination vector was transiently expressed in rice protoplasts isolated from rice 9-day-old seedlings as described [32]. Cell nuclei were stained with 100 µg/mL 4′,6-diamidino-2-phenylindole (DAPI) for 15–30 minutes. Fluorescence was observed using an Olympus fluorescent microscope and visualized with Olympus DP2-BSW software.
Detection of H202 by DAB staining
Rice tissues from seedlings with 3–5 leaves (tillering stage) after inoculation were collected at 2 hpi, and then immersed in DAB solution (1 mg/mL) at pH 3.8 for three hours as described [33] and transferred to the light for 8 h. Arabidopsis tissues were treated in a similar fashion, except the vacuum treatment was reduced to 1.5 h, and then transferred to light for 5 h. Plant tissues was then cleared by boiling for 10 minutes in absolute ethanol, and then photographed with a Canon camera.
Results
Expression pattern and subcellular localization of OsPFA-DSP2
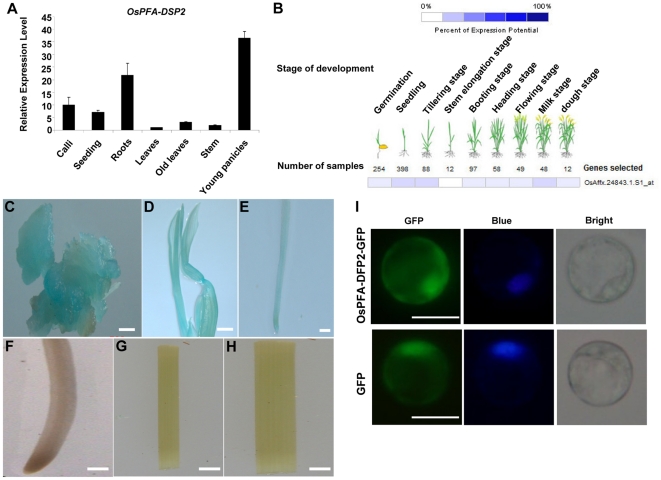
OsPFA-DSP2 is a 205 amino acid protein that has been annotated as a putative dual-specificity phosphatase (DSPs) belonging to the PFA-DSP subfamily; it contains four conserved motifs characteristic of members of the PFA-DSP subfamily [23]. To determine the expression pattern of OsPFA-DSP2, qRT-PCR and GENEVESTIGATOR analysis were carried out, and transgenic plants expressing the GUS gene under control of the OsPFA-DAP2 promoter were generated. Expression of OsPFA-DSP2 was highest in young panicles, followed by roots, calli, old leaves, stem and leaves (10-days-old) as determined by qRT-PCR (Figure 1A). The probe Os.Affx24843.1.S1_at from OsPFA-DSP2 was used to analyze an O. sativa microarray database (OS_51K: Rice Genome 51K array) in GENEVESTIGATOR V3 [34]. The results of this analysis indicated taht OsPFA-DSP2 was mainly expressed in seedlings, at the tillering stage and milk stage, and was similar to results of the qRT-PCR assay (Figure 1B).
Figure 1. Expression pattern and subcellular localization.
(A) Expression profile of OsPFA-DSP2 in different tissues of wild type rice using qRT-PCR. (B) Expression of OsPFA-DSP2 at different developmental periods using GENEVESTIGATOR V3. (C–H) Histochemical localization of GUS expression in different tissues of transgenic rice carrying the OsPFA-DSP2 promoter fused to GUS; (C) callus, (D) seedling (five day), (E) young root (five day), (F) old root (flowering stage), (G) stem, and (H) old leaf (flowering stage). (I) Subcellular localization of OsPFA-DSP2 in rice protoplasts by transient transformation, the fluorescence of GFP and DAPI are indicated by green and blue, respectively. Bars = 10 µm.
In order to further demonstrate the expression pattern of OsPFA-DSP2, the activitiy of the Escherichia coli β-glucuronidase (GUS) gene under control of the OsPFA-DSP2 promoter region (1750 bp) was examined in transgenic rice plants. A total of 15 independent transgenic lines were analyzed, and all exhibited the same pattern of GUS staining. GUS activity was strongest in calli, young roots and seedling, with very little expression detected in old roots, stems and old leaves (Figure 1C–H). Based on these results, we concluded that OsPFA-DSP2 is mainly expressed in young tissues.
To investigate the subcellular localization of the OsPFA-DSP2 protein in rice protoplasts, we created constructs for expression of OsPFA-DSP2 fused to the green fluorescence protein (GFP) under control of the 35S-CaMV promoter. The fluorescence emission of GFP was monitored in rice protoplasts using an Olympus fluorescence microscope. Nuclei were counter-stained with DAPI dye. The GFP fluorescence was distributed in the cytoplasm and nucleus (Figure 1I). OsPFA-DSP2-GFP fusion protein fluorescence was mainly localized to the cytoplasm and nucleus, which could be seen by blue DAPI staining. This result is in agreement with several previous studies that showed protein dual-specificity phosphatases such as AtIBR5, AtMKP2, AtPP2C5, OsIBR5 and OsPFA-DSP1, also localized to the cytoplasm and nucleus when analyzed as protein fusions with fluorescent protein tags [18], [23], [35], [36], [37]. These data indicate that OsPFA-DSP2 may target to both the cytoplasm and nucleus.
OsPFA-DSP2 is involved in the host response to M. grisea infection
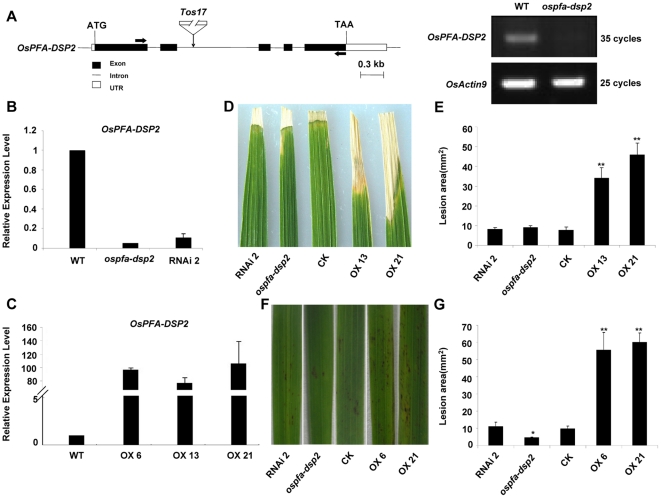
To demonstrate the function of the OsPFA-DSP2 gene in rice, the OsPFA-DSP2 deficiency mutant (seed stock number: NG8142, named as ospfa-dsp2) was chosen from the Tos17 rice database [38]. In this mutant, a retrotransposon (Tos17) has inserted into the second intron. Transcription of OsPFA-DSP2 in the mutant was not detected by semi-reverse transcription polymerase chain reaction (RT-PCR) (Figure 2A). We also generated RNAi transgenic plants to specificly silence OsPFA-DSP2; expression of OsPFA-DSP2 in the mutant and silenced plant (named RNAi2) was suppressed approximately 95% and 89% compared with that in wild type (WT) rice as detected by qRT-PCR (Figure 2B). The expression level of OsPFA-DSP2 in overexpression lines (named OX6, OX13 and OX21) was increased about 102.5-fold, 83-fold and 112.7-fold, respectively, as compared to WT (Figure 2C). To observe the phenotypes, the F2 generation of transgenic rice plants was treated with an M. grisea conidial suspension, containing 0.01% Tween 20, by two different methods. Compared with CK, which was transformed with the empty vector (pCXUN-flag vector), the overexpression lines OX13 and OX21 were sensitive to M. grisea at 7 days post inoculation (dpi); the lesion area was approximately 4.5-fold and 6-fold larger than that observed on CK (p<0.01) (Figure 2D and E). However, the symptoms of the ospfa-dsp2 mutant and RNAi2 plant did not differ appreciably from those observed on CK at 7 dpi, with the lesion area being similar (Figure 2D and E). Many more hypersensitive response (HR) spots were observed on the surface of OX6 and OX21 leaves than on CK, ospfa-dsp2 mutant and RNAi2 rice leaves at 7 days after spraying with the M. grisea conidial suspension; the lesion area of OX6 and OX21 leaves were ∼5.7-fold and 6.2-fold (p<0.01) larger than the area on CK (Figure 2F and G). The number of HR spots on the ospfa-dsp2 mutant was less than that of CK, and the area of the lesions was reduced by about half (p<0.05) (Figure 2F and G). However, the HR spots and lesion area observed on the RNAi2 line was not obviously different from CK (Figure 2F and G). These results indicated that OsPFA-DSP2 could be considered as a negative regulator involved in the plant response to M. grisea.
Figure 2. Phenotypes of the ospfa-dsp2 mutant and OsPFA-DSP2 transgenic rice plants following M. grisea infection.
(A) Genomic structure of OsPFA-DSP2 and transcription level. The retrotransposon Tos17 was inserted into the second intron; expression of OsPFA-DSP2 was detected using RT-PCR. (B) and (C) Expression of OsPFA-DSP2 in wild type rice, mutant, OsPFA-DSP2-RNAi and OsPFA-DSP2-overexpression plants was detected using qRT-PCR. (D) and (E) Phenotype and lesion area of CK, ospfa-dsp2 mutant, OsPFA-DSP2-overexpression and OsPFA-DSP2-RNAi plants at 7 days after inoculation with M. grisea conidial suspension. The experiment was repeated with three biological replicates, *P<0.05, **P<0.01.
OsPFA-DSP2 inhibits accumulation of H2O2 and expression of PR genes in response to M. grisea infection
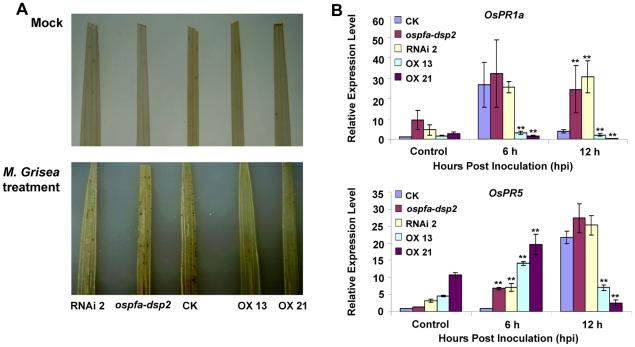
In order to further study the biological function of phosphatase OsPFA-DSP2, DAB staining was used to detect H2O2 accumulation in the leaves after infection. Leaves were stained for H2O2 at 2 hours post inoculation (hpi) with M. grisea (3–5×105 conidia/mL containing 0.01% Tween 20) and distilled-water containing 0.01% Tween 20 (control treatment). We observed that the production and accumulation of H2O2 in rice leaves was not detected by DAB staining in the control, a low level of H2O2 accumulated in the leaves of CK, the ospfa-dsp2 mutant and RNAi2 plants at 2 hpi, and H2O2 accumulation in OX13 and OX21 was minimal (Figure 3A). Also, when compared with CK, the expression level of OsPR1a in the OX13 and OX21 plants was reduced by ∼88.2% and 93% at 6 hpi (p<0.01), but the expression level of OsPR1a in the ospfa-dsp2 mutant and RNAi2 plant was increased by approximately 1.5-fold at 6 hpi (Figure 3B). The expression profile of OsPR5 in the ospfa-dsp2 mutant and RNAi2 plants was also elevated, approximately 1.2-fold compared with CK at 12 hpi, but its expression in OX13 and OX21 was reduced by ∼68% and 88% compared with CK (p<0.01) (Figure 3B). These results indicated that OsPFA-DSP2 inhibited the production and accumulation of H2O2 and suppressed the transcription of PR genes, further demonstrating that OsPFA-DSP2 plays a key role in the host response to M. grisea infection.
Figure 3. Histochemical detection of H2O2 by 3,3-diaminobenzidine (DAB at 1 mg/mL) staining and detection of defense-related genes using qRT-PCR.
(A) Production and accumulation of H2O2 in leaves at 2 hpi after M. grisea infection and mock inoculation (distilled water containing 0.01% Tween 20). (B) Detection of OsPR1a and OsPR5 expression at 6 hpi and 12 hpi following M. grisea infection by qRT-PCR. Each experiment consistd of three-independent replicates, given the representative group, **P<0.01.
AtPFA-DSP4 is involved in the host response to Pst DC3000 infection
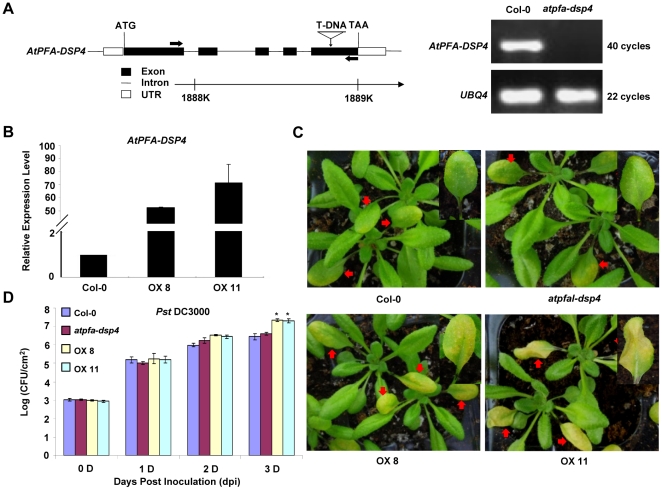
The homolog of OsPFA-DSP2 in Arabidopsis is AtPFA-DSP4; their protein sequences share a high degree of similarity (identifies = 67%). Because OsPFA-DSP2 is involved in the pathogen response, we were interested to know whether AtPFA-DSP4 has similar function. An AtPFA-DSP4 deficiency mutant (stock name: salk_016876, named as atpfa-dsp4 mutant) was obtained from the Arabidopsis Biological Resource Center (ABRC) at The Ohio State University. The T-DNA was determined to be inserted in the fifth exon by analyzing the information from database and the gene sequence; transcription of AtPFA-DSP4 in the mutant was not detected by RT-PCR (Figure 4A). We also generated AtPFA-DSP4-overexpressing transgenic plants; two independent overexpression lines, OX8 and OX11, were used in our experiments. The expression levels of AtPFA-DSP4 in OX8 and OX11 were increased ∼55.5-fold and 74.5-fold compared with Col-0, respectively (Figure 4B). The Arabidopsis plants were treated with a Pst DC3000 suspension in distilled water. Compared with Col-0, OX8 and OX11 lines were sensitive to Pst DC3000 at 3 dpi; based on color, the lesions on OX8 and OX11 leaves which were injected with the Pst DC3000 suspension were more extensive than those observed on Col-0 (Figure 4C). However, the lesion area on the atfpa-dsp4 mutant was not appreciably different than Col-0 (Figure 4C). We also calculated the amount of Pst DC3000 per unit area in leaves infected with Pst DC3000. The bacterial cell counts in OX8 and OX11 plants were 1.14-fold and 1.13-fold higher, respectively, than in Col-0 at 3 dpi, but the bacterial cell count in the mutant was not distinct from Col-0 at 3 dpi (Figure 4D). These results indicated that AtPFA-DSP4 is involved in Pst DC3000 response and negatively regulated the process.
Figure 4. Phenotypes of atpfa-dsp4 mutant, Col-0 and AtPFA-DSP4-overexpression plants following Pst DC3000 infection.
(A) Genomic structure and transcription of AtPFA-DSP4 detected using RT-PCR. The T-DNA was inserted into the fifth exon. (B) Expression of AtPFA-DSP4 in Col-0 and overexpression plants was quantified using qRT-PCR. (C) Phenotype of Col-0, atpfa-dsp4 mutant, and AtPFA-DSP4-overexpression plants at 3 days after Pst DC3000 infection. (D) Bacterial counts in leaves of Col-0, atpfa-dsp4 mutant, and AtPFA-DSP4-overexpression plants at different days after inoculation with Pst DC3000. The experiment was repeated with three biological replicates *P<0.05.
AtPFA-DSP4 inhibited accumulation of H2O2 and photosynthesis in the Pst DC3000 response
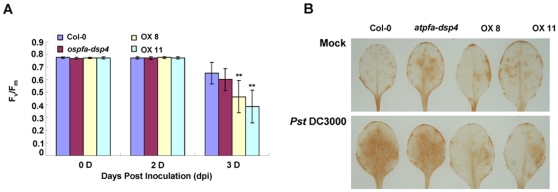
We examined the effects of AtPFA-DSP4 on the photosynthesic capability of Arabidopsis after Pst DC3000 infection. The results of this study showed that AtPFA-DSP4 inhibited photosynthesis in all plants at 3 dpi, but the inhibition observed in OX8 and OX11 plants was reduced by approximately 29% and 41% compared with Col-0 (Figure 5A). Inhibition of photosynthesis in the atfpa-dsp4 mutant was not obviously different from Col-0 (Figure 5A). We also detected the generation and accumulation of H2O2 in the leaves at 2 hpi using DAB staining. The mock treatment (distilled water) did not have an obvious affect on the production and accumulation of H2O2 in leaves at 2 hpi. However, infection with Pst DC3000 resulted in a marked increase in the accumulation of H2O2 in leaves of Col-0 and the atpfa-dsp4 mutant, but not in leaves of the OX8 and OX11 plants (Figure 5B). Based on these results, we concluded that AtPFA-DSP4 inhibited photosynthesis and suppressed production and accumulation of H2O2 during the response to bacterial infection.
Figure 5. Photosynthesic inhibition and accumulation of H2O2.
(A) The effects of AtPFA-DSP4 on inhibition of photosynthesis. The photosynthesic capacity of Col-0, atpfa-dsp4 mutant and AtPFA-DSP4-overexpression plants was detected using the IMAGE-PAM system at days zero, two and three following Pst DC3000 inoculation. The experiment was repeated with three replicates (plants: n = 10), and averaged, **P<0.01, compared with Col-0 under same treatment condition. (B) Detection of H2O2 in leaves at 2 hpi after mock (distilled water) treatment and Pst DC3000 inoculation. The experiment was repeated with three independent replicates (leaves: n = 4).
Discussion
Protein phosphatases dephosphorylate activated protein kinases, and play important roles in plant processes such as growth and the reponse to abiotic and biotic stresses [12], [39], [40], [41]. The results presented here show that OsPFA-DSP2 negatively regulates the pathogen response in rice. In the ospfa-dsp2 mutant and RNAi2 plants, the expression profiles of OsPR1a and OsPR5 were enhanced in the early stages of M. grisea infection compared with CK, and the lesion area was similar to that of CK at 7 dpi (Figure 2D–G and 3B). The phenotype of the ospfa-dsp2 mutant and RNAi2 plants was not obviously different from the CK at 7 dpi, leading us to suggest that the homologous genes exist in rice. The expression of OsPR1a and OsPR5 in OX13 and OX21 plants was decreased compared with CK, and the lesion area was more extensive than in CK, because of overexpression of OsPFA-DSP2 in rice (Figure 2D–G and 3B). A previous study showed that a mutant deficient in AtMKP1 expression suppressed the proliferation of Pst DC3000 and elevated the resistance to Pst DC3000 through the SA-mediated signalling pathway [20]. The function of OsPFA-DSP2, which negatively regulates the response to M. grisea infection, was consistent with the function of AtMKP1 [20]. However, rice plants lacking the phosphatase XB15 (a member of the PP2C subfamily) were sensitive to M. grisea, and its function was contrary to that of phosphatase OsPFA-DSP2 [17].
We also demonstrated that AtPFA-DSP4 acts as a negative regulator in the host response to Pst DC3000 infection. The AtPFA-DSP4 mutant showed reduced bacterial proliferation and enhanced resistance to Pst DC3000, but OX8 and OX11 plants were susceptible to Pst DC3000 at 3 dpi (Figure 4C and D). Several studies have shown that AtMKP1 and AtPTP1 negatively regulate the AtMPK3/6 signaling pathway, and act to inhibit biosynthesis of salicylic acid (SA). However, the AtMKP1 mutant displayed growth defects and enhanced resistance to pathogen Pst DC3000 [20], [42]. The function of AtPFA-DSP4 was found to be similar to that of AtMKP1, and was contrary to the function of AtMKP2 [18], [20].
Pathogen infection leads to the generation and accumulation of ROS in plants; H2O2 is an important component of ROS, and plays key roles in plants, such as regulating growth and development, signal transduction and oxidative damage [4], [43], [44], [45]. The results of our study showed that OsPFA-DSP2 and AtPFA-DSP4 inhibited the production and accumulation of H2O2 in leaves of overexpression lines in the early stages of infection. Recent studies have reported that endogenous and exogenous H2O2 inhibited pathogen cell growth, penetration and proliferation at the infection site by inducing PCD [3], [8], [9]. H2O2 levels in transgenic plants overexpressing OsPFA-DSP2 or AtPFA-DSP4 did not accumulate to control levels, which lead to inactivation of downstream signaling molecules and reduced expression of defense-related genes, such as PR genes. Pst DC3000 grew and proliferated rapidly in the overexpression lines, which were more susceptible to pathogens. However, because the appearance of black spots on the leaf surface of OX13 and OX21 plants was observed at 7 dpi, we suggest that H2O2 in the overexpression lines is produced during the later stages of fungal infection, leading to the appearance of HR symptoms (Figure 2F).
The results presented here show that AtPFA-DSP4 and OsPFA-DSP2 (data not shown) inhibited the photosynthesic capacity during the later stages of infection. Pathogen inoculation may result in the production and accumulation of H2O2, but a certain percentage of H2O2 is derived from the chloroplast [4], [5], [7], [45]. H2O2 as a signaling molecule can negatively regulate photosynthesic capacity [46]. Therefore, we presumed that the integrity of chloroplasts was impaired by intense H2O2 production during the later stages of infection, leading to the observed decrease in photosynthesis in the overexpression lines. However, the production and accumulation of H2O2 in overexpression plants was not synchronous with the inhibition of photosynthesis. On the one hand, as a first defense system, the burst of H2O2 is rapid; on the other hand, chloroplast act as a semi-independent organelle, have the ability of auto-repair, resulting in delaying the inhibition of photosynthesic capacity. H2O2 is an upstream signaling molecule, and is involved in regulating the MAPK cascade [20], [42], [47].
In conclusion, we have demonstrated that two novel homologous genes from rice and Arabidopsis, OsPFA-DSP2 and AtPFA-DSP4, function as negative regulators in the pathogen response through the H2O2-mediated pathway. However, H2O2 plays critical roles in different signaling cascades, and which H2O2-mediated pathway is modulated by OsPFA-DSP2 and AtPFA-DSP4 is not clear. To further understand the functions of OsPFA-DSP2 and AtPFA-DSP4 in response to biotic stress, it will be critical to identify the upstream and downstream effectors which function in the OsPFA-DSP2 and AtPFA-DSP4 signaling pathways, and to demonstrate whether OsPFA-DSP2 and AtPFA-DSP4 function in biotic response.
Acknowledgments
We thank Professor Nan Yao (Sun Yat-sen University, Guangzhou, China) and Dr Jingli Lin (South China Agriculture University, Guangzhou, China) for providing pathogen strains.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by grants from the National Natural Science Foundation of China (no. 30800600 and no. 30970237), the Natural Science Foundation of Guangdong Province, People's Republic of China (no. 8151027501000016) and the Fundamental Research Funds for the Central Universities (10lgpy34). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ribot C, Hirsch J, Balzergue S, Tharreau D, Notteghem JL, et al. Susceptibility of rice to the blast fungus, Magnaporthe grisea. J Plant Physiol. 2008;165:114–124. doi: 10.1016/j.jplph.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 3.Mellersh DG, Foulds IV, Higgins VJ, Heath MC. H2O2 plays different roles in determining penetration failure in three diverse plant-fungal interactions. Plant J. 2002;29:257–268. doi: 10.1046/j.0960-7412.2001.01215.x. [DOI] [PubMed] [Google Scholar]
- 4.Nanda AK, Andrio E, Marino D, Pauly N, Dunand C. Reactive oxygen species during plant-microorganism early interactions. J Integr Plant Biol. 2010;52:195–204. doi: 10.1111/j.1744-7909.2010.00933.x. [DOI] [PubMed] [Google Scholar]
- 5.Quan LJ, Zhang B, Shi WW, Li HY. Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol. 2008;50:2–18. doi: 10.1111/j.1744-7909.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- 6.Torres MA. ROS in biotic interactions. Physiol Plant. 2010;138:414–429. doi: 10.1111/j.1399-3054.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 7.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Kotchoni SO, Gachomo EW. The reactive oxygen species network pathways:an essential prerequisite for perception of pathogen attack and the acquired disease resistance in plants. J Biosci. 2006;31:389–404. doi: 10.1007/BF02704112. [DOI] [PubMed] [Google Scholar]
- 9.Qin G, Liu J, Cao B, Li B, Tian S. Hydrogen peroxide acts on sensitive mitochondrial proteins to induce death of a fungal pathogen revealed by proteomic analysis. PLoS One. 2011;6:e21945. doi: 10.1371/journal.pone.0021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartels S, Gonzalez Besteiro MA, Lang D, Ulm R. Emerging functions for plant MAP kinase phosphatases. Trends Plant Sci. 2010;15:322–329. doi: 10.1016/j.tplants.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Luan S. Protein phosphatases in plants. Annu Rev Plant Biol. 2003;54:63–92. doi: 10.1146/annurev.arplant.54.031902.134743. [DOI] [PubMed] [Google Scholar]
- 12.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 13.Pitzschke A, Schikora A, Hirt H. MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol. 2009;12:421–426. doi: 10.1016/j.pbi.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 15.Ishihama N, Yamada R, Yoshioka M, Katou S, Yoshioka H. Phosphorylation of the Nicotiana benthamiana WRKY8 transcription factor by MAPK functions in the defense response. Plant Cell. 2011;23:1153–1170. doi: 10.1105/tpc.110.081794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao G, Meng X, Liu Y, Zheng Z, Chen Z, et al. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell. 2011;23:1639–1653. doi: 10.1105/tpc.111.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CJ, Peng Y, Chen X, Dardick C, Ruan D, et al. Rice XB15, a protein phosphatase 2C, negatively regulates cell death and XA21-mediated innate immunity. PLoS Biol. 2008;6:e231. doi: 10.1371/journal.pbio.0060231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumbreras V, Vilela B, Irar S, Sole M, Capellades M, et al. MAPK phosphatase MKP2 mediates disease responses in Arabidopsis and functionally interacts with MPK3 and MPK6. Plant J. 2010;63:1017–1030. doi: 10.1111/j.1365-313X.2010.04297.x. [DOI] [PubMed] [Google Scholar]
- 19.Vilela B, Pages M, Lumbreras V. Regulation of MAPK signaling and cell death by MAPK phosphatase MKP2. Plant Signal Behav. 2010;5:1497–1500. doi: 10.4161/psb.5.11.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson JC, Bartels S, Gonzalez Besteiro MA, Shahollari B, Ulm R, et al. Arabidopsis MAP Kinase Phosphatase 1 (AtMKP1) negatively regulates MPK6-mediated PAMP responses and resistance against bacteria. Plant J. 2011;67:258–268. doi: 10.1111/j.1365-313X.2011.04588.x. [DOI] [PubMed] [Google Scholar]
- 21.Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Roma-Mateo C, Rios P, Tabernero L, Attwood TK, Pulido R. A novel phosphatase family, structurally related to dual-specificity phosphatases, that displays unique amino acid sequence and substrate specificity. J Mol Biol. 2007;374:899–909. doi: 10.1016/j.jmb.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Fan J, Zhang Y, Mu P, Wang P, et al. OsPFA-DSP1, a rice protein tyrosine phosphatase, negatively regulates drought stress responses in transgenic tobacco and rice plants. Plant Cell Rep. 2012 doi: 10.1007/s00299-011-1220-x. doi: 10.1007/s00299-012-1231-2. [DOI] [PubMed] [Google Scholar]
- 24.Aceti DJ, Bitto E, Yakunin AF, Proudfoot M, Bingman CA, et al. Structural and functional characterization of a novel phosphatase from the Arabidopsis thaliana gene locus At1g05000. Proteins. 2008;73:241–253. doi: 10.1002/prot.22041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Songkumarn P, Liu J, Wang GL. A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol. 2009;150:1111–1121. doi: 10.1104/pp.109.137125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toki S, Hara N, Ono K, Onodera H, Tagiri A, et al. Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J. 2006;47:969–976. doi: 10.1111/j.1365-313X.2006.02836.x. [DOI] [PubMed] [Google Scholar]
- 27.Earley KW, Haag JR, Pontes O, Opper K, Juehne T, et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006;45:616–629. doi: 10.1111/j.1365-313X.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- 28.Torii KU, McNellis TW, Deng XW. Functional dissection of Arabidopsis COP1 reveals specific roles of its three structural modules in light control of seedling development. EMBO J. 1998;17:5577–5587. doi: 10.1093/emboj/17.19.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuoka S, Saka N, Koga H, Ono K, Shimizu T, et al. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science. 2009;325:998–1001. doi: 10.1126/science.1175550. [DOI] [PubMed] [Google Scholar]
- 30.He P, Shan L, Lin NC, Martin GB, Kemmerling B, et al. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell. 2006;125:563–575. doi: 10.1016/j.cell.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Su J, Duan S, Ao Y, Dai J, et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods. 2011;7:30. doi: 10.1186/1746-4811-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato T, Tanabe S, Nishimura M, Ohtake Y, Nishizawa Y, et al. Differential responses of rice to inoculation with wild-type and non-pathogenic mutants of Magnaporthe oryzae. Plant Mol Biol. 2009;70:617–625. doi: 10.1007/s11103-009-9495-9. [DOI] [PubMed] [Google Scholar]
- 34.Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, et al. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics. 2008;2008:420747. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brock AK, Willmann R, Kolb D, Grefen L, Lajunen HM, et al. The Arabidopsis mitogen-activated protein kinase phosphatase PP2C5 affects seed germination, stomatal aperture, and abscisic acid-inducible gene expression. Plant Physiol. 2010;153:1098–1111. doi: 10.1104/pp.110.156109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JS, Wang S, Sritubtim S, Chen JG, Ellis BE. Arabidopsis mitogen-activated protein kinase MPK12 interacts with the MAPK phosphatase IBR5 and regulates auxin signaling. Plant J. 2009;57:975–985. doi: 10.1111/j.1365-313X.2008.03741.x. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Feng D, Zhang D, Su J, Yang Z, et al. Rice MAPK phosphatase IBR5 negatively regulates drought stress tolerance in transgenic Nicotiana tabacum. Plant Sci. 2012;188–189:10–18. doi: 10.1016/j.plantsci.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Hirochika H, Sugimoto K, Otsuki Y, Tsugawa H, Kanda M. Retrotransposons of rice involved in mutations induced by tissue culture. Proc Natl Acad Sci U S A. 1996;93:7783–7788. doi: 10.1073/pnas.93.15.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta R, Ting JT, Sokolov LN, Johnson SA, Luan S. A tumor suppressor homolog, AtPTEN1, is essential for pollen development in Arabidopsis. Plant Cell. 2002;14:2495–2507. doi: 10.1105/tpc.005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monroe-Augustus M, Zolman BK, Bartel B. IBR5, a dual-specificity phosphatase-like protein modulating auxin and abscisic acid responsiveness in Arabidopsis. Plant Cell. 2003;15:2979–2991. doi: 10.1105/tpc.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh A, Giri J, Kapoor S, Tyagi AK, Pandey GK. Protein phosphatase complement in rice: genome-wide identification and transcriptional analysis under abiotic stress conditions and reproductive development. BMC Genomics. 2010;11:435. doi: 10.1186/1471-2164-11-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartels S, Anderson JC, Gonzalez Besteiro MA, Carreri A, Hirt H, et al. MAP kinase phosphatase1 and protein tyrosine phosphatase1 are repressors of salicylic acid synthesis and SNC1-mediated responses in Arabidopsis. Plant Cell. 2009;21:2884–2897. doi: 10.1105/tpc.109.067678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neill S, Desikan R, Hancock J. Hydrogen peroxide signalling. Curr Opin Plant Biol. 2002;5:388–395. doi: 10.1016/s1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 44.Overmyer K, Brosche M, Kangasjarvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 45.Swanson S, Gilroy S. ROS in plant development. Physiol Plant. 2010;138:384–392. doi: 10.1111/j.1399-3054.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 46.Mullineaux P, Karpinski S. Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol. 2002;5:43–48. doi: 10.1016/s1369-5266(01)00226-6. [DOI] [PubMed] [Google Scholar]
- 47.Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci U S A. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]