Abstract
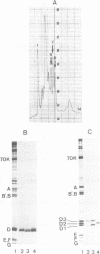
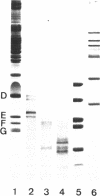
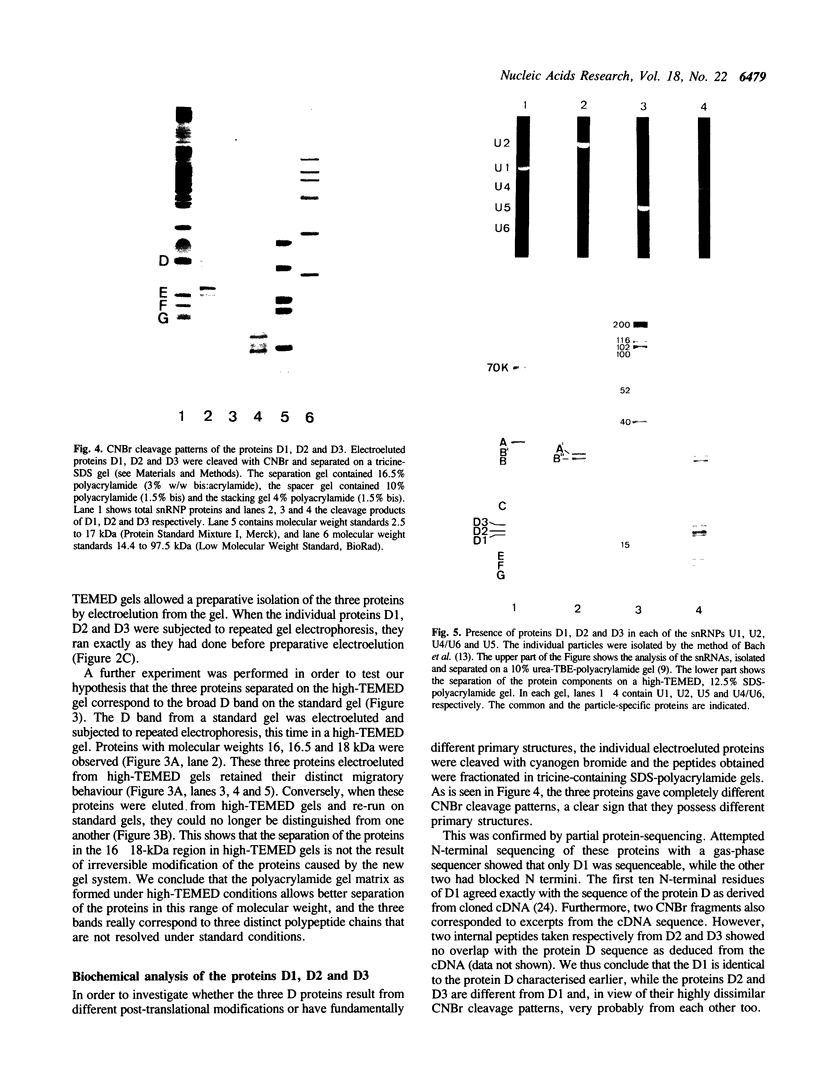
Electrophoresis of the mixture of proteins from purified snRNPs U1, U2, U4/U6 and U5 on SDS-polyacrylamide gels that had been allowed to polymerise in the presence of high TEMED concentrations have revealed the presence of proteins in the snRNPs that previously had eluded detection. The most striking case is that of protein D, heretofore generally observed as a single broad band; in high-TEMED gels, this splits into three clearly-separated bands, identified as three distinct proteins. We have denoted these proteins D1 (16 kDa), D2 (16.5 kDa) and D3 (18 kDa). Chemical and immunological studies have shown that D1 is identical with the common snRNP protein D, whose structure was recently resolved by cDNA cloning (Rokeach et al. (1988), Proc. Natl. Acad. Sci. USA, 85, 4832-4836) and that D2 and D3 are clearly distinct from D1 and very probably from each other. In addition to D1, proteins D2 and D3 are present in purified U1, U2, U4/U6 and U5 snRNPs isolated from HeLa cells, so these also belong to the group of common snRNP proteins. They are also found in snRNPs isolated from mouse cells, indicating that the role of these proteins in the structure and/or function of UsnRNPs has been conserved in evolution. Interestingly, patients with systemic lupus erythematosus produce populations of anti-Sm autoantibodies that react differentially with the D proteins; some recognise all of them and others only a subset. The high-TEMED gels allow improved resolution not only of the D proteins, but also of some of the U5-specific proteins contained in 20S U5 snRNPs, in particular the 15-kDa protein. In addition, under these conditions, the common G protein, previously observed as a single band, appears as a doublet. Whether the additional band represents a distinct common snRNP protein or a post-translationally modified version of G is not yet known.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M., Winkelmann G., Lührmann R. 20S small nuclear ribonucleoprotein U5 shows a surprisingly complex protein composition. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6038–6042. doi: 10.1073/pnas.86.16.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings P. B., Barton J. R., Hoch S. O. A murine monoclonal antibody recognizes the 13,000 molecular weight polypeptide of the Sm small nuclear ribonucleoprotein complex. J Immunol. 1985 Jul;135(1):428–432. [PubMed] [Google Scholar]
- Bochnig P., Reuter R., Bringmann P., Lührmann R. A monoclonal antibody against 2,2,7-trimethylguanosine that reacts with intact, class U, small nuclear ribonucleoproteins as well as with 7-methylguanosine-capped RNAs. Eur J Biochem. 1987 Oct 15;168(2):461–467. doi: 10.1111/j.1432-1033.1987.tb13439.x. [DOI] [PubMed] [Google Scholar]
- Bringmann P., Lührmann R. Purification of the individual snRNPs U1, U2, U5 and U4/U6 from HeLa cells and characterization of their protein constituents. EMBO J. 1986 Dec 20;5(13):3509–3516. doi: 10.1002/j.1460-2075.1986.tb04676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. E., Conner G. E., Reeves W. H., Wisniewolski R., Blobel G. Small nuclear ribonucleoprotein particle assembly in vivo: demonstration of a 6S RNA-free core precursor and posttranslational modification. Cell. 1985 Oct;42(3):751–758. doi: 10.1016/0092-8674(85)90271-5. [DOI] [PubMed] [Google Scholar]
- Fritz A., Parisot R., Newmeyer D., De Robertis E. M. Small nuclear U-ribonucleoproteins in Xenopus laevis development. Uncoupled accumulation of the protein and RNA components. J Mol Biol. 1984 Sep 15;178(2):273–285. doi: 10.1016/0022-2836(84)90144-x. [DOI] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Habets W. J., Sillekens P. T., Hoet M. H., McAllister G., Lerner M. R., van Venrooij W. J. Small nuclear RNA-associated proteins are immunologically related as revealed by mapping of autoimmune reactive B-cell epitopes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4674–4678. doi: 10.1073/pnas.86.12.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets W., Hoet M., Bringmann P., Lührmann R., van Venrooij W. Autoantibodies to ribonucleoprotein particles containing U2 small nuclear RNA. EMBO J. 1985 Jun;4(6):1545–1550. doi: 10.1002/j.1460-2075.1985.tb03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs V., Bach M., Winkelmann G., Lührmann R. U1-specific protein C needed for efficient complex formation of U1 snRNP with a 5' splice site. Science. 1990 Jan 5;247(4938):69–72. doi: 10.1126/science.2136774. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Konigsberg W. H., Henderson L. Removal of sodium dodecyl sulfate from proteins by ion-pair extraction. Methods Enzymol. 1983;91:254–259. doi: 10.1016/s0076-6879(83)91022-4. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Klein E. S., Russo A. F., Simmons D. M., Rosenfeld M. G. Isolation of cDNA clones encoding small nuclear ribonucleoparticle-associated proteins with different tissue specificities. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9778–9782. doi: 10.1073/pnas.86.24.9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986 Sep 12;46(6):905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- McAllister G., Amara S. G., Lerner M. R. Tissue-specific expression and cDNA cloning of small nuclear ribonucleoprotein-associated polypeptide N. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5296–5300. doi: 10.1073/pnas.85.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Pettersson I., Hinterberger M., Mimori T., Gottlieb E., Steitz J. A. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984 May 10;259(9):5907–5914. [PubMed] [Google Scholar]
- Reuter R., Lührmann R. Immunization of mice with purified U1 small nuclear ribonucleoprotein (RNP) induces a pattern of antibody specificities characteristic of the anti-Sm and anti-RNP autoimmune response of patients with lupus erythematosus, as measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8689–8693. doi: 10.1073/pnas.83.22.8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter R., Rothe S., Habets W., Van Venrooij W. J., Lührmann R. Autoantibody production against the U small nuclear ribonucleoprotein particle proteins E, F and G in patients with connective tissue diseases. Eur J Immunol. 1990 Feb;20(2):437–440. doi: 10.1002/eji.1830200231. [DOI] [PubMed] [Google Scholar]
- Rokeach L. A., Haselby J. A., Hoch S. O. Molecular cloning of a cDNA encoding the human Sm-D autoantigen. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4832–4836. doi: 10.1073/pnas.85.13.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauterer R. A., Goyal A., Zieve G. W. Cytoplasmic assembly of small nuclear ribonucleoprotein particles from 6 S and 20 S RNA-free intermediates in L929 mouse fibroblasts. J Biol Chem. 1990 Jan 15;265(2):1048–1058. [PubMed] [Google Scholar]
- Schmauss C., McAllister G., Ohosone Y., Hardin J. A., Lerner M. R. A comparison of snRNP-associated Sm-autoantigens: human N, rat N and human B/B'. Nucleic Acids Res. 1989 Feb 25;17(4):1733–1743. doi: 10.1093/nar/17.4.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Venrooij W. J., Sillekens P. T. Small nuclear RNA associated proteins: autoantigens in connective tissue diseases. Clin Exp Rheumatol. 1989 Nov-Dec;7(6):635–645. [PubMed] [Google Scholar]
- Zeller R., Nyffenegger T., De Robertis E. M. Nucleocytoplasmic distribution of snRNPs and stockpiled snRNA-binding proteins during oogenesis and early development in Xenopus laevis. Cell. 1983 Feb;32(2):425–434. doi: 10.1016/0092-8674(83)90462-2. [DOI] [PubMed] [Google Scholar]
- Zieve G., Penman S. Subnuclear particles containing a small nuclear RNA and heterogeneous nuclear RNA. J Mol Biol. 1981 Jan 25;145(3):501–523. doi: 10.1016/0022-2836(81)90542-8. [DOI] [PubMed] [Google Scholar]
- van Dam A., Winkel I., Zijlstra-Baalbergen J., Smeenk R., Cuypers H. T. Cloned human snRNP proteins B and B' differ only in their carboxy-terminal part. EMBO J. 1989 Dec 1;8(12):3853–3860. doi: 10.1002/j.1460-2075.1989.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]