Abstract
Extra-intestinal pathogenic Escherichia coli (ExPEC) strains cause many diseases in humans and animals. While remaining asymptomatic, they can colonize the intestine for subsequent extra-intestinal infection and dissemination in the environment. We have previously identified the fos locus, a gene cluster within a pathogenicity island of the avian ExPEC strain BEN2908, involved in the metabolism of short-chain fructooligosaccharides (scFOS). It is assumed that these sugars are metabolized by the probiotic bacteria of the microbiota present in the intestine, leading to a decrease in the pathogenic bacterial population. However, we have previously shown that scFOS metabolism helps BEN2908 to colonize the intestine, its reservoir. As the fos locus is located on a pathogenicity island, one aim of this study was to investigate a possible role of this locus in the virulence of the strain for chicken. We thus analysed fos gene expression in extracts of target organs of avian colibacillosis and performed a virulence assay in chickens. Moreover, in order to understand the involvement of the fos locus in intestinal colonization, we monitored the expression of fos genes and their implication in the growth ability of the strain in intestinal extracts of chicken. We also performed intestinal colonization assays in axenic and Specific Pathogen-Free (SPF) chickens. We demonstrated that the fos locus is not involved in the virulence of BEN2908 for chickens and is strongly involved in axenic chicken cecal colonization both in vitro and in vivo. However, even if the presence of a microbiota does not inhibit the growth advantage of BEN2908 in ceca in vitro, overall, growth of the strain is not favoured in the ceca of SPF chickens. These findings indicate that scFOS metabolism by an ExPEC strain can contribute to its fitness in ceca but this benefit is fully dependent on the bacteria present in the microbiota.
Introduction
Extra-intestinal pathogenic E. coli (ExPEC) strains are responsible for a wide range of diseases in humans and animals. These strains have been isolated either from urinary tract infections, neonatal meningitis, cases of septicaemia of various origins, pneumonia, deep surgical wound infections and mastitis, or from colibacillosis in poultry, a systemic infection that starts in the respiratory tract [1]–[5]. This respiratory disease is characterized by fibrinopurulent lesions of internal organs such as air sacculitis, perihepatitis and pericarditis and is often associated with septicaemia and mortality [6], [7]. These extra-intestinal diseases represent a serious economic, medical and veterinary burden [8].
ExPEC strains can asymptomatically colonize the intestinal tract of humans and animals as commensal bacteria. Consequently, the intestine serves as a reservoir for pathogenic strains. Intestinal colonization by ExPEC is thus a potential risk factor for a subsequent extra-intestinal infection in the same host or for dissemination of pathogens in the environment, thus leading to a potential zoonotic risk [9]–[14]. The establishment of ExPEC in the intestine appears to play an important role in their future establishment in the urinary or respiratory tract. For instance, strains involved in urinary tract infections gain access to the periurethral area from the anus and establish infection in an ascending manner [15]. Strains involved in neonatal meningitis could translocate from the intestinal lumen of the neonate to the blood stream, and poultry inhale pathogenic E. coli in dust derived from faeces [6], [7], [16].
ExPEC strains mainly belong to the phylogenetical lineage B2 [2], [17]. It has been shown that strains belonging to this phylogenetic group have a greater ability to persist in the intestinal tract of healthy or infected people [18]–[21]. It has been suggested that virulence factors of ExPEC such as adhesins, or iron acquisition factors could confer a higher capacity to colonize their reservoir [18], [19], [22]. For example, the K5 capsule and P fimbriae enhance intestinal colonization in gnotobiotic rats [23], [24]. Moreover, the frz operon of the avian ExPEC strain BEN2908 and the pathogenicity islands of the uropathogenic strain 536 are fitness elements involved in intestinal colonization [25], [26]. Maintenance of intestinal colonization thus requires many properties, one of the most important being metabolic competence, in addition to virulence factors. When two strains compete for a limited nutrient, the one that is able to use it more efficiently should outcompete the other [26], [27].
We have recently shown that the ability of the strain BEN2908 to metabolize short-chain fructooligosaccharides (scFOS) enhances colonization of the chicken intestine by bacteria during the first 8 days post-inoculation [28]. scFOS are natural linear polymers comprising two to four β-(2-1)-linked fructosyl units, usually attached to a terminal glucose residue [29]. Like many complex plant carbohydrates, these sugars are not hydrolyzed by digestive enzymes, and therefore they reach the distal parts of the intestine intact where they are assimilated by the intestinal microbiota, particularly probiotic bacteria [30], [31].
The genomic region responsible for scFOS metabolism in the BEN2908 strain, called the fos locus (GenBank accession no. AY857617), is found on the AGI-3 pathogenicity island [32]. This locus is composed of six genes organized as an operon encoding for a putative MFS (Major Facilitator Superfamily) sugar transporter (FosT), two glycoside hydrolases of family 32 (FosE1 and FosE2), two proteins of unknown function (FosX and FosY), a fructokinase (FosK), and of a divergently transcribed gene encoding for a putative transcriptional regulator of the LacI/GalR family (FosR). We previously defined a regulatory model of scFOS metabolism in BEN2908 [33]. In the absence of scFOS, FosR is able to bind to the promoter of the fos operon on two operator sequences, suppressing fos gene expression. Moreover, fos gene expression relies on catabolite repression, and the presence of glucose represses this expression. It has also been shown that fos gene expression depends on the presence of scFOS in the medium.
Due to the presence of the fos locus of the ExPEC strain BEN2908 on the AGI-3 pathogenicity island, one aim of this study was to investigate a possible role of these genes in the virulence of BEN2908 for chickens by analysing fos gene expression in extracts of target organs of avian colibacillosis and by performing a virulence assay in chickens. Moreover, in order to study the involvement of the fos locus in the colonization of the strain's reservoir, we analyzed fos gene expression and the implication of these genes in the growth ability of BEN2908 in different intestinal extracts. Finally, we performed intestinal colonization assays in axenic and SPF (Specific Pathogen-Free) chickens. We found that the fos locus is not involved in the virulence of BEN2908 for chickens and that, even if this locus is strongly involved in axenic chicken intestinal colonization in the ceca, it does not significantly contribute to cecal colonization of SPF chickens.
Results
The fos locus is not involved in virulence
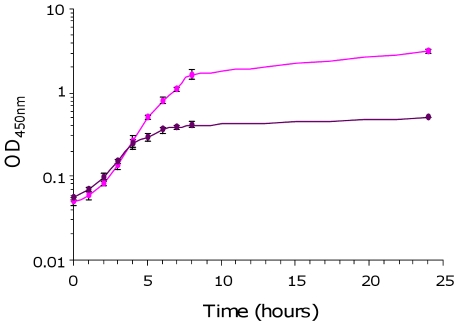
As the fos locus is located on the AGI-3 pathogenicity island [32], we investigated a possible role of this locus in the virulence of the BEN2908 strain for chicken. Firstly, we examined fos gene expression in minimal media containing extracts of target organs of avian colibacillosis including the lung, liver, spleen and pericardial fluid. As the average body temperature of chicken is 41.5°C [34], we first checked the growth ability of the strain BEN2908 in M9 minimal medium containing scFOS at 41.5°C. Surprisingly, this strain was impaired in its ability to grow in this minimal medium at 41.5°C compaired to 37°C (Fig. 1). This impairment was also observed in M9 minimal medium containing glucose but not in a more complex medium such as LB-Miller medium (data not shown). As growth is altered in M9 minimal medium at 41.5°C, we thus performed the experiments at 37°C.
Figure 1. Growth of E. coli strain BEN2908 in the presence of scFOS at 37°C and 41.5°C.
Growth curves (OD450 nm) of strain BEN2908 grown with shaking at 37°C (pink circle) or 41.5°C (purple circle) in M9 minimal medium supplemented with 0.2% scFOS. The average values and standard deviations result from three independent experiments.
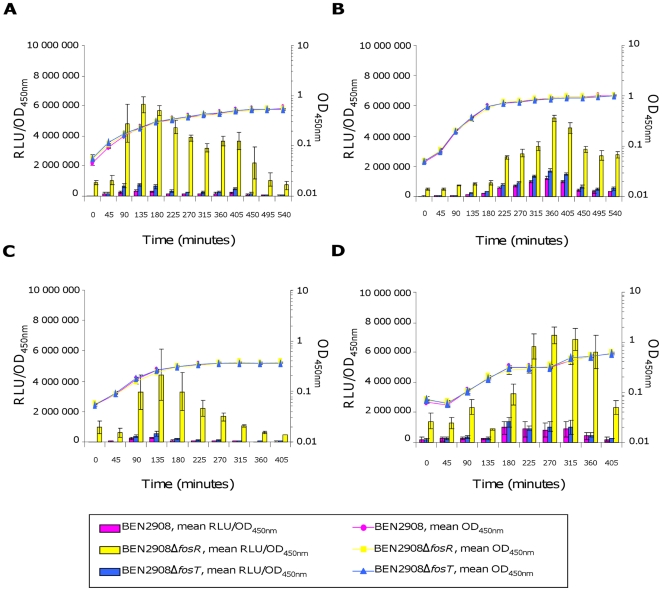
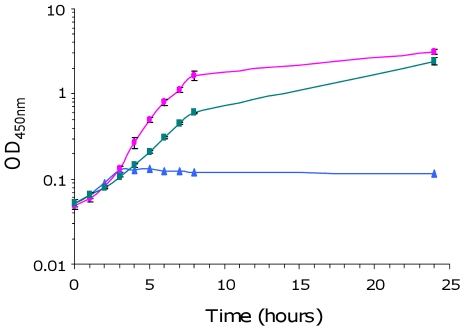
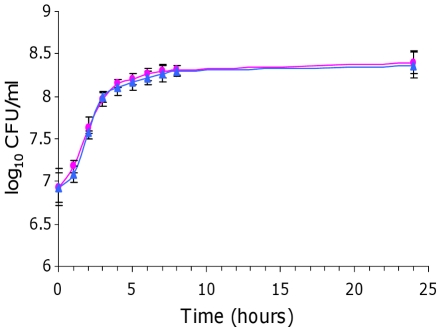
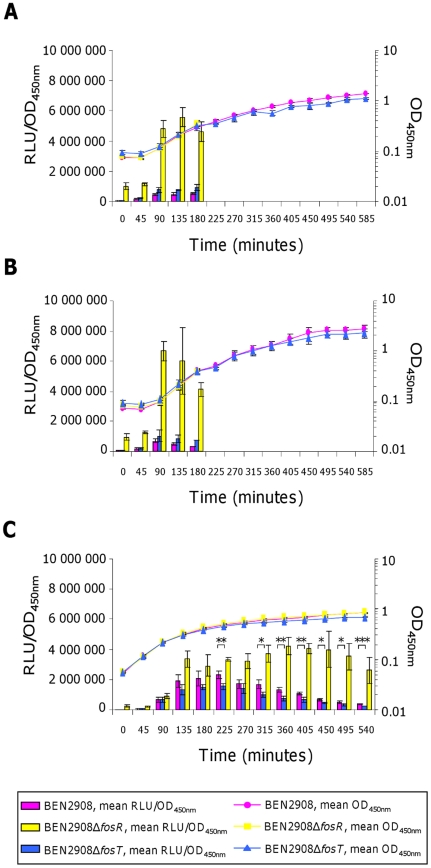
Expression of fos genes was monitored by measuring the fos promoter-mediated expression of luciferase from plasmid pQF52 during the growth of the BEN2908, BEN2908ΔfosT (unable to metabolize scFOS) and BEN2908ΔfosR (unable to repress fos gene expression) strains. As shown in Fig. 2, luciferase expression in BEN2908 and BEN2908ΔfosT was always lower than in BEN2908ΔfosR in all the media tested (5- to 31-fold higher in BEN2908ΔfosR than in BEN2908 in the lung, 3- to 14-fold higher in the liver, 14- to 36-fold higher in the spleen, and 3- to 14-fold higher in pericardial fluid; 5- to 17-fold higher in BEN2908ΔfosR than in BEN2908ΔfosT in the lung, 2- to 11-fold higher in the liver, 7- to 22-fold higher in the spleen, and 2- to 12-fold higher in pericardial fluid). This indicates that FosR repressed fos gene expression in these organ extracts. Nevertheless, fos genes were expressed slightly more in both BEN2908 and BEN2908ΔfosT in minimal media containing liver extract and pericardial fluid (a maximum of 1.22±0.11×106 and 1.74±0.1×106 RLU/OD450 in the liver, 1±0.42×106 and 1.35±0.3×106 RLU/OD450 in pericardial fluid, versus 3.34±0.4×105 and 7.07±0.7×105 RLU/OD450 in the lung and 2.7±0.3×105 and 5.38±1.19×105 RLU/OD450 in spleen extracts, respectively) (Fig. 2). This expression was not due to the presence in the media of sugars metabolized by the fos locus because it was not higher in BEN2908 than in BEN2908ΔfosT and there was no difference in the growth of these strains. We then verified that the fos locus, whose genes were slightly expressed in a minimal medium containing liver extract, did not give a growth advantage to strain BEN2908 in the presence of this colibacillosis target organ. Before, we checked that the impairment of the BEN2908ΔfosT strain in scFOS metabolism was only due to deletion of the fosT gene. We then introduced in this strain a pGEM-T derivative containing the whole fos locus [28]. As shown in Fig. 3, the recombinant BEN2908ΔfosT strain was able to grow with scFOS as the sole carbon source. However, this plasmid is unstable in M9 minimal medium containing organ extracts (70–80% of the recombinant bacteria maintain the plasmid during the growth, data not shown). As we performed co-cultures to determine the implication of the fos locus in the growth on such media, this instability did not allow us to realize complementation assays in the presence of organ extracts. To check the involvement of the fos locus in the presence of liver extract, BEN2908 and BEN2908ΔfosT were inoculated together in equal amounts in a minimal medium containing liver extract, and the proportion of each strain was monitored during their growth. As shown in Fig. 4, BEN2908 did not outcompete BEN2908ΔfosT in the presence of liver extract.
Figure 2. fos operon promoter activity in extracts of target organs of avian colibacillosis.
Growth curves (OD450 nm) and relative luminescence intensities (RLU/OD450 nm) of strains BEN2908, BEN2908ΔfosR and BEN2908ΔfosT carrying pQF52 grown without shaking at 37°C in M9 minimal medium supplemented with (A) 10% of lung extract, (B) 4% of liver extract, (C) 4% of spleen extract, (D) 4% of pericardial fluid. The RLU average values and standard deviations result from three independent experiments.
Figure 3. Growth of E. coli strains BEN2908, BEN2908ΔfosT and BEN2908ΔfosT/pGEM::fos in the presence of scFOS.
Growth curves (OD450 nm) of strains BEN2908 (pink circle), BEN2908ΔfosT (blue triangle) and BEN2908ΔfosT/pGEM::fos (green square) grown with shaking at 37°C in M9 minimal medium supplemented with 0.2% scFOS. The average values and standard deviations result from three independent experiments.
Figure 4. Competition between E. coli strains BEN2908 and BEN2908ΔfosT for growth in the presence of liver extract.
Growth curves (log10 CFU/ml) of strains BEN2908 (pink circle) and BEN2908ΔfosT (blue triangle) grown without shaking at 37°C in M9 minimal medium supplemented with 4% of liver extract. The average values and standard deviations result from three independent experiments.
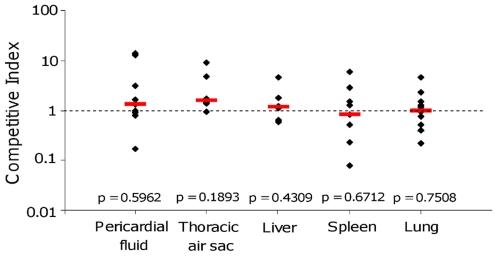
While these results strongly suggest that the fos locus is not involved in virulence, the expression of fos genes was not studied in blood and air sacs, which are other target organs/fluids of colibacillosis. We thus examined the impact of the fos locus on the virulence of the BEN2908 strain in vivo. To that end, equal amounts of BEN2908 and BEN2908ΔfosT were co-inoculated into the air-sac of SPF chickens (5×106 CFU of each strain/chicken), and the proportion of each strain in organs was monitored. As shown in Fig. 5, there was no difference in the colonization of target organs of avian colibacillosis (lung, thoracic air sac, liver, spleen and pericardial fluid) by BEN2908 or BEN2908ΔfosT, as the competitive indexes did not differ significantly from 1. Overall, these results demonstrate that the fos locus is not involved in the virulence of BEN2908 for chicken.
Figure 5. Competition between E. coli strains BEN2908 and BEN2908ΔfosT for colonization of chicken organs during avian colibacillosis.
Twenty five-day-old SPF White Leghorn chickens were inoculated in the right air sac with strains BEN2908 and BEN2908ΔfosT together (each at 5×106 CFU/chicken). Animals were euthanized 48 h post-inoculation by injection of Nesdonal and then necropsied. The proportion of each strain in organs was monitored and CI were calculated. Horizontal red bars indicate the median of CI and diamonds indicate individual CI. Statistical analyses were conducted using the Mann-Whitney U-test, measuring the difference between CI in organs and in the inoculum. The calculated P values are presented.
fos genes are expressed in chicken cecal content and not in intestinal mucus
The involvement of the fos locus in chicken intestinal colonization has previously been demonstrated by monitoring the proportion of BEN2908 and BEN2908ΔfosT in faeces [28]. However, identifying viable bacteria in faeces does not enable the site(s) along the gastrointestinal tract in which the fos locus is important to be determined. We thus investigated whether fos genes were expressed in different parts of the distal intestine, i.e. in intestinal mucus collected from the ileum or colon and in cecal content of axenic chickens. As shown in Fig. 6A and B, luciferase expression in intestinal mucus from the ileum and colon was much lower in BEN2908 and BEN2908ΔfosT than in BEN2908ΔfosR (7.5- to 12-fold higher in BEN2908ΔfosR than in BEN2908 in the ileum, and 7- to 12.5-fold higher in the colon; 5- to 7-fold higher in BEN2908ΔfosR than in BEN2908ΔfosT in the ileum, and 5.5- to 7-fold higher in the colon). This indicates that no inducer was present in these media to lift the FosR repression and to allow fos gene expression. In cecal content, luciferase expression in BEN2908 and BEN2908ΔfosT was much higher (a maximum of 2.3±0.25×106 and 1.53±0.2×106 RLU/OD450 in cecal content, respectively, versus 5.32±0.49×105 and 9.19±2.21×105 RLU/OD450 in mucus from the ileum and 7.03±1.45×105 and 1.05±0.4×106 RLU/OD450 in mucus from the colon, respectively), although lower than expression in BEN2908ΔfosR (1.5- to 7-fold higher, and 1.5- to 11-fold higher in BEN2908ΔfosR, respectively) (Fig. 6C). This indicates that expression in BEN2908 and BEN2908ΔfosT was not fully activated. Moreover, growth of BEN2908ΔfosT was less than that of BEN2908 and BEN2908ΔfosR and luciferase expression was significantly higher in BEN2908 than in BEN2908ΔfosT at different times (Fig. 6C) [2.30±0.25×106 and 1.53±0.2×106 RLU/OD450 at 225 min (p = 0.014); 1.67±0.28×106 and 9.92±1.94×105 RLU/OD450 at 315 min (p = 0.027); 1.31±0.15×106 and 7.51±1.46×105 RLU/OD450 at 360 min (p = 0.01); 1.08±0.05×106 and 6.71±1.63×105 RLU/OD450 at 405 min (p = 0.014); 6.46±1.05×105 and 4.64±0.32×105 RLU/OD450 at 450 min (p = 0.045); 4.97±0.87×105 and 3.32±0.44×105 RLU/OD450 at 495 min (p = 0.042); 3.79±0.44×105 and 2.43±0.23×105 RLU/OD450 at 540 min (p = 0.009), respectively]. This suggests that inducers of fos gene expression are present in cecal content to support BEN2908 growth and that this strain is able to metabolize cecal nutrients via the fos locus.
Figure 6. fos operon promoter activity in extracts of chicken intestine.
Growth curves (OD450 nm) and relative luminescence intensities (RLU/OD450 nm) of strains BEN2908, BEN2908ΔfosR and BEN2908ΔfosT carrying pQF52 grown without shaking at 37°C in M9 minimal medium supplemented with (A) intestinal mucus from the ileum at 2 mg/ml of proteins, (B) intestinal mucus from the colon at 2 mg/ml of proteins, (C) 2% of cecal content. The RLU average values and standard deviations result from three independent experiments. Asterisks indicate significant differences in mean luciferase activity between BEN2908 and BEN2908ΔfosT determined by a Student's t-test. *** P<0.005; ** P<0.02; * P<0.05.
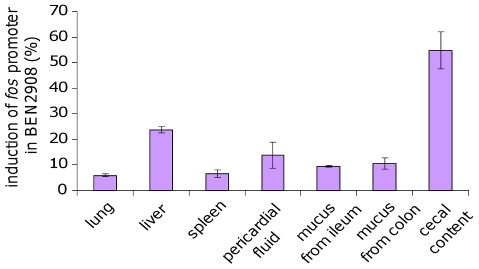
For a better comparison of activation of fos gene expression under all the conditions tested, we calculated the ratio of maximal luciferase activity in BEN2908 to the maximal luciferase activity in BEN2908ΔfosR. As shown in Fig. 7, maximal activation of the fos promoter was observed in cecal content (54.7±7.3%). The fos promoter was less activated in the presence of mucus from the ileum and colon (9.5±0.4% and 10.6±2.2%, respectively) than in the liver (23.7±1.4%), and was activated to the same extent in the presence of pericardial fluid (13.9±5.1%). It was activated least in the presence of lung and spleen extracts (5.8±0.5% and 6.5±1.5%, respectively).
Figure 7. Activation of fos promoter in chicken extracts or physiological fluids.
Percentage of fos promoter activation was obtained by dividing the maximum luciferase expression in BEN2908 by the maximum luciferase expression in BEN2908ΔfosR. The average values and standard deviations result from three independent experiments.
The fos locus is involved in growth on cecal content from axenic chicken in vitro and in vivo
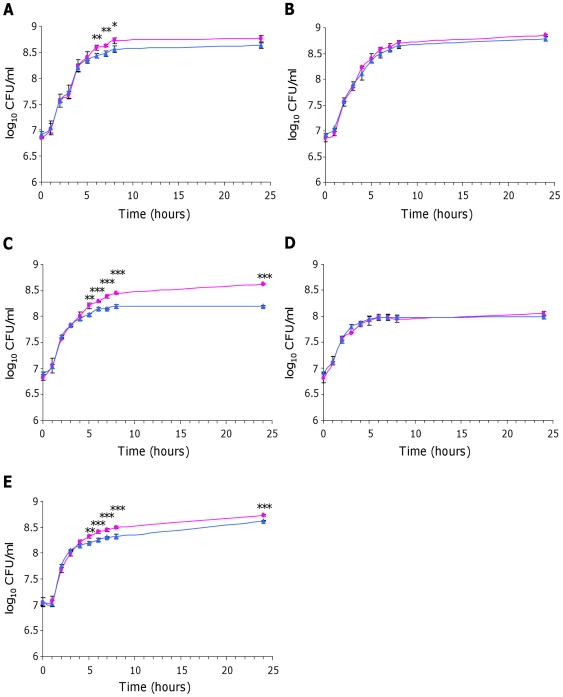
As fos genes were expressed in cecal content, we then investigated whether the fos locus benefited BEN2908 growth in cecal content. We also investigated a possible role of this locus in the growth of BEN2908 on intestinal mucus from the ileum and colon. Indeed, although fos genes were not expressed in these media, BEN2908 grew better than BEN2908ΔfosT in these biological extracts when monitored independently during expression analyses (Fig. 6). To monitor the involvement of the fos locus in the growth of BEN2908 in the presence of samples from the distal intestine, equal amounts of BEN2908 and BEN2908ΔfosT were inoculated together into a minimal medium containing either intestinal mucus from the ileum or colon, or cecal content from axenic chickens. In the medium containing intestinal mucus from the ileum, there was no difference between BEN2908 and BEN2908ΔfosT during the exponential growth phase. From six hours of growth, strain BEN2908 had a significant advantage over BEN2908ΔfosT [8.59±0.04 and 8.43±0.05 log10 CFU/ml at 6 hours (p = 0.017); 8.63±0.02 and 8.48±0.06 log10 CFU/ml at 7 hours (p = 0.015); 8.73±0.06 and 8.56±0.06 log10 CFU/ml at 8 hours (p = 0.023), respectively]. However, at 24 hours of growth, this difference was no longer significant (Fig. 8A). In the medium containing intestinal mucus from the colon, there was no significant difference between BEN2908 and BEN2908ΔfosT during the entire growth curve (Fig. 8B). Finally, in the medium containing cecal content, there was a significant difference from five hours of growth with a distinct advantage of BEN2908 over BEN2908ΔfosT [8.21±0.06 and 8.03±0.02 log10 CFU/ml at 5 hours (p = 0.008); 8.28±0.02 and 8.14±0.03 log10 CFU/ml at 6 hours (p = 0.002); 8.38±0.03 and 8.14±0.02 log10 CFU/ml at 7 hours (p = 0.0003); 8.44±0.01 and 8.19±0.04 log10 CFU/ml at 8 hours (p = 0.0003); 8.62±0.02 and 8.2±0.01 log10 CFU/ml at 24 hours (p = 9.9×10−6), respectively] (Fig. 8C). These results indicate that the fos locus confers a slight growth advantage to BEN2908 in ileal mucus and a more marked advantage in cecal content from axenic chicken.
Figure 8. Competition between E. coli strains BEN2908 and BEN2908ΔfosT to grow in the presence of extracts of chicken intestine.
Growth curves (log10 CFU/ml) of strains BEN2908 (pink circle) and BEN2908ΔfosT (blue triangle) grown without shaking at 37°C in M9 minimal medium supplemented with (A) intestinal mucus from the ileum at 2 mg/ml of proteins, (B) intestinal mucus from the colon at 2 mg/ml of proteins, (C) 2% of cecal content from axenic chicken, (D) 2% of cecal content from SPF chicken, (E) 4% of cecal content from axenic chicken supplemented with cecal bacteria from SPF chicken. The average values and standard deviations result from three independent experiments. Asterisks indicate significant differences in mean number of CFU between BEN2908 and BEN2908ΔfosT determined by a Student's t-test. *** P<0.005; ** P<0.02; * P<0.05.
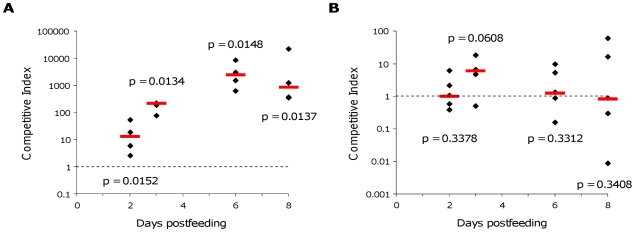
To confirm this involvement in this intestinal compartment, we performed an in vivo experiment. BEN2908 and BEN2908ΔfosT were fed together in equal amounts to axenic chickens (5×107 CFU of each strain/chicken), and the proportion of each was monitored in ceca. The results showed that the BEN2908 strain had a strong advantage in cecal colonization compared to the BEN2908ΔfosT strain up to eight days post-inoculation (median of CI of 12.73 at 2 days post-feeding; 215 at 3 days; 2382.5 at 6 days and 844.5 at 8 days) (Fig. 9A). This clearly indicates that the fos locus is involved in the colonization of axenic chicken ceca.
Figure 9. Chicken cecal colonization by E. coli strains BEN2908 and BEN2908ΔfosT.
Eleven-day-old axenic (A) and SPF (B) White Leghorn chickens were fed with strains BEN2908 and BEN2908ΔfosT together (each at 5×107 CFU/chicken). Animals were euthanized on days 2, 3, 6 and 8 post-inoculation by injection of Nesdonal and then necropsied. The proportion of each strain in animal cecal content was monitored over time and CI were calculated. Horizontal red bars indicate the median of CI and diamonds indicate individual CI. Statistical analyses were conducted using the Mann-Whitney U-test, measuring the difference between CI in cecal content and in the inoculum. The calculated P values are presented, with values below 0.05 considered significant.
The fos locus is involved in growth on cecal content in the presence of a microbiota in vitro but not in vivo
All the previous experiments were conducted with biological extracts from axenic chicken and do not represent the real conditions encountered by the bacteria in vivo. Indeed, sugars present in these extracts have not undergone any catabolism or degradation by bacteria. The sugar contents in the chicken intestine with a normal microbiota could be different. We thus analyzed the involvement of the fos locus in the growth of BEN2908 in cecal content from SPF chicken. When BEN2908 and BEN2908ΔfosT were inoculated together in a minimal medium containing previously sterilized cecal content from SPF chicken, there was no competition between the two strains, indicating that the sugars metabolized via the fos locus had previously been metabolized by the cecal microbiota (Fig. 8D). This also suggests that competition observed in cecal content from axenic chicken is a consequence of sugar metabolism mediated by the fos locus.
In order to avoid the use of sugars metabolized by the fos locus before sampling of cecal content but while looking at whether the presence of cecal microbiota could inhibit the benefit conferred by the fos locus, equal amounts of BEN2908 and BEN2908ΔfosT were inoculated together into a minimal medium containing cecal content from axenic chicken supplemented with cecal bacteria from SPF chicken. We first verified that these cecal bacteria were able to grow in a minimal medium containing cecal content from axenic chicken, although the experiments were not carried out in anaerobic conditions (8.97 log10 CFU/ml culturable on LB-Miller medium at 24 h) (data not shown). As shown in Fig. 8E, there was a significant difference after five hours of growth, with BEN2908 having a distinct advantage over BEN2908ΔfosT [8.32±0.03 and 8.2±0.02 log10 CFU/ml at 5 hours (p = 0.007); 8.41±0.02 and 8.26±0.04 log10 CFU/ml at 6 hours (p = 0.003); 8.45±0.03 and 8.3±0.02 log10 CFU/ml at 7 hours (p = 0.002); 8.50±0.03 and 8.32±0.04 log10 CFU/ml at 8 hours (p = 0.002); 8.73±0.02 and 8.63±0.004 log10 CFU/ml at 24 hours (p = 0.0005), respectively]. These results demonstrate that the BEN2908 strain is able to compete with a complex microbiota that also metabolizes the substrate of the fos locus.
We then checked if these results could be observed in vivo. To that end, BEN2908 and BEN2908ΔfosT were fed together in equal amounts to SPF chickens (5×107 CFU of each strain/chicken), and the proportion of each was monitored in ceca. We also enumerated the total E. coli population in cecal content of each animal. This population varied from one animal to the other (from 3.41×105 to 1.24×108 CFU/g of cecal content) (Table 2). As shown in Fig. 9B, the BEN2908 strain had a slight advantage, but not significant, in cecal colonization compared to the BEN2908ΔfosT strain at 3 days post-feeding but no advantage was observed on other days (median of CI of 1 at 2 days post-feeding; 5.96 at 3 days; 1.24 at 6 days and 0.85 at 8 days) (Fig. 9B). These results indicate that, in the presence of a complex microbiota, the fos locus does not provide a benefit to the BEN2908 strain to colonize the intestine.
Table 2. Proportion of the BEN2908 and BEN2908ΔfosT strains in the total E. coli population in the cecal content of each SPF chicken.
| Chicken | Days postfeeding | Total E. coli (CFU/g of cecal content) | Proportion of BEN2908 in the total E. coli population (%) | Proportion of BEN2908ΔfosT in the total E. coli population (%) | Competitive index |
| 75 | 2 | 3.33×107 | 61.43 | 9.11 | 5.92 |
| 76 | 2 | 1.00×108 | 0.07 | 0.06 | 1.00 |
| 77 | 2 | 1.67×107 | 2.57 | 6.07 | 0.37 |
| 78 | 2 | 1.68×107 | 43.12 | 18.17 | 2.08 |
| 92 | 2 | 4.60×107 | 5.95 | 9.41 | 0.56 |
| 79 | 3 | 2.25×107 | 0.70 | 0.04 | 17.38 |
| 82 | 3 | 1.05×108 | 5.59 | 0.78 | 6.28 |
| 83 | 3 | 1.46×107 | 50.70 | 7.47 | 5.96 |
| 84 | 3 | 2.18×107 | 2.76 | 5.07 | 0.48 |
| 91 | 3 | 4.36×107 | 0.18 | 0.03 | 4.55 |
| 85 | 6 | 9.45×106 | 56.23 | 9.90 | 4.99 |
| 87 | 6 | 6.85×106 | 0.60 | 3.59 | 0.15 |
| 88 | 6 | 4.25×106 | 0.45 | 0.47 | 0.84 |
| 89 | 6 | 9.09×106 | 7.54 | 5.34 | 1.24 |
| 93 | 6 | 1.24×108 | 0.11 | 0.01 | 9.10 |
| 80 | 8 | 1.85×107 | 3.32 | 10.44 | 0.28 |
| 81 | 8 | 7.55×107 | 1.85 | 0.03 | 56.05 |
| 86 | 8 | 3.05×107 | 0.63 | 0.04 | 15.38 |
| 90 | 8 | 8.91×107 | 0.10 | 10.71 | 0.01 |
| 94 | 8 | 3.41×105 | 24.82 | 25.62 | 0.85 |
Discussion
In this study, we demonstrated both in vitro and in vivo that the E. coli strain BEN2908 had a strong growth advantage in cecal content from axenic chicken over a strain that does not metabolize scFOS (Fig. 6C, 8C and 9A). In the presence of a microbiota in cecal content, we observed that, in vitro, the ability to metabolize scFOS gave significant competitive advantage to the BEN2908 strain (Fig. 8E). However, in vivo in SPF chickens, overall, no significant competitive advantage was observed but it is notable that competitive indexes considerably varied from one animal to another (Fig. 9B). As the cecum is an intestinal site where non-digestible carbohydrates such as cellulose, inulin and FOS are assimilated by the microbiota [35], [36], we can assume that the substrates giving a growth advantage to BEN2908 are scFOS. These results thus suggest that scFOS metabolism by the BEN2908 strain could contribute to its implantation in ceca. Nevertheless, one of the most important factors determining the nutrients found in the gastrointestinal tract is the presence of the intestinal microbiota. The number of bacteria found in the ceca of chicken is approximately 1011 CFU/g, including Clostridiaceae, Sporomusa, Enterobacteriaceae, Fusobacterium, Bacteroides, Lactobacillus, Streptococcus, Ruminococcus and Bifidobacterium [37]–[40]. The presence of these bacteria allows extensive bacterial fermentation, resulting in further nutrient absorption, detoxification of harmful substances and prevention of pathogen colonization [35], [38], [41]. Among these bacteria, some strains of Lactobacillus, Bifidobacterium, Bacteroides and Fusobacterium prausnitzii are able to metabolize nutrients such as FOS [37], [42]–[47]. These strains can therefore compete with the BEN2908 strain to metabolize FOS nutrients in vivo. We have previously shown that the fos locus of the BEN2908 strain was able to give an advantage to this strain to colonize the intestine of SPF chickens [28]. In this latter experiment, SPF chickens were obtained by inoculating axenic chickens with a complex bacterial inoculum consisting of a suspension of faeces from adult SPF hens that have been conserved during several years at −80°C. In this case, it is likely that there were no strict anaerobes in the faeces, therefore any anaerobes inoculated to chickens. Similarly, in the in vitro experiment performed in this study with the cecal content from axenic chickens supplemented with cecal bacteria from SPF chickens, it is possible that strict anaerobes did not survive during sampling of cecal content and/or did not survive in the medium because experiment was not achieve in anaerobic conditions. This suggests that a strict anaerobic bacterium is able to metabolize scFOS more efficiently than E. coli strain BEN2908.
During the intestinal colonization experiment in SPF chickens, we observed that the E. coli population varied from one animal to the other (Table 2). This suggests that, despite the fact that chickens had the same diet and same environmental conditions, the intestinal colonization by E. coli is highly variable. This fluctuation is also observed for the strains BEN2908 and BEN2908ΔfosT, the proportions of which in this total population were inconstant (from 0.07 to 61.43% for BEN2908 and from 0.01 to 25.62% for BEN2908ΔfosT) (Table 2). Finally, we can also observe that, in some animals, the BEN2908 strain was highly dominant in the total E. coli population (61.43%, 43.12%, 50.70% and 56.23% in chickens 75, 78, 83 and 85, respectively) whereas the BEN2908ΔfosT strain proportion was lower (a maximum of 25.62% in the chicken 94) (Table 2). This observation suggests that the fos locus, according to the bacteria present in the microbiota, and more particularly in the absence of a particular anaerobic bacterium able to metabolize scFOS most efficiently, is able to contribute to intestinal colonization. It can nevertheless be noted that, in few animals, and especially in chicken 90, the BEN2908ΔfosT strain is able to outcompete the BEN2908 strain (Table 2). As we have never observed this trend before, even in minimal media containing only one carbon source, we cannot currently explain this result in these animals.
It is noteworthy that the E. coli strain BEN2908 is fully able to colonize experimentally the chicken intestine, even in SPF animals. Conversely, commensal E. coli colonization cannot be studied experimentally in conventional animals due to colonization resistance, which results when all niches are filled by the microbiota. Streptomycin-treated animals are thus routinely used to study E. coli intestinal colonization [48]. The fact that BEN2908 is able to colonize SPF chicken intestine, without antibiotic treatment and on a long range, strongly suggests that ExPEC strains are better intestinal colonizers than commensal E. coli strains. This is consistent with previous observations demonstrating that strains belonging to phylogenetical lineage B2 have a greater ability to persist in the intestine [18]–[21].
In the present study, we investigated fos gene expression in different biological extracts and we showed that fos gene expression in cecal content was high in both the BEN2908 and BEN2908ΔfosT strain (Fig. 6C). This result is surprising since we previously showed that fos gene expression depends on the presence of scFOS [with β-(2-1) links] in the medium and that BEN2908ΔfosT is not able to metabolize these carbohydrates [28], [33]. Several hypotheses can therefore be considered. Different types of fructans exist: inulin and its derivatives, with one linear β(2-1)-linked fructosyl chain attached to the fructosyl residue of the sucrose starter; neo-series inulin with two linear β(2-1)-linked fructosyl chains, one attached to the fructosyl residue of the sucrose, the other to the glycosyl residue; levan with one linear β(2-6)-linked fructosyl chain attached to the fructosyl residue of the sucrose starter; and graminan with both β(2-1) and β(2-6) types of fructosyl linkages [49]. One interesting hypothesis is that another type of fructan induces fos gene expression. This inducer could enter via a transporter other than FosT, explaining fos gene expression in BEN2908ΔfosT. Another hypothesis to explain this result could be the action of an activator of fos gene expression. We previously demonstrated that fos gene expression also depends on catabolite repression and the binding of CRP-cAMP complex to the fos promoter region [33]. This binding enhances the ability of RNA polymerase to bind and initiate transcription of fos genes. cAMP synthesis is mediated by adenylate cyclase which is activated by phosphorylated EIIAGlc (IIA component of the glucose-specific phosphoenolpyruvate:carbohydrate phosphotransferase system) [50]–[53]. The phosphorylation state of EIIAGlc depends on the [phosphoenolpyruvate]/[pyruvate] ratio and is thus completely dependent on the substrates metabolized by the cell and differs according to the substrate [54]. In this study, fos gene expression in the BEN2908ΔfosT strain was up to 36% in cecal content and 33% in liver extract (compared to the expression in the BEN2908ΔfosR strain). Therefore, some substrates found in these media, entering via the FosT transporter in the BEN2908 and BEN2908ΔfosR strains, could lead to a lower [phosphoenolpyruvate]/[pyruvate] ratio and thus lower concentrations of phosphorylated EIIAGlc and cAMP synthesis in these strains. This could also explain the high levels of expression in the BEN2908ΔfosT strain compared to the expression in the BEN2908ΔfosR strain. Another surprising result in this study is that fos gene expression was always lower in BEN2908 than in BEN2908ΔfosR, even in cecal content (Fig. 2 and 6). This indicates that in none of the biological extracts tested, corresponding to the conditions encountered by the bacteria in vivo, was there a sufficient inducer concentration to allow a complete lift of FosR repression, whereas previously we observed this situation in vitro [33].
When this study was initiated, the fos locus had not been identified in bacteria other than E. coli and its prevalence was low (only 10 of the 133 E. coli strains tested possessed this locus [28]). The subsequent release of newly discovered genome sequences in databases enabled us to identify a truncated locus similar to the fos locus in the genome of several pathogenic bacteria. Several Klebsiella pneumoniae strains and Klebsiella variicola strain At-22 possess a truncated locus including a transcriptional regulator sharing 77% identity with FosR, an MFS transporter sharing 85% identity with FosT, and a glycosyl-hydrolase sharing 73% identity with FosE1. This locus also comprises an intergenic region between the regulator and the transporter that is very similar to that of the fos locus (sharing 54% identity throughout the whole region), including the same regulatory elements of operator 1 and 2 sequences and a CRP-cAMP recognition sequence. Moreover, the Enterobacteriaceae bacterium 9_2_54FAA strain, isolated from inflamed biopsy tissue from a patient with Crohn's disease [Enterobacteriaceae bacterium 9_2_54FAA Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/)], possesses a truncated locus similar to the fos locus with a transcriptional regulator (73% identity with FosR), an MFS transporter (89% identity with FosT), a glycosyl-hydrolase (67% identity with FosE1), a protein of unknown function, and a fructokinase (66% identity with FosK). It is well known that the gastrointestinal tract of humans and animals is the reservoir of commensal and pathogenic Enterobacteriaceae strains such as Klebsiella [55]–[58]. This suggests that other pathogenic bacteria could assimilate scFOS and that this metabolism could be controlled by the same regulation mechanism as that of the fos locus of BEN2908. Finally, we can thus postulate that, like the BEN2908 strain, the ability to metabolize scFOS could also enhance the ability of these pathogens to colonize their reservoir according to the bacteria present in the intestinal microbiota.
In sum, as the importance of the fos locus is dependent on the presence of the microbiota, and particularly on the presence of specific anaerobic bacteria, it could be of interest to study the involvement of this locus on intestinal colonization in different avian lineage and in chicken fed with different diets, thus with different intestinal microbiota, to determine if some conditions are more favorable to a pathogenic strain to colonize its reservoir then leading to greater dissemination of this strain in the local environment, dust for example, and hence colonization of the respiratory tract and pathogenesis.
Materials and Methods
Ethics statement
The housing, husbandry and slaughtering conditions complied with European Union guidelines for the care and use of laboratory animals. The experimental protocol for experimental colibacillosis was approved by the regional ethics committee [Comité d'Ethique en Expérimentation Animale (CEEA) Val de Loire] under number CL2007-44. The experimental protocol for intestinal colonization of chickens was approved by the regional ethics committee (CEEA Val de Loire) under number CL2007-43.
Bacterial strains, plasmids and growth conditions
Bacterial strains and plasmids used in this study are listed in Table 1.
Table 1. Bacterial strains and plasmids used in this study.
| Strain or plasmid | Relevant characteristics | Reference |
| E. coli strains | ||
| BEN2908 | Extraintestinal pathogenic strain; O2:K1:H5; Nalr Fos+ Fim+ Iut+ IbeA+ AGI-3+, avian origin | [59], [60] |
| BEN2908ΔfosT | Isogenic deletion mutant of BEN2908; Nalr Kanr Fos− | [28] |
| BEN2908ΔfosR | Isogenic deletion mutant of BEN2908; Nalr Fos+ | [33] |
| Plasmids | ||
| pQF52 | oripMB1 oripRO1600 Ampr; luc under the control of the fos promoter | [33] |
| pGEM::fos | pGEM-T easy vector containing the whole fos locus; Ampr | [28] |
E. coli strain BEN2908, O2:K1:H5 is a nalidixic acid-resistant derivative of strain MT78 isolated from the trachea of a chicken with a respiratory infection [59], [60]. The fosT and fosR isogenic mutants of BEN2908 were also used in this study [28], [33]. Strains were routinely grown in LB-Miller medium at 37°C with agitation [61]. When necessary, antibiotics were used at the following concentrations: nalidixic acid 30 µg/ml, ampicillin 100 µg/ml, or kanamycin 50 µg/ml. For growth monitoring experiments, overnight LB-Miller cultures of strains were washed twice and resuspended in the same volume of M9 minimal medium [61]. The strains were then inoculated to an optical density (OD) at 450 nm of 0.05 and cultured at 37°C or 41.5°C with agitation in 20 ml of M9 medium supplemented with 0.2% scFOS (Profeed P95; Beghin Meiji, France). scFOS powder is a mixture containing small quantities of glucose, fructose and sucrose (5%), and larger amounts of kestose (37%), nystose (63%) and fructofuranosyl nystose (10%).
For expression analyses, overnight LB-Miller cultures of strains carrying the pQF52 plasmid [33] were washed as described previously. The strains were then inoculated to an OD at 450 nm of 0.05 and cultured at 37°C without agitation in 20 ml of M9 medium supplemented with either 2% of cecal content, 4% of liver or spleen extract, or 10% of lung extract, in 2.5 ml of M9 medium supplemented with 4% of pericardial fluid, or in 1 ml of M9 medium supplemented with intestinal mucus adjusted at a final protein concentration of 2 mg/ml as described by Edelman et al. [62]. In these experiments, biological extracts were collected from axenic chickens. For media containing pericardial fluid and intestinal mucus, bacterial growth was monitored by plating serial dilutions onto LB-agar plates supplemented with ampicillin at 100 µg/ml at different times.
For co-cultures of BEN2908 and BEN2908ΔfosT, overnight LB-Miller cultures were washed as described previously. The strains were then inoculated in equivalent numbers (each corresponding to an OD at 450 nm of 0.025) and cultured at 37°C without agitation in 10 ml of M9 medium supplemented with either 2% of cecal content from axenic or SPF chicken, 4% of cecal content from axenic chicken supplemented with cecal microbiota of SPF chicken, or 4% of liver extract, or in 0.3 ml of M9 medium supplemented with intestinal mucus adjusted to a final protein concentration of 2 mg/ml. At different times, serial dilutions were plated onto LB-agar plates supplemented with nalidixic acid at 30 µg/ml (selection of BEN2908 and BEN2908ΔfosT strains) or with kanamycin at 50 µg/ml (selection of the BEN2908ΔfosT strain) for bacterial quantification. The number of CFU of BEN2908 was calculated by subtracting the number of kanamycin-resistant bacteria from the number of nalidixic acid-resistant bacteria.
In all the experiments, the media supplemented with the biological extracts were vigorously vortexed, centrifuged for 5 min at 4,000× g, and supernatants were sterilized by filtration.
For the experiment with cecal bacteria, 100 µl of M9 minimal medium containing cecal microbiota were inoculated in 10 ml of M9 minimal medium supplemented with cecal content from axenic chickens (representing 5×105 CFU/ml, culturable on LB-agar medium).
Collection of chicken organs, pericardial fluid, cecal content, intestinal mucus and cecal microbiota
Liver, lung, spleen, pericardial fluid, cecal content and intestinal mucus were collected from 25-day-old axenic PA12 White Leghorn chickens from the Institut National de la Recherche Agronomique experimental platform for infectious diseases. Axenic PA12 White Leghorn chickens were obtained using the method described by Le Bars [63]. Cecal content was also collected from 25-day-old SPF PA12 White Leghorn chickens. None antibiotic resistant E. coli strain was present in the intestine of SPF chickens. Animals were euthanized by Nesdonal (Rhône-Mérieux, Lyon, France) injection in the occipital sinus. Organs were collected and homogenized in sterile saline. Pericardial fluid was collected with a Pasteur pipette and ceca were collected and drilled to recover cecal content. Aliquots containing extracts of organ, pericardial fluid or cecal content from axenic chicken were stored at −20°C. Mucus was isolated from the ileum and from the colon of chicken as described previously [64], [65]. The animals were fasted 20 h before isolating intestinal mucus. Briefly, colon and ileum were collected and gently rinsed with PBS to remove intestinal content. Mucus was isolated from the intestinal walls by gentle scraping with the back of a scalpel, diluted 1∶3 with 25 mM HEPES (pH 7.2 to 7.5; Invitrogen) and vigorously vortexed. Epithelial cells and large cell components were removed by centrifugation at 11,000× g for 10 min at 4°C. The supernatant was then centrifuged at 26,000× g for 15 min at 4°C, and aliquots of the supernatant containing the crude mucus were stored at −20°C. The protein concentration of the mucus preparation was determined using a Bradford protein assay (Biorad).
Cecal content from SPF chicken was preserved in glycerol at −80°C to conserve cecal bacteria. To collect cecal microbiota, this cecal content was washed to remove cecal matter while maintaining cecal bacteria. To that end, one volume of cecal content was first diluted 1∶10 in M9 minimal medium and centrifuged 1 min at 200× g. Supernatant was recovered, centrifuged for 1 min at 300× g, then 2 min at 300× g. Finally, the supernatant was centrifuged for 2 min at 16,000× g and the pellet was resuspended with the same volume of M9 minimal medium.
Luciferase measurements
Promoter activities of the fos operon in different media were determined by firefly luciferase expression along the growth curve as described previously [33]. Briefly, samples of 100 µl were taken every 45 min, and light emission (relative light unit, RLU) was recorded with a luminometer (Lumat LB 9507, Berthold). A luciferase Assay System kit (Promega) was used with some modifications. Samples were incubated with 300 µl of freshly lysed buffer [1× CCLR (Cell Culture Lysis Reagent, Promega), 1.25 mg/ml lysozyme (Sigma), 2.5 mg/ml BSA (Sigma)] for 10 min with agitation at room temperature. Solutions were quick-frozen in liquid nitrogen and then immediately incubated at 37°C. After thawing, samples were incubated for 10 min with agitation at room temperature. Finally, RLU was measured by incubating 25 µl of cell lysate with 50 µl of Luciferase Assay Reagent.
To compare levels of luciferase expression, a correlation curve between OD at 450 nm and the number of bacterial CFU was plotted for each strain. Numbers of CFU obtained in media containing pericardial fluid and intestinal mucus were then converted into OD values to normalize results.
Experimental colibacillosis
An in vivo virulence assay was conducted as described previously with some modifications [32]. SPF chickens were obtained from the Institut National de la Recherche Agronomique experimental platform for infectious diseases. Fifteen 25-day-old SPF PA12 White Leghorn chickens were inoculated in the right thoracic air sac with a 0.1 ml suspension containing a mixture of equal numbers of BEN2908 and BEN2908ΔfosT (each approximately at 5×106 CFU) in LB-Miller medium. The inoculum was prepared from an overnight culture of each strain grown in 20 ml LB-Miller medium at 37°C without agitation. Animals were euthanized 48 h post-inoculation by injection with Nesdonal (Rhône-Mérieux, Lyon, France) in the occipital sinus and necropsied. A swab of the left thoracic air sac was taken, and samples of the left lung, liver, spleen and pericardial fluid were collected. After homogenization in sterile saline, serial dilutions were plated onto Drigalski agar plates (Biorad) supplemented with nalidixic acid at 30 µg/ml (selection of BEN2908 and BEN2908ΔfosT strains) or with kanamycin at 50 µg/ml (selection of the BEN2908ΔfosT strain) for bacterial quantification. The number of CFU of BEN2908 was calculated by subtracting the number of kanamycin-resistant bacteria from the number of nalidixic-resistant bacteria. Competition indices (CI) were calculated following Freter et al.'s method using BEN2908 as the reference strain [CI = (number of BEN2908 CFU/number of BEN2908ΔfosT CFU)/(number of BEN2908 CFU/number of BEN2908ΔfosT CFU in the initial inoculum)] [66]. By definition, a CI of >1 indicates out-competition of the mutant strain (BEN2908ΔfosT) by the wild-type reference strain (BEN2908). A CI equal to 1 indicates no difference in colonization of organs, and a CI of <1 indicates out-competition of the wild-type reference strain (BEN2908) by the mutant (BEN2908ΔfosT).
Intestinal colonization of chickens
An intestinal colonization assay was conducted as described previously with some modifications [28]. Briefly, 16 axenic and 20 SPF 11-day-old PA12 White Leghorn chickens from the Institut National de la Recherche Agronomique experimental platform for infectious diseases were fed with a 0.5 ml suspension containing a mixture of equal numbers of BEN2908 and BEN2908ΔfosT (each approximately at 5×107 CFU) in LB-Miller medium. None antibiotic resistant E. coli strain was present in the intestine of SPF chickens. The inoculum was prepared from an overnight culture of each strain grown in 20 ml LB-Miller medium at 37°C without agitation. Animals were euthanized on days 2, 3, 6 and 8 post-inoculation by injection with Nesdonal in the occipital sinus and necropsied. Ceca were collected and cecal contents were weighed and then homogenized in sterile saline (9 ml/g of cecal content). Viable E. coli cells were counted by plating 10-fold dilutions in sterile saline on Drigalski agar plates (Biorad) supplemented with nalidixic acid at 30 µg/ml (selection of BEN2908 and BEN2908ΔfosT strains) or with kanamycin at 50 µg/ml (selection of the BEN2908ΔfosT strain). The number of CFU of BEN2908 was calculated by subtracting the number of kanamycin-resistant bacteria from the number of nalidixic-resistant bacteria. The numbers of cecal CFU were calculated per gram of cecal content. Competition indices (CI) were calculated as described before for experimental colibacillosis.
In the intestinal colonization experiment of SPF chicken, total E. coli population of cecal contents was counted by plating 10-fold dilutions of cecal contents in sterile saline on Drigalski agar plates (Biorad). In this experiment, we also verified that the kanamycin-resistant E. coli population was also nalidixic acid-resistant and unable to metabolize scFOS. To that end, kanamycin-resistant colonies were picked on LB-Miller agar plates containing nalidixic acid at 30 µg/ml. All the colonies tested were nalidixic acid-resistant. Moreover, for each animal, kanamycin-resistant colonies were pooled, resuspended in 10 ml of LB-Miller medium containing kanamycin at 50 µg/ml and incubated overnight at 37°C with agitation. Overnight LB-Miller cultures were washed twice in M9 minimal medium and resuspended in the same volume of M9 minimal medium. Five milliliters of M9 minimal medium supplemented with 0.2% scFOS were then inoculated with 50 µl of the washed culture and incubated at 37°C with agitation. No growth was observed for any of the pools tested.
Statistical analysis
Statistical analyses of CI were performed using the Mann-Whitney U test. Exact P values were calculated with StatXact software (version 5.0; Cytel Inc., Cambridge, MA). Statistical analyses of luciferase expression and growth ability were performed using a Student's t-test.
Acknowledgments
We thank Nathalie Lallier for her invaluable assistance in the practical aspects of the work. We thank Fanny Foussier for her technical assistance with competition experiments. We thank Bruno Campone, Patrice Cousin and Edouard Guitton from the INRA experimental platform for infectious diseases for their expert assistance with in vivo experiments, and the poultry technicians for rearing the chickens.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the Era-NET PathoGenoMics European program (grant ANR-06-PATHO-002-01), the Institut National de la Recherche Agronomique and the Institut Fédératif de Recherche 136 (Agents Transmissibles et Infectiologie). GP is a pre-doctoral fellow of INRA (MICA)/Région Centre (France). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Russo TA, Johnson JR. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000;181:1753–1754. doi: 10.1086/315418. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JR, Russo TA. Extraintestinal pathogenic Escherichia coli: “the other bad E. coli”. J Lab Clin Med. 2002;139:155–162. doi: 10.1067/mlc.2002.121550. [DOI] [PubMed] [Google Scholar]
- 3.Smith JL, Fratamico PM, Gunther NW. Extraintestinal pathogenic Escherichia coli. Foodborne Pathog Dis. 2007;4:134–163. doi: 10.1089/fpd.2007.0087. [DOI] [PubMed] [Google Scholar]
- 4.Ron EZ. Host specificity of septicemic Escherichia coli: human and avian pathogens. Curr Opin Microbiol. 2006;9:28–32. doi: 10.1016/j.mib.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Shpigel NY, Elazar S, Rosenshine I. Mammary pathogenic Escherichia coli. Curr Opin Microbiol. 2008;11:60–65. doi: 10.1016/j.mib.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Barnes HJ, Vaillancourt JP, Gross WB. Colibacillosis. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE, editors. Diseases of poultry. Ames, IA: Iowa State University Press; 2003. pp. 631–652. [Google Scholar]
- 7.Dho-Moulin M, Fairbrother JM. Avian pathogenic Escherichia coli (APEC). Vet Res. 1999;30:299–316. [PubMed] [Google Scholar]
- 8.Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–456. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 9.Belanger L, Garénaux A, Harel J, Boulianne M, Nadeau E, et al. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol Med Microbiol. 2011;62:1–10. doi: 10.1111/j.1574-695X.2011.00797.x. [DOI] [PubMed] [Google Scholar]
- 10.Ewers C, Antao EM, Diehl I, Philipp HC, Wieler LH. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Appl Environ Microbiol. 2009;75:184–192. doi: 10.1128/AEM.01324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harry EG, Hemsley LA. The association between the presence of septicaemia strains of Escherichia coli in the respiratory and intestinal tracts of chickens and the occurrence of coli septicaemia. Vet Rec. 1965;77:35–40. [PubMed] [Google Scholar]
- 12.Wiles TJ, Kulesus RR, Mulvey MA. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85:11–19. doi: 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jantunen ME, Saxen H, Lukinmaa S, Ala-Houhala M, Siitonen A. Genomic identity of pyelonephritogenic Escherichia coli isolated from blood, urine and faeces of children with urosepsis. J Med Microbiol. 2001;50:650–652. doi: 10.1099/0022-1317-50-7-650. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JR, Stell AL, Delavari P. Canine feces as a reservoir of extraintestinal pathogenic Escherichia coli. Infect Immun. 2001;69:1306–1314. doi: 10.1128/IAI.69.3.1306-1314.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto S. Molecular epidemiology of uropathogenic Escherichia coli. J Infect Chemother. 2007;13:68–73. doi: 10.1007/s10156-007-0506-y. [DOI] [PubMed] [Google Scholar]
- 16.Pluschke G, Mercer A, Kusecek B, Pohl A, Achtman M. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun. 1983;39:599–608. doi: 10.1128/iai.39.2.599-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard B, Garcia JS, Gouriou S, Duriez P, Brahimi N, et al. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun. 1999;67:546–553. doi: 10.1128/iai.67.2.546-553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno E, Johnson JR, Perez T, Prats G, Kuskowski MA, et al. Structure and urovirulence characteristics of the fecal Escherichia coli population among healthy women. Microbes Infect. 2009;11:274–280. doi: 10.1016/j.micinf.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Nowrouzian FL, Adlerberth I, Wold AE. Enhanced persistence in the colonic microbiota of Escherichia coli strains belonging to phylogenetic group B2: role of virulence factors and adherence to colonic cells. Microbes Infect. 2006;8:834–840. doi: 10.1016/j.micinf.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Nowrouzian FL, Wold AE, Adlerberth I. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. J Infect Dis. 2005;191:1078–1083. doi: 10.1086/427996. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Foxman B, Marrs C. Both urinary and rectal Escherichia coli isolates are dominated by strains of phylogenetic group B2. J Clin Microbiol. 2002;40:3951–3955. doi: 10.1128/JCM.40.11.3951-3955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schierack P, Walk N, Ewers C, Wilking H, Steinruck H, et al. ExPEC-typical virulence-associated genes correlate with successful colonization by intestinal E. coli in a small piglet group. Environ Microbiol. 2008;10:1742–1751. doi: 10.1111/j.1462-2920.2008.01595.x. [DOI] [PubMed] [Google Scholar]
- 23.Herias MV, Midtvedt T, Hanson LA, Wold AE. Role of Escherichia coli P fimbriae in intestinal colonization in gnotobiotic rats. Infect Immun. 1995;63:4781–4789. doi: 10.1128/iai.63.12.4781-4789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herias MV, Midtvedt T, Hanson LA, Wold AE. Escherichia coli K5 capsule expression enhances colonization of the large intestine in the gnotobiotic rat. Infect Immun. 1997;65:531–536. doi: 10.1128/iai.65.2.531-536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouquet G, Porcheron G, Barra C, Reperant M, Chanteloup NK, et al. A metabolic operon in extraintestinal pathogenic Escherichia coli promotes fitness under stressful conditions and invasion of eukaryotic cells. J Bacteriol. 2009;191:4427–4440. doi: 10.1128/JB.00103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diard M, Garry L, Selva M, Mosser T, Denamur E, et al. Pathogenicity-associated islands in extraintestinal pathogenic Escherichia coli are fitness elements involved in intestinal colonization. J Bacteriol. 2010;192:4885–4893. doi: 10.1128/JB.00804-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freter R, Brickner H, Fekete J, Vickerman MM, Carey KE. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983;39:686–703. doi: 10.1128/iai.39.2.686-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schouler C, Taki A, Chouikha I, Moulin-Schouleur M, Gilot P. A genomic island of an extraintestinal pathogenic Escherichia coli strain enables the metabolism of fructooligosaccharides, which improves intestinal colonization. J Bacteriol. 2009;191:388–393. doi: 10.1128/JB.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 30.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 31.Roberfroid MB. Prebiotics: preferential substrates for specific germs? Am J Clin Nutr. 2001;73:406S–409S. doi: 10.1093/ajcn/73.2.406s. [DOI] [PubMed] [Google Scholar]
- 32.Chouikha I, Germon P, Bree A, Gilot P, Moulin-Schouleur M, et al. A selC-associated genomic island of the extraintestinal avian pathogenic Escherichia coli strain BEN2908 is involved in carbohydrate uptake and virulence. J Bacteriol. 2006;188:977–987. doi: 10.1128/JB.188.3.977-987.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porcheron G, Kut E, Canepa S, Maurel MC, Schouler C. Regulation of fructooligosaccharide metabolism in an extra-intestinal pathogenic Escherichia coli strain. Mol Microbiol. 2011;81:717–733. doi: 10.1111/j.1365-2958.2011.07725.x. [DOI] [PubMed] [Google Scholar]
- 34.Whittow GC. Regulation of body temperature. In: Sturkie PD, editor. Avian Physiology. New York, Berlin, Heidelberg, Tokyo: Springer-Verlag; 1986. pp. 221–252. [Google Scholar]
- 35.Clench MH, Mathias JR. The avian cecum: a review. Wilson Bull. 1995;107:93–121. [Google Scholar]
- 36.Turk DE. The anatomy of the avian digestive tract as related to feed utilization. Poult Sci. 1982;61:1225–1244. doi: 10.3382/ps.0611225. [DOI] [PubMed] [Google Scholar]
- 37.Bjerrum L, Engberg RM, Leser TD, Jensen BB, Finster K, et al. Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques. Poult Sci. 2006;85:1151–1164. doi: 10.1093/ps/85.7.1151. [DOI] [PubMed] [Google Scholar]
- 38.Gong J, Forster RJ, Yu H, Chambers JR, Sabour PM, et al. Diversity and phylogenetic analysis of bacteria in the mucosa of chicken ceca and comparison with bacteria in the cecal lumen. FEMS Microbiol Lett. 2002;208:1–7. doi: 10.1111/j.1574-6968.2002.tb11051.x. [DOI] [PubMed] [Google Scholar]
- 39.Olsen KN, Henriksen M, Bisgaard M, Nielsen OL, Christensen H. Investigation of chicken intestinal bacterial communities by 16S rRNA targeted fluorescence in situ hybridization. Antonie Van Leeuwenhoek. 2008;94:423–437. doi: 10.1007/s10482-008-9260-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhu XY, Zhong T, Pandya Y, Joerger RD. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl Environ Microbiol. 2002;68:124–137. doi: 10.1128/AEM.68.1.124-137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnes EM. The avian intestinal flora with particular reference to the possible ecological significance of the cecal anaerobic bacteria. Am J Clin Nutr. 1972;25:1475–1479. doi: 10.1093/ajcn/25.12.1475. [DOI] [PubMed] [Google Scholar]
- 42.Barrangou R, Altermann E, Hutkins R, Cano R, Klaenhammer TR. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc Natl Acad Sci U S A. 2003;100:8957–8962. doi: 10.1073/pnas.1332765100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falony G, Lazidou K, Verschaeren A, Weckx S, Maes D, et al. In vitro kinetic analysis of fermentation of prebiotic inulin-type fructans by Bifidobacterium species reveals four different phenotypes. Appl Environ Microbiol. 2009;75:454–461. doi: 10.1128/AEM.01488-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan H, Hutkins RW. Metabolism of fructooligosaccharides by Lactobacillus paracasei 1195. Appl Environ Microbiol. 2003;69:2217–2222. doi: 10.1128/AEM.69.4.2217-2222.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan SM, Fitzgerald GF, van Sinderen D. Transcriptional regulation and characterization of a novel beta-fructofuranosidase-encoding gene from Bifidobacterium breve UCC2003. Appl Environ Microbiol. 2005;71:3475–3482. doi: 10.1128/AEM.71.7.3475-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, et al. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141:1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan SH, Hold GL, Harmsen HJ, Stewart CS, Flint HJ. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst Evol Microbiol. 2002;52:2141–2146. doi: 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- 48.Leatham-Jensen MP, Frimodt-Moller J, Adediran J, Mokszycki ME, Banner ME, et al. The Streptomycin-Treated Mouse Intestine Selects Escherichia coli envZ Missense Mutants that Interact with a Dense and Diverse Intestinal Microbiota. Infect Immun. 2012 doi: 10.1128/IAI.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritsema T, Smeekens S. Fructans: beneficial for plants and humans. Curr Opin Plant Biol. 2003;6:223–230. doi: 10.1016/s1369-5266(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 50.Brückner R, Titgemeyer F. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett. 2002;209:141–148. doi: 10.1111/j.1574-6968.2002.tb11123.x. [DOI] [PubMed] [Google Scholar]
- 51.Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol. 2008;11:87–93. doi: 10.1016/j.mib.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Görke B, Stülke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 53.Saier MH., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989;53:109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hogema BM, Arents JC, Bader R, Eijkemans K, Yoshida H, et al. Inducer exclusion in Escherichia coli by non-PTS substrates: the role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol Microbiol. 1998;30:487–498. doi: 10.1046/j.1365-2958.1998.01053.x. [DOI] [PubMed] [Google Scholar]
- 55.Cain JR, Mayoral LG, Lotero H, Bolanos O, Duque E. Enterobacteriaceae in the jejunal microflora prevalence and relationship to biochemical and histological evaluations in healthy Colombian men. Am J Clin Nutr. 1976;29:1397–1403. doi: 10.1093/ajcn/29.12.1397. [DOI] [PubMed] [Google Scholar]
- 56.Hennequin C, Forestier C. oxyR, a LysR-type regulator involved in Klebsiella pneumoniae mucosal and abiotic colonization. Infect Immun. 2009;77:5449–5457. doi: 10.1128/IAI.00837-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schierack P, Walk N, Reiter K, Weyrauch KD, Wieler LH. Composition of intestinal Enterobacteriaceae populations of healthy domestic pigs. Microbiology. 2007;153:3830–3837. doi: 10.1099/mic.0.2007/010173-0. [DOI] [PubMed] [Google Scholar]
- 58.Selden R, Lee S, Wang WL, Bennett JV, Eickhoff TC. Nosocomial klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med. 1971;74:657–664. doi: 10.7326/0003-4819-74-5-657. [DOI] [PubMed] [Google Scholar]
- 59.Dho M, Lafont JP. Escherichia coli colonization of the trachea in poultry: comparison of virulent and avirulent strains in gnotoxenic chickens. Avian Dis. 1982;26:787–797. [PubMed] [Google Scholar]
- 60.Chanteloup NK, Porcheron G, Delaleu B, Germon P, Schouler C, et al. The extra-intestinal avian pathogenic Escherichia coli strain BEN2908 invades avian and human epithelial cells and survives intracellularly. Vet Microbiol. 2010;147:435–439. doi: 10.1016/j.vetmic.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Miller JH. Experiments in molecular genetics. xvi, 466. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 62.Edelman S, Leskela S, Ron E, Apajalahti J, Korhonen TK. In vitro adhesion of an avian pathogenic Escherichia coli O78 strain to surfaces of the chicken intestinal tract and to ileal mucus. Vet Microbiol. 2003;91:41–56. doi: 10.1016/s0378-1135(02)00153-0. [DOI] [PubMed] [Google Scholar]
- 63.Le Bars J. Demonstration of a protocol for obtaining germ-free chickens. Ann Rech Vet. 1976;7:383–396. [PubMed] [Google Scholar]
- 64.Gusils C, Oppezzo O, Pizarro R, Gonzalez S. Adhesion of probiotic lactobacilli to chick intestinal mucus. Can J Microbiol. 2003;49:472–478. doi: 10.1139/w03-055. [DOI] [PubMed] [Google Scholar]
- 65.Hermans D, Martel A, Van Deun K, Verlinden M, Van Immerseel F, et al. Intestinal mucus protects Campylobacter jejuni in the ceca of colonized broiler chickens against the bactericidal effects of medium-chain fatty acids. Poult Sci. 2010;89:1144–1155. doi: 10.3382/ps.2010-00717. [DOI] [PubMed] [Google Scholar]
- 66.Freter R, Allweiss B, O'Brien PC, Halstead SA, Macsai MS. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981;34:241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]