Abstract
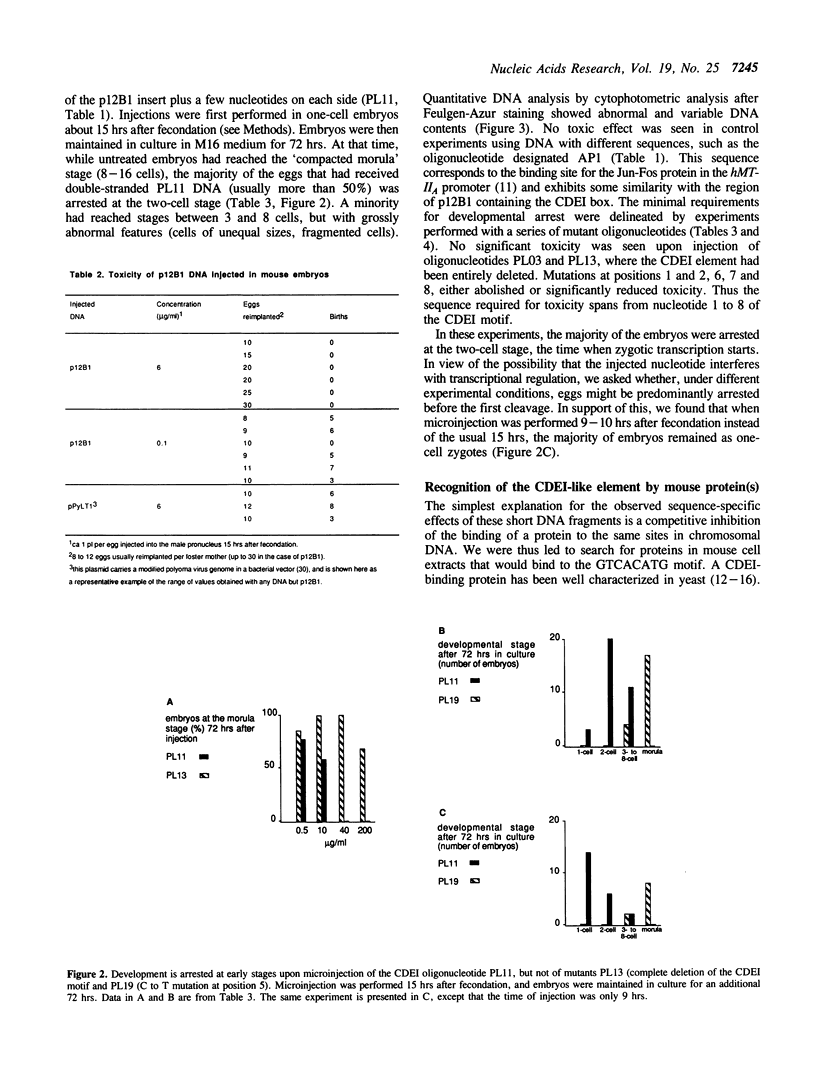
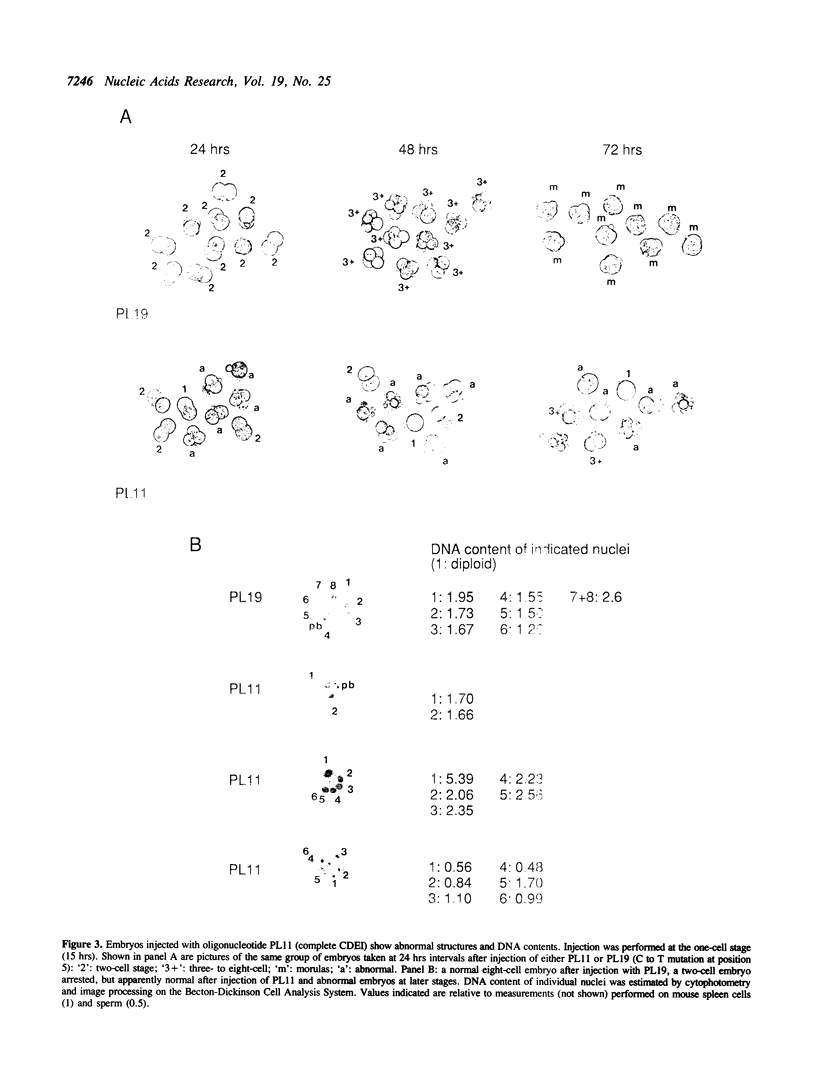
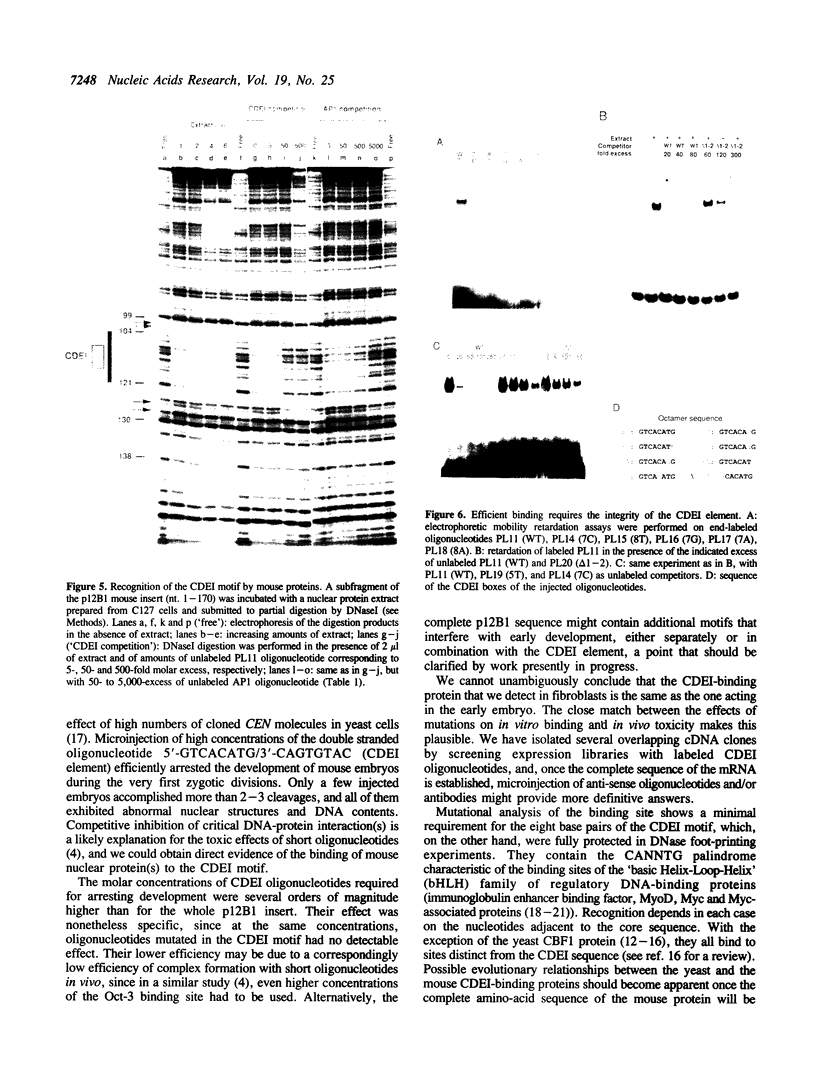
We have reported previously (1) two unexpected consequences of the microinjection into fertilized mouse eggs of a recombinant plasmid designated p12B1, carrying a 343 bp insert of non-repetitive mouse DNA. Injected at very low concentrations, this plasmid could be established as an extrachromosomal genetic element. When injected in greater concentration, an early arrest of embryonic development resulted. In the present work, we have studied this toxic effect in more detail by microinjecting short synthetic oligonucleotides with sequences from the mouse insert. Lethality was associated with the nucleotide sequence GTCACATG, identical with the CDEl element of yeast centromeres. Development of injected embryos was arrested between the one-cell and the early morula stages, with abnormal structures and DNA contents. Electrophoretic mobility shift and DNAse foot-printing assays demonstrated the binding of mouse nuclear protein(s) to the CDEl-like box. Base changes within the CDEl sequence prevented both the toxic effects in embryos and the formation of protein complex in vitro, suggesting that protein binding at such sites in chromosomal DNA plays an important role in early development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati B. B., Gasser S. M. Chromosomal ARS and CEN elements bind specifically to the yeast nuclear scaffold. Cell. 1988 Sep 23;54(7):967–978. doi: 10.1016/0092-8674(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Baker R. E., Masison D. C. Isolation of the gene encoding the Saccharomyces cerevisiae centromere-binding protein CP1. Mol Cell Biol. 1990 Jun;10(6):2458–2467. doi: 10.1128/mcb.10.6.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- Blackwood E. M., Eisenman R. N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- Bloom K., Hill A., Kenna M., Saunders M. The structure of a primitive kinetochore. Trends Biochem Sci. 1989 Jun;14(6):223–227. doi: 10.1016/0968-0004(89)90031-5. [DOI] [PubMed] [Google Scholar]
- Bram R. J., Kornberg R. D. Isolation of a Saccharomyces cerevisiae centromere DNA-binding protein, its human homolog, and its possible role as a transcription factor. Mol Cell Biol. 1987 Jan;7(1):403–409. doi: 10.1128/mcb.7.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M. J., Davis R. W. Purification of a yeast centromere-binding protein that is able to distinguish single base-pair mutations in its recognition site. Mol Cell Biol. 1989 Jun;9(6):2544–2550. doi: 10.1128/mcb.9.6.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M., Davis R. W. Yeast centromere binding protein CBF1, of the helix-loop-helix protein family, is required for chromosome stability and methionine prototrophy. Cell. 1990 May 4;61(3):437–446. doi: 10.1016/0092-8674(90)90525-j. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Raymondjean M., Carranca A. G., Herbomel P., Yaniv M. Factors involved in control of tissue-specific expression of albumin gene. Cell. 1987 Aug 14;50(4):627–638. doi: 10.1016/0092-8674(87)90036-5. [DOI] [PubMed] [Google Scholar]
- Clarke L. Centromeres of budding and fission yeasts. Trends Genet. 1990 May;6(5):150–154. doi: 10.1016/0168-9525(90)90149-z. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Weiss M., Darnell J. E., Jr Liver-specific RNA metabolism in hepatoma cells: variations in transcription rates and mRNA levels. Mol Cell Biol. 1985 Oct;5(10):2633–2641. doi: 10.1128/mcb.5.10.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futcher B., Carbon J. Toxic effects of excess cloned centromeres. Mol Cell Biol. 1986 Jun;6(6):2213–2222. doi: 10.1128/mcb.6.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galup C., Léopold P., Trejo-Avila L., el Baze P., Rassoulzadegan M., Gaudray P., Cuzin F. High affinity binding of the large T protein of polyoma virus to a genomic mouse DNA sequence. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1053–1062. doi: 10.1016/s0006-291x(87)80238-3. [DOI] [PubMed] [Google Scholar]
- Haslinger A., Karin M. Upstream promoter element of the human metallothionein-IIA gene can act like an enhancer element. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8572–8576. doi: 10.1073/pnas.82.24.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. H. The molecular and cellular basis of preimplantation mouse development. Biol Rev Camb Philos Soc. 1981 Aug;56(3):463–498. doi: 10.1111/j.1469-185x.1981.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly W., Buchman A., Kornberg R., Rine J. Roles of two DNA-binding factors in replication, segregation and transcriptional repression mediated by a yeast silencer. EMBO J. 1988 Jul;7(7):2241–2253. doi: 10.1002/j.1460-2075.1988.tb03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léopold P., Vailly J., Cuzin F., Rassoulzadegan M. Germ line maintenance of plasmids in transgenic mice. Cell. 1987 Dec 24;51(6):885–886. doi: 10.1016/0092-8674(87)90575-7. [DOI] [PubMed] [Google Scholar]
- Magnuson T., Epstein C. J. Genetic control of very early mammalian development. Biol Rev Camb Philos Soc. 1981 Aug;56(3):369–408. doi: 10.1111/j.1469-185x.1981.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pluta A. F., Cooke C. A., Earnshaw W. C. Structure of the human centromere at metaphase. Trends Biochem Sci. 1990 May;15(5):181–185. doi: 10.1016/0968-0004(90)90158-8. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Léopold P., Vailly J., Cuzin F. Germ line transmission of autonomous genetic elements in transgenic mouse strains. Cell. 1986 Aug 15;46(4):513–519. doi: 10.1016/0092-8674(86)90876-7. [DOI] [PubMed] [Google Scholar]
- Rosner M. H., De Santo R. J., Arnheiter H., Staudt L. M. Oct-3 is a maternal factor required for the first mouse embryonic division. Cell. 1991 Mar 22;64(6):1103–1110. doi: 10.1016/0092-8674(91)90265-z. [DOI] [PubMed] [Google Scholar]
- Thomas D., Cherest H., Surdin-Kerjan Y. Elements involved in S-adenosylmethionine-mediated regulation of the Saccharomyces cerevisiae MET25 gene. Mol Cell Biol. 1989 Aug;9(8):3292–3298. doi: 10.1128/mcb.9.8.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F. Centromeres of mammalian chromosomes. Trends Genet. 1990 Dec;6(12):410–416. doi: 10.1016/0168-9525(90)90302-m. [DOI] [PubMed] [Google Scholar]
- Zhu Z. Y., Veldman G. M., Cowie A., Carr A., Schaffhausen B., Kamen R. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984 Jul;51(1):170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]