Abstract
Aim
Previous economic literature on the cost-effectiveness of antiretroviral treatment (ART) programs has been mainly focused on the microeconomic consequences of alternative use of resources devoted to the fight against the HIV pandemic. We rather aim at forecasting the consequences of alternative scenarios for the macroeconomic performance of countries.
Methods
We used a micro-simulation model based on individuals aged 15–49 selected from nationally representative surveys (DHS for Cameroon, Tanzania and Swaziland) to compare alternative scenarios : 1-freezing of ART programs to current levels of access, 2- universal access (scaling up to 100% coverage by 2015, with two variants defining ART eligibility according to previous or current WHO guidelines). We introduced an “artificial” ageing process by programming methods. Individuals could evolve through different health states: HIV negative, HIV positive (with different stages of the syndrome). Scenarios of ART procurement determine this dynamics. The macroeconomic impact is obtained using sample weights that take into account the resulting age-structure of the population in each scenario and modeling of the consequences on total growth of the economy.
Results
Increased levels of ART coverage result in decreasing HIV incidence and related mortality. Universal access to ART has a positive impact on workers' productivity; the evaluations performed for Swaziland and Cameroon show that universal access would imply net cost-savings at the scale of the society, when the full macroeconomic consequences are introduced in the calculations. In Tanzania, ART access programs imply a net cost for the economy, but 70% of costs are covered by GDP gains at the 2034 horizon, even in the extended coverage option promoted by WHO guidelines initiating ART at levels of 350 cc/mm3 CD4 cell counts.
Conclusion
Universal Access ART scaling-up strategies, which are more costly in the short term, remain the best economic choice in the long term. Renouncing or significantly delaying the achievement of this goal, due to “legitimate” short term budgetary constraints would be a misguided choice.
Introduction
In its strategy for 2011–2015, UNAIDS states that “a renewed advocacy effort must be launched to encourage the continued commitment of the global North to support development efforts in the global South, with a focus on long-term predictable financing, particularly through multilateral mechanisms” [1]. Indeed following the 2001 Declaration of Commitment of the United Nations General Assembly Special Session on HIV/AIDS (UNGASS) and its further 2006 recommendation to scale up services and interventions “towards the goal of providing universal access to HIV prevention, treatment and care by 2015”, global funding for HIV programs has spectacularly increased from US$ 1.4 billion in 2000 to 15.6 billion in 2009. It is estimated that approximately 70% of total spending for HIV in low and middle-income countries comes in the form of international assistance [2]. Significant advances in the fight against the pandemic have been obtained from these efforts. Globally, HIV infections are fewer now than ten years ago, reflecting several factors that include the natural course of the epidemic and the impact of HIV prevention efforts. By the end of 2009, over five million people in low- and middle-income countries were reported to be receiving antiretroviral treatment (ART), including eight countries providing ART to at least 80% of patients in need and 21 additional ones with coverage rates higher than 50%, an achievement that would not have been deemed possible five years ago [3]. Progress has been most noticeable in sub-Saharan Africa the world's region most hardly hit by the epidemic [4].
In the context of the worst financial and economic crisis since the 1930's, there are growing concerns that global HIV funding will be flat-lined or even reduced in the next future [5]. Short-term sustainability of scaling up ART coverage to reach universal access is particularly under question since WHO 2010 recommendations to initiate ART at an earlier stage of the disease have mechanically increased the number of people eligible for treatment from an estimated 10.1 million to 14.6 million worldwide [6], and since growing number of patients are in need of more expensive new first-line and second-line antiretroviral regimens [7]. Pledges by governments and private donors for the 2011–2013 third voluntary replenishment of the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM), although amounting to US$ 11.7 billion, fell short of the minimum target set by the GFATM (US$ 13 billion) and from its own needs estimates to keep on track with the goal of universal access in 2015 (US$ 18–20 billion) [8]. As acknowledged by the Executive Director of GFATM, “the replenishment will enable further significant scale up, but not at the same pace as in recent years and is insufficient to meet anticipated demand” [9]. Moreover, the fiscal-year 2011 budget of the US President's Emergency Plan for AIDS Relief (PEPfAR), the other major donor for funding of ART, proposes only a 2% increase, which may result in future treatment enrollment freezes [10]. Indeed, the long-term sustainability and economic rationale of scaling up access to ART is increasingly questioned in both academic and policy circles [11]. Exploring the macroeconomic impact on GDP growth and human development of renouncing to -or delaying- the goal of universal access to HIV care and treatment is therefore key for “making sense of the money” devoted to HIV by bilateral and multilateral donor programs [12].
In previous economic literature, there have been a number of exercises aimed at estimating future funding needs for scaling up HIV programs in low and middle income countries based on extrapolation of current expenditures and epidemiologic modeling [13], [14]. On the other hand, there have been macroeconomic estimations of the impact of HIV/AIDS integrating epidemiological dynamics into neoclassical or “endogenous growth” models [15]–[18]. However, proper estimates of the economic impact of alternative funding scenarios for HIV treatment have to be based on the potential consequences of each scenario for the future life-history of affected individuals and, jointly, on an aggregation of these effects for the whole dynamics of the economy. This is now made possible by the availability of individual data on socio-economic characteristics and health-seeking behaviors of nationally representative population samples through Demographic and Health Surveys (DHS) and the use of dynamic micro-simulation methods[19]. To our knowledge, this paper is the first attempt to compare the long term (mid 2030's) economic consequences of two archetypal “extreme” scenarios for funding access to ART: the UNGASS inspired universal access scenario (scaling-up to 100% coverage by 2015, with two variants defining ART eligibility according to previous or current WHO guidelines), versus a “freezing” scenario in which the currently observed ART coverage is maintained without further increase in ART provision. This modeling exercise was carried out in the case of three African countries where high-quality DHS were available and which differ in terms of HIV prevalence and current level of wealth: Cameroon, Tanzania and Swaziland. Per capita Gross Domestic Product (GDP) ranges from US$ 347 in Tanzania and US$ 906 in Cameroon, to US$ 2659 for Swaziland [20]. In Cameroon and Tanzania HIV prevalence rates are relatively high but lower than Swaziland where it is one of the highest in the world [2].
Materials and Methods
Overview
To estimate the future impact of the HIV epidemic under different levels of ART procurement in Cameroon, Swaziland and Tanzania, the following steps were carried out.
We design a micro-simulation model based on individuals aged 15–49 selected from nationally representative surveys. By this process each individual may be seen as “representative” of a portion of the general population of the country.
We introduced an “artificial” ageing process, by computer programming methods. Individuals could evolve through four different health states: HIV negative, HIV positive (with two stages of the disease) and death. Four ART procurement scenarios determine these dynamics through their consequences on survival rates of the affected population and on infection rates in the general population: No Access (S0), Aid Freeze (S1), Universal Access (all patients with CD4 cell counts <200/µl)(S2a), and Extended Universal Access (all patients with CD4 cell counts <350/µl) (S2b).
At the end of the artificial ageing process, we created a “picture” for each given country in the mid-2030's not only in terms of ART need and procurement, lives saved, and HIV infections prevented (compared to S0), but also in terms of macroeconomic aggregates, derived for each scenario (costs and economic benefits). The macroeconomic impact is obtained using sample weights that take into account the resulting age-structure of the population in each scenario.
Datasources: Representative Agents
The simulations of this paper are based on three databases from the worldwide MEASURE Demographic and Health Surveys (DHS) program: the Tanzania HIV/AIDS Indicator Survey 2003–04 (THIS), the Cameroon Demographic and Health Survey 2004 (EDSC), and the Swaziland Demographic and Health Survey 2006–07 (SDHS) [21]–[23].THIS, SDHS and EDSC are the first nationwide surveys to provide HIV prevalence estimates: in addition to the data collected through interviews, respondents aged 15–49 were asked to voluntary provide a blood sample for subsequent HIV testing (regardless the severity of HIV). The HIV testing protocols have been approved by ORC Macro ethical comity. They were based on the anonymous linked protocol developed by DHS, which allows for the linking of the HIV test results to the socio-demographic data, provided that information potentially identifying an individual is destroyed before the linking takes place. Among all respondents, 80.5% in Tanzania (TZ), 82.7% in Swaziland (SZ) and 91.0% in Cameroon (CM) agreed to participate to the HIV testing protocol. The sampling procedure was determined before HIV testing agreement in order to avoid biases in the representativeness of the general population. Besides, non-participation to the HIV testing protocol has been shown to have negligible bias on HIV prevalence estimates [21]–[23].The final samples for the simulations comprised 10,747 individuals in TZ, 8,187 observations in SZ and 9,751 records in CM, characterized by gender, 5 years age group and HIV status (divided into two stages, asymptomatic and symptomatic; see Appendix S1 for methodology).
Ageing Process: A Micro-Simulation Model
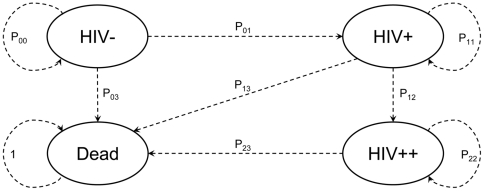
An individual's future health status was forecasted using a discrete-time micro-simulation model. In the model, an individual is, at a given period, either HIV negative (HIV−) in the asymptomatic stage of HIV (HIV+), in the symptomatic stage of HIV (HIV++) or deceased (D). At a time t, an individual is described by his/her health status. At the next period t+1 (one period lasting 5 years), the same person's health status is determined by a transition rate matrix –age and gender specific- according to his/her previous health status. This Markovian process can be summarized by Figure 1. We opted for five years periods in the model shorter intervals (e.g. one year).Indeed, DHS exhibit considerable sampling variations and non-robustness which could lead to spurious annual transition rates. It would follow that results at the 2040 horizon, resulting from 30 simulation cycles, may be severely biased.
Figure 1. State transition diagram of the Markov chain.
Ellipses define the four different health states of the model (HIV−, HIV+, HIV++ and Death). The dashed arrows represent the probabilities of going from one state to another in the subsequent period.
Appendix S1 describes how we compute each transition probability Pk;j (the probabilities of going from HIV− to HIV−(P00), from HIV− to HIV+ (P01), etc). HIV being a chronic and “progressive” disease, the probabilities of going from HIV+ to HIV− (P10), from HIV++ to HIV− (P20) and from HIV++ to HIV+ (P21) are null. P33is equal to 1 (death being an absorbing state).
An essential characteristic of our micro modelling is that this set of probabilities changes along with the policy scenario (e.g., P23, probability from HIV++ to death, is reduced in the case of access to treatment). They are computed on the basis of DHS surveys and litterature estimates (e.g. WHO/UNAIDS estimates of the HIV epidemic[2]; refer to Appendix S1 for details on the methodology).
During the simulation process, individuals becoming older than 54 are removed from the simulation sample (i.e. those aged 45–49 in the initial period are censored after the second period, those aged 40–44 in the first period are censored after the third period,…), as we cannot establish transition probabilities nor the future of these age groups. When the “oldest” cohort (aged 50–54 in t) is censored in t+5 it is automatically replaced by the preceding cohort (aged 45-49 in t, becoming 50–54 in t+5). This issue is similar for the youngest cohort (aged 15–19 in t, becoming 20–24 in t+5). A new cohort of individuals aged 15–19, taken from the original DHS dataset, is introduced at every time period in the simulation sample to mimic real-life population dynamics. In order to take into account the demographic evolution, the 15–19 population's five-year growth rate is estimated and allows us to include a new “demographic-adjusted size” cohort at each period (see Table 1 for values) [24].
Table 1. Model Key Parameters.
| Parameter | Swaziland | Tanzania | Cameroon | ||
| Demographic | 15–-24 Population | Growth Rate | 14.18% | 13.56% | 11.71% |
| 15–-49 Population | (In Thousands) | 582 | 16,220 | 7,708 | |
| Sample Weights | 71 | 1,521 | 790 | ||
| Sample Sizes | 8,187 | 10,747 | 9,751 | ||
| Economics | Employment rates: | Age [15–-19] | Reference | ||
| Estimated | Age [20–-24] | 1.538 | 1.339 | 1.299 | |
| Coefficients from the | Age [25–-29] | 2.378 | 2.284 | 2.025 | |
| Logit Model | Age [30–-34] | 2.649 | 2.514 | 2.618 | |
| Age [35] –[39] | 2.836 | 2.702 | 2.929 | ||
| Age [40] –[44] | 2.696 | 2.934 | 3.094 | ||
| Age [45] –[49] | 2.691 | 2.677 | 2.977 | ||
| Woman | −0.7567 | -0.369 | −0.596 | ||
| Intercept | −1.580 | 0.196 | −0.663 | ||
| Absenteeism Rates | HIV − | 100% | |||
| HIV+ | 100% | ||||
| HIV++ w/ ART | 95% | ||||
| HIV++ w/o ART | 75% | ||||
| GDP at Market Price | In billions USD | 2.648 | 11.351 | 15.775 | |
| Annual Avg. Wage | Per Worker | $10,381 | $870 | $3,223 | |
| Epidemiology | τ (ART need; 2006 Guidelines ) | 31.05% | 30.00% | 29.91% | |
| TC (ART coverage; 2006 Guidelines ) | T0 (Initial Stage) | 42.37% | 0.71% | 8.75% | |
| T0-T1 | Unknown | 16% | 18% | ||
| T1 (First Period) | Unknown | 31% | 26% | ||
| α (HIV history) | 0.1 | ||||
| v (HIV+ Mortality Gap) | 0.005 | ||||
| u (HIV++ Mortality Gap) | 0.2 for S1 & S2a ; 0.1 for S2b | ||||
| γ ( HIV Diffusion Parameter) | 0.518 | 0.388 | 0.511 | ||
| ϕ (Prevention Parameter) | 0 for S1 & S2a ; 0.7 for S2b | ||||
| β (Transition Booster) | 1.30 | 1.27 | 1.30 | ||
| ψ (Transition Restrainer) | 0.7 for asymptomatic patients ; 1 otherwise | ||||
Microsimulating the impact of ART Procurement Strategies
Micro-simulation methods allowed us to compare two ARV access scenarios: “Aid Freeze” (S1) and “Universal Access” (S2), the latter being broken down into two scenarios -whether 2006 (for S2a) or 2010 (for S2b, “Extended Universal Access”) ART eligibility criteria are considered [25], [26]. The WHO recommended in 2006 that HIV infected people with a CD4 cell count ≤200 cells/µl, those in clinical stage III with a CD4 cell count ≤350 cells/µl and those with a diagnosis of WHO stage IV disease should start ART [25]. Since 2010 the WHO recommends that people with a CD4 cell count ≤350 cells/µl should start ART regardless of the presence or absence of clinical symptoms and that those with a diagnosis of WHO stage III or IV should start ART irrespective of their CD4 cell count [26]. These scenarios are contrasted between each other, but also versus a baseline scenario (“No Access”, S0) which provides a picture of what the world would have looked like if ARVs procurement had not been introduced in the three studied countries and remained null during the whole period. “No-Access” and “Universal Access” scenarios can be considered as hypothetical boundary scenarios: the “Universal Access” scenarios –i.e. all those who need ART have access to it- show what could happen if the scaling-up of ART program was achieved in 2015. In S2b, the target population is 1.49 times greater than in S2a [27]: in addition to every HIV++ individual under ART in S2a, an additional subset of the HIV+ population with higher CD4 cell counts (200<CD4<350) receives ARVs. The “Aid-Freeze” scenario represents what could be the extreme consequences of renouncing to further ART scale up due to the budgetary constraints of the financial crisis: the current number of PLWHIV receiving ARVs remains constant (i.e. a “freeze” in the absolute number of ARV treatments delivered).
Thanks to the explicit modeling of individual ageing we can include the consequences of each scenario for the future life-history of individuals. Roughly speaking, we deform the transition matrix in order to capture the main effects of a program. HIV++ individuals live longer when receiving ARVs, and the earlier they start ART, the higher their survival rate is (see Appendix S1 and Table 1). Moreover ARVs do not only reduce mortality: when a large proportion of the infected population is treated, as in scenarios S2a and S2b, ART is also thought to have a preventative effect, in terms of an individual's infectiousness, which is negatively correlated to the delay between HIV infection and ART initiation [28]. This reduction in HIV−transmission rates (ϕ, see Appendix S1) is thus assumed to be higher in S2b than in S2a. For instance, the baseline infection probabilities are multiplied by one in S2a (a conservative assumption) and by 0.3 in S2b (a rather optimistic hypothesis; Table 1). The outcomes' sensitivity to these assumptions will also be assessed.
Economic assumptions
When assessing the cost-benefit of a healthcare program, the costs and benefits components must be determined. The latter component can include two aspects: the gains in the economic production and those attached with better health outcomes. This paper being driven by an accounting approach, we only measure the economic benefit as production gains.
DHS include a module on respondents' employment status: male and female aged 15 and above were asked whether they were working in the 7 days preceding the interview. We propose to estimate probabilities of participating to the labor market and to integrate this dynamic behavior into the micro-simulation model. Using a binary Logit model, labor participation is explained by the respondent's age group and gender (Table 1). Employment rate is a concave function of age and is higher among men than among women. Several authors examined the impact of HIV on economic activity in terms of productivity loss [29]–[32]. Regarding these works, we assume that compared to HIV− workers (exhibiting a null absenteeism rate), HIV+ individuals (asymptomatic stage) are not statistically more absent, while HIV++ (symptomatic without ART) workers are 25% more absent (but solely 5% more absent with ART; Table 1). Although the impact of HIV on productivity may be more severe as individuals get older, the model's absenteeism rates were assumed age-invariant due to the lack of appropriate calibration data. The age-gender specific employment rates were then multiplied by health condition-specific presence-at-work rates to obtain individual productivity rates.
We also estimate country-specific maximal average incomes per working adult (at full productivity rate) by dividing the national GDP by the number of working adults aged 15–49 in the country (Table 1) [20], [24]. The product between productivity rates & potential income provides a vector of age/gender & health condition specific wages that reproduce GDP per capita national levels. Aggregate GDP levels were computed by aggregating individual wages (multiplied by the representative weight of each observation, i.e. the number of “real” agents one DHS interviewee represents). GDP dynamics only result in the microsimulation model from epidemiologic and demographic changes: no exogenous GDP growth rate is included (a conservative assumption).
In order to compute program costs, we assume for all three scenarios that patients receive first-line ARVs for five years (210$ per patient per year) before receiving second-line ARVs until their death (590$ per patient per year) [33], [34]. In developing countries, 1.9% of 1st line drugs patients switch to 2nd line regimens each year [35]. Thus the model's regimens switching rate is probably overestimated, even for the future (after 2015). However, this assumption is voluntary conservative: the lower the switching rate, the lower programs associated costs would be. Laboratory tests (191$ per patient per year) and service delivery costs (72$) were also included in the costs component [34]. We do not make any additional hypothesis about ART characteristics in the future e.g. price variations, innovative or more effective treatment strategies, etc. Yet, the decreasing trend of ARVs prices suggests that our cost data may over-estimate future costs [33], [36]. The total cost of each scenario can be computed by aggregating individual costs and will be used for the macro-economic evaluation.
Health and macroeconomic outcomes
The micro-simulation model offers a large panel of outcomes, providing public health and economic “snapshots” for each country and each five-year period: in addition to several epidemiological indicators (number of deaths averted, HIV prevalent and incident cases (and prevented cases), and treatment need; see Table 2), program characteristics and associated economic outcomes were estimated for the alternative scenarios (volume of ARV procurement, programs' costs and economic benefits; see Table 3). Time series of GDP and GDP gains (relative to S0) are computed for each alternative scenario by using standard aggregations methods throughout the surviving agents (the age-structure of the population and the total labor supply are endogenously determined by the aging process).
Table 2. Epidemiological Impacts of 4 ART Coverage Scenarios in 3 Countries.
| Epidemiology | Late 2000's | Mid 2030's | |||||
| Country | Indicators | Initial Characteristics | Scenario S0 No Access | Scenario S1 Aid Freeze | Scenario S2a Universal Access | Scenario S2b Extended Universal Access | |
| Swaziland | Prevalence | HIV Prevalence (in percent) | 25.88 | 21.91 | 22.45 | 26.81 | 12.86 |
| 2007 – 2037 | PLWHIV (in thousands) | 151 | 350 | 362 | 458 | 223 | |
| Proportion of HIV++ among PLWHIV | 0.310 | 0.290 | 0.312 | 0.459 | 0.462 | ||
| Number of Adults aged 15-49 (in thousands) | 582 | 1,600 | 1,611 | 1,708 | 1,737 | ||
| Deaths | Deaths from 2032 onwards (in thousands) | n/a | 111 | 108 | 67 | 52 | |
| Deaths from 2032 onwards due to HIV (in percent) | n/a | 79.9 | 79.3 | 67.4 | 29.6 | ||
| Cum. Deaths from 2007 onwards (in thousands) | n/a | 439 | 412 | 252 | 201 | ||
| Infections | Cum. Infections from 2007 onwards (in thousands) | n/a | 615 | 615 | 615 | 272 | |
| Tanzania | Prevalence | HIV Prevalence (in percent) | 6.35 | 5.35 | 5.47 | 6.68 | 3.38 |
| 2009 – 2034 | PLWHIV (in thousands) | 1,195 | 1,972 | 2,021 | 2,500 | 1,270 | |
| Proportion of HIV++ among PLWHIV | 0.32 | 0.26 | 0.28 | 0.41 | 0.45 | ||
| Number of Adults aged 15-49 (in thousands) | 18,828 | 36,894 | 36,952 | 37,428 | 37,612 | ||
| Deaths | Deaths from 2029 onwards (in thousands) | n/a | 643 | 617 | 441 | 345 | |
| Deaths from 2029 onwards due to HIV (in percent) | n/a | 57.2 | 56.5 | 37.4 | 16 | ||
| Cum. Deaths from 2009 onwards (in thousands) | n/a | 3,144 | 2,989 | 2,135 | 1,811 | ||
| Infections | Cum. Infections from 2009 onwards (in thousands) | n/a | 3,655 | 3,655 | 3,655 | 1,811 | |
| Cameroon | Prevalence | HIV Prevalence (in percent) | 5.13 | 4.67 | 4.78 | 6 | 2.90 |
| 2009 – 2034 | PLWHIV (in thousands) | 471 | 863 | 884 | 1,124 | 545 | |
| Proportion of HIV++ among PLWHIV | 0.3 | 0.27 | 0.29 | 0.44 | 0.48 | ||
| Number of Adults aged 15–49 (in thousands) | 9,169 | 18,480 | 18,504 | 18,733 | 18,825 | ||
| Deaths | Deaths from 2029 onwards (in thousands) | n/a | 413 | 404 | 311 | 264 | |
| Deaths from 2029 onwards due to HIV (in percent) | n/a | 45 | 43.9 | 26.3 | 9.7 | ||
| Cum. Deaths from 2009 onwards (in thousands) | n/a | 1,817 | 1,765 | 1,376 | 1,219 | ||
| Infections | Cum. Infections from 2009 onwards (in thousands) | n/a | 1,584 | 1,584 | 1,584 | 760 | |
Table 3. Economic Impacts of 4 ART Coverage Scenarios in 3 Countries.
| Economics | Late 2000's | Mid 2030's | |||||
| Country | Indicator | Initial Characteristics | Scenario S0 No Access | Scenario S1 Aid Freeze | Scenario S2a Universal Access | Scenario S2b Extended Universal Access | |
| Swaziland 2007 –2037 | ART Coverage | ART Coverage of HIV++ population (in percent) | 42 | 0 | 17 | 100 | 149 |
| Individuals under ART (in thousands) | 20 | 0 | 20 | 210 | 154 | ||
| ART Costs | Total ART Cost from 2032 onwards (in millions USD) | n/a | 0 | 72 | 753 | 590 | |
| Total ART Cost from 2007 onwards (in millions USD) | n/a | 0 | 496 | 3,115 | 3,336 | ||
| GDP | GDP per Capita (in USD) | 4,466 | 4,905 | 4,921 | 5,068 | 5,115 | |
| Overall GDP (in billions USD) | 2.6 | 7.85 | 7.93 | 8.66 | 8.88 | ||
| GDP gap from 2007 onwards (in millions USD) | n/a | Reference | 2,668 | 16,067 | 19,630 | ||
| GDP gains / ART Costs from 2007 onwards | n/a | Reference | 5.37 | 5.16 | 5.88 | ||
| Tanzania 2009 – 2034 | ART Coverage | ART Coverage of HIV++ population (in percent) | 31 | 0 | 19 | 100 | 149 |
| Individuals under ART (in thousands) | 119 | 0 | 110 | 1,103 | 856 | ||
| ART Costs | Total ART Cost from 2029 onwards (in millions USD) | n/a | 0 | 402 | 3,670 | 3,320 | |
| Total ART Cost from 2009 onwards (in millions USD) | n/a | 0 | 2,672 | 15,980 | 18,432 | ||
| GDP | GDP per Capita (in USD) | 699.5 | 704.2 | 704.5 | 707.2 | 708.3 | |
| Overall GDP (in billions USD) | 13.17 | 25.98 | 26.03 | 26.47 | 26.64 | ||
| GDP gap from 2009 onwards (in millions USD) | n/a | Reference | 1,696 | 10,138 | 12,712 | ||
| GDP gains / ART Costs from 2009 onwards | n/a | Reference | 0.64 | 0.63 | 0.69 | ||
| Cameroon 2009 – 2034 | ART Coverage | ART Coverage of HIV++ population (in percent) | 25 | 0 | 14 | 100 | 149 |
| Individuals under ART (in thousands) | 35 | 0 | 36 | 492 | 390 | ||
| ART Costs | Total ART Cost from 2029 onwards (in millions USD) | n/a | 0 | 131 | 1,769 | 1,516 | |
| Total ART Cost from 2009 onwards (in millions USD) | n/a | 0 | 848 | 7,239 | 8,060 | ||
| GDP | GDP per Capita (in USD) | 2,062 | 2,159 | 2,160 | 2,171 | 2,176 | |
| Overall GDP (in billions USD) | 18.9 | 39.9 | 39.96 | 40.67 | 40.95 | ||
| GDP gap from 2009 onwards (in millions USD) | n/a | Reference | 1,911 | 14,989 | 18,973 | ||
| GDP gains / ART Costs from 2009 onwards | n/a | Reference | 2.27 | 2.07 | 2.35 | ||
In order to compare the ART access scenarios with the counterfactual S0 (no access to ART) in terms of economic sustainability, the common indicator “self-financing ratio” (SFR) was computed –the quotient between the total economic gains generated by treating HIV-infected workers and its associated costs. Therefore, a SFR superior to 1 means that the HIV program is cost-saving: its net contribution to the economy is positive because its global macroeconomic benefit is superior to its total costs. Alternatively, a SFR inferior to 1 means the program has a net cost for the economy but this does not necessarily means it is not worthwhile since this net cost translates, beyond its macroeconomic impact on GDP, in welfare improvements due to increase in life expectancy and quality of life of the HIV-infected population.
Results
Microsimulation of life paths: an illustration
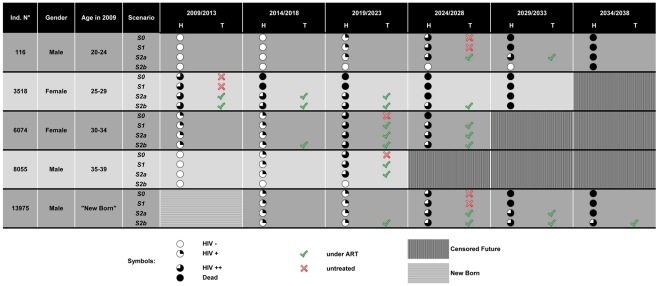
Figure 2 presents health trajectories from a THIS 2003–04 subsample according to the four alternative scenarios. This ageing process creates one possible future from many others for every DHS representative agent (the Markov process is a random process). Individual No. 116 is a man aged between 20 and 24 years in 2009; he contracts HIV in 2019 in scenarios S0, S1 & S2a. He progresses to advanced HIV in 2024 and receives ARVs solely in S2a (he lives one more period than in S0 & S1). If the S2b program had been set up, he would never have contracted HIV (preventative effect of ART ϕ > 0) and would have died in 2034 from other causes than HIV. Individual No. 3,518 is a HIV++ woman in 2009. She dies in the subsequent period in S0 & S1. With S2a & S2b she remains under ART until her death (due to ART failure or external causes), respectively in 2024 and 2029. Individual No. 6,074 is HIV+ at the initial stage. She meets 2010 eligibility criteria in the 2nd period and starts ART in S2b. In the subsequent period she shifts to HIV++ and obtains ART in S2a, before exiting from the observation window (we could not establish transition probabilities for individuals older than 54). Individual No. 8,055 contracts HIV between 2010 and 2014 in all scenarios except S2b and then receives ARVs in S1 & S2a before being censored; his observed survival is equal in all scenarios. Individual No. 13,975 is introduced in the simulation process in 2014. He starts ART earlier in S2b than in S2a and thus lives longer in S2b (lower mortality with ART when initiated early). The epidemiological results at national levels are based on the aggregation of all individual simulated life paths.
Figure 2. Life Paths.
Simulated health trajectories of a THIS 03–04 subsample as per the four ART procurement scenarios. In columns 2009/13,…,2034/38, the full-white circles refer to individuals being HIV negative at a given period, the one-quarter black circles to HIV+, the three-quarter black circles to HIV++ and the full-black circles to deceased individuals. The ART circles columns provide information on whether or not individuals are treated with ARVs.
Epidemiologic Impact
As shown in Table 2, not having provided ARVs (S0) would have had a catastrophic impact on life expectancy through HIV-related mortality: in Swaziland (SZ), 439,000 individuals would die during the course of the simulation, 83% of them being HIV infected at the time of death. Tanzania (TZ) would experience 3.1 million deaths over 25 years and Cameroon (CM) 1.8 million. Compared with the absence of ART (S0), extended Universal Access (S2b) is the scenario that saves the greatest number of lives (238,000 in SZ –i.e. 54% of S0's deaths are avoided-, 1.3 million in TZ and 598,000 in CM), followed by the Universal Access (S2a) scenario (187,000 in SZ, 1 million in TZ and 441,000 in CM). The “Aid Freeze” scenario would only avoid 27,000 deaths in SZ (respectively 155,000 & 52,000 in TZ & CM) compared with S0.
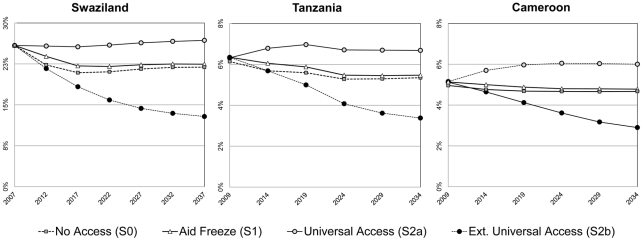
Because of the high mortality associated with HIV in the absence of ART, a decrease in HIV prevalence rates, shown in Figure 3, can be predicted for the “No Access” Scenario (S0) in the three examined countries (by 4% in SZ, 1% in TZ and 0·5% in CM). Treating individuals with ART produces a mechanical increase of HIV prevalence: HIV-infected individuals live longer, which results in a higher number of HIV carriers than in the scenario S0: the prevalence rate of S0 is slightly lower than in S1 but considerably lower than in S2a (Figure 3). In the Extended Universal Access Scenario (S2b), the differential in prevalence rates due to the surviving HIV-infected population (these survival rates are even higher in S2b than in S2a) is reversed by the reduction of incidence: 343,000 new infections can be avoided over 30 years in SZ, 1.8 million in TZ and 824,000 in CM over 25 years (due to the preventive effect of the program), such that HIV prevalence is reduced by half at the end of the micro-simulation process (it diminishes by 13 % in SZ, 3% in TZ and 2% in CM). Since mortality and infection rates of HIV- individuals are equal in S0, S1 and S2a, the cumulated numbers of new infections observed over the simulation process are, by construction, equal across these options.
Figure 3. HIV prevalence rates.
Evolution of HIV prevalence rates overtime in the three studied countries as per the four hypothetical ART access scenarios.
Economic Impact
The annual costs associated with the “Aid Freeze” scenario remain stable in the three countries as the number of treated individuals remains constant (Table 3). For the Universal Access scenario (S2a), 753 million USD would be disbursed in SZ to cover 210,000 individuals for the final period 2032–2037 (the annual costs of the program are multiplied by 5 over 30 years), while the expenditures reach respectively 3.7 and 1.8 billion USD in TZ and CM for the final period (respectively three and four times the first period's costs). The overall spending involved by the Extended Universal Access scenario S2b are up to 15% higher than the previous figures. However the time-structure of these costs differs in this latter scenario: annual costs are important in the first period of the simulation (the program initiates ART for an increased portion of the HIV+ population) but increase more slowly afterwards (they only double in the three countries) as the preventive effect of earlier treatment implies that new HIV infections are diminished.
Due to the general growth of the economy (resulting from demographic & epidemiological changes solely), an increase in the GDP per capita occurs in the “No Access” Scenario (S0) at the end of the micro-simulation process (by 9·8% in SZ, 0·7% in TZ and 4·7% in CM), but GDP growth-rates are higher when ART programs are initiated: a surplus, positively correlated with the coverage rate of the population can be generated through increased productivity of treated HIV-infected workers.
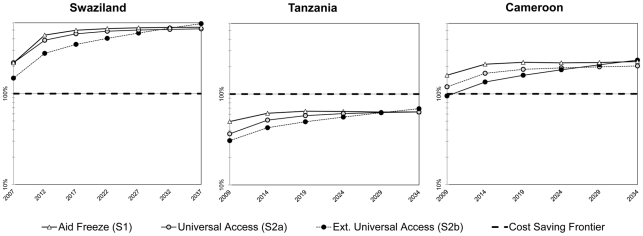
To what extent ART programs may be cost-saving when such positive macroeconomic impact is taken into account? The answer depends on the countries' GDP levels (as ARVs prices were assumed constant across the three countries of our modeling exercise). For both Swaziland and Cameroon, when compared to the counterfactual benchmark (no access to ART at all), all scenarios of ART coverage appear to be cost saving: the production gains they allow are higher than their cumulated costs (as shown in Figure 4, the self-financing ratios exceed 100% in CM and SZ). In both countries, the aid freeze scenario (S1) is the most cost saving at the beginning of the simulation but after 2030, the extended universal access scenario (S2b) provides the maximal net benefit and dominates (in terms of SFR) the other scenarios in the long run (Figure 4 and Table 3). In Tanzania, the extended universal access scenario (S2b) also dominates over the long run. However, since the GDP per capita is far lower than in Cameroon and Swaziland, GDP gains compensate only partially the total costs of ART in the three scenarios: 69% for S2b, and respectively 64% and 63% for S1 and S2a. Financing ART programs in TZ clearly implies a net cost for the economy: only 69% of total costs are covered by GDP gains in 2034 in S2b. However, in all scenarios, the net-cost per life year saved is smaller than 1000$, even when using the very conservative assumption that each life saved only represents on average five years of additional life expectancy.
Figure 4. Self Financing Ratios.
Time trends in the cost-benefit measures of ART programs in the three examined countries. The Y axis shows, on a logarithm 10 scale, the ratio between the cumulated GDP gains (compared with the No Access scenario S0) and the ART program cumulated costs.
The long term dominance of the Extended Universal Access Scenario on the others requires further examination of the model's hypotheses. Indeed, this scenario has, compared to the others, three relative advantages: the spread of the epidemic, the progression pace to the symptomatic stage of HIV and the mortality rate under treatment are all decreased. These three modifications have notable effects on the number of live saved and the self financing ratio (through program costs and/or GDP gains). We propose here eight sensitivity tests, wherein each key parameter from the microsimulation is altered. Table 4 provides estimates of both SFR and lives saved -compared to S0- at the end of the simulation process for the core analysis described previously and the alternative stipulations in the three studied countries. Sensitivity analyses V1-V3 weaken (separately) each relative advantage of S2b. In V1, ϕ, the strength of the prevention effect, is lowered from 0.7 to 0.5. S2b becomes less profitable than S1 (and sometimes S2a). When ψ (restraining the progression to HIV++) is decreased from 0.3 to 0.1 in V2, S2b becomes dominated by S1 except in SZ but remains more profitable than S2a. The same conclusion can be drawn when the mortality under ART when initiated early, u*, is increased from 0.1 to 0.15. We then symmetrically assume that the mortality rates under treatment (late initiation) are augmented from 0.2 to 0.3: S1 and S2a are even less efficient than in the core analysis (V4). If we assume that a preventive effect exists for S2a (raising the parameter from zero to 0·2), S2a slightly dominates S2b (and thus S1) in the long run (V5). Thanks to the preventative effect of treatment, S2b saves additional lives and becomes more efficient when the mortality rate of the asymptomatic HIV population is increased (v parameter, V6). We finally conduct two robustness tests on the microeconomic components of the model that conserve policy rankings (a decrease of presence rates under ART, V7, and a decrease in the price of 2nd line HIV medicines). These eight sensitivity analyses demonstrate two things: the financial sustainability of ART programs is robust to any change, and seems to depend more on the initial GDP-per-capita, rather than on the parameter used for the transition-matrix (programs are always cost-effective in Cameroon and Swaziland, but not in Tanzania); the precise ranking of S1, S2a and S2b can change depending on parameter values, but the general sketch is that S2b tends to dominate the alternatives in the long run in terms of SFR and is anyway the most life-saving option throughout the simulation.
Table 4. Sensitivity Analyses.
| Last Period Outcomes | Main Analysis | V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | ||||||||||
| Φ(S2b) from 0.7 to 0.5 | Ψ(S2b) from 0.3 to 0.1 | u (S2b) from 0.1 to 0.15 | u (S1 & S2a) from 0.2 to 0.3 | Φ (S2a) from 0 to 0.2 | v from 0.005 to 0.05 | Presence of patients from 0.95 to 0.85 | 2nd line costs from $600 to $400 | ||||||||||||
| SFR | LS | SFR | LS | SFR | LS | SFR | LS | SFR | LS | SFR | LS | SFR | LS | SFR | LS | SFR | LS | ||
| SZ 2037 | S1 | 5.37 | 27 | 5.37 | 27 | 5.37 | 27 | 5.37 | 27 | 5.16 | 24 | 5.37 | 27 | 5.37 | 27 | 4.52 | 27 | 6.54 | 27 |
| S2a | 5.16 | 187 | 5.16 | 187 | 5.16 | 187 | 5.16 | 187 | 4.90 | 148 | 5.92 | 196 | 5.16 | 187 | 4.25 | 187 | 5.98 | 187 | |
| S2b | 5.88 | 238 | 5.02 | 233 | 5.56 | 236 | 5.53 | 221 | 5.88 | 238 | 5.88 | 238 | 6.38 | 250 | 5.30 | 238 | 7.04 | 238 | |
| CM 2034 | S1 | 2.27 | 52 | 2.27 | 52 | 2.27 | 52 | 2.27 | 52 | 2.15 | 45 | 2.27 | 52 | 2.27 | 52 | 1.87 | 52 | 2.82 | 52 |
| S2a | 2.07 | 441 | 2.07 | 441 | 2.07 | 441 | 2.07 | 441 | 2.02 | 361 | 2.37 | 468 | 2.07 | 441 | 1.71 | 441 | 2.42 | 446 | |
| S2b | 2.35 | 598 | 2.10 | 584 | 2.18 | 591 | 2.21 | 490 | 2.35 | 598 | 2.35 | 598 | 2.51 | 609 | 2.10 | 598 | 2.87 | 598 | |
| TZ 2034 | S1 | 0.64 | 155 | 0.64 | 155 | 0.64 | 155 | 0.64 | 155 | 0.60 | 134 | 0.64 | 155 | 0.64 | 155 | 0.52 | 155 | 0.76 | 155 |
| S2a | 0.63 | 1010 | 0.63 | 1010 | 0.63 | 1010 | 0.63 | 1010 | 0.60 | 800 | 0.70 | 1041 | 0.63 | 1010 | 0.52 | 1010 | 0.72 | 1010 | |
| S2b | 0.69 | 1334 | 0.62 | 1318 | 0.63 | 1315 | 0.65 | 1243 | 0.69 | 1334 | 0.69 | 1334 | 0.73 | 1388 | 0.61 | 1334 | 0.82 | 1334 | |
Discussion
The use of micro-simulation techniques combined with the availability of detailed data on economic behaviors at individual levels in three African countries allowed us to forecast the impact of alternative scenarios for access to ART throughout the whole economy. Previous economic literature on the cost-effectiveness of ART programs has mainly focused on the microeconomic consequences of alternative use of resources devoted to the fight against the HIV pandemic: trade-off between prevention and treatment or trade-off between alternative threshold criteria for initiating ART or switching to second line regimens [37]–[41]. Although such approaches are very useful to help optimize treatment and comprehensive strategies and adapt them to low-resource settings [42], they ignore de facto the macroeconomic benefits induced by procuring treatment to the labor force. Our micro-simulation based modeling improves the contribution of economic evaluations to policy making decisions by adopting a concept of “net total costs and benefit” integrating GDP-gains, as in Resch et al. [43].
Not surprisingly, the human toll that would be paid by freezing ART coverage at the current levels, rather than pursuing the way forward the UNGASS goal of universal access, would be quite enormous. In such freezing scenario, the situation in terms of future AIDS-related deaths would not be very different in the 2030's from the counterfactual scenario of no-access at all to ART (by comparison, no more than 6% of additional deaths could be avoided by the current level of coverage). In contrast, universal access programs could save six times more lives. Our results also show that although universal access scenarios are logically more costly in the short term they are indeed the more cost-beneficial in the long term, when taking into account their macroeconomic consequences. The evaluations performed for Swaziland and Cameroon show that Universal Access scenarios would imply net cost-savings at the scale of the society.
The growth rate of an economy and future national wealth depend on the choices made (i.e. the people saved have a productive value which adds to the social value of human lives). The extra economic value created provides funding for treatment access programs such that Universal Access is found to be the most economically feasible option. In purely economic terms, Universal Access may be considered as an investment in productive human capital, with a positive 'Keynesian-flavored' multiplier (public spending with higher returns than initial expenditures). Reciprocally, scenarios dealing with freezing programs (e.g. reduced international aid because of the world financial crisis) despite (initially) yielding higher tax returns generate smaller benefits in the long term and have recessive effects on the economy.
Our results convey two additional messages to ongoing international debates. First, they tend to confirm [44] that in spite of the mechanical increase of direct treatment costs, the extended coverage implied by the recent revision of WHO guidelines, wherein ART is initiated at higher levels of CD4 cell counts (350 cc/mm3), is cost-effective when including its whole macroeconomic impact. Second, they confirm that the macroeconomic consequences of universal access to ART are highly sensitive to its potential impact on HIV transmission and consequently on the trajectory of the HIV epidemic globally [45]. This brings an additional argument for the urgent need of large scale randomized experiments to provide definitive data about the impact on the epidemic of the so-called “test and treat” or “treatment as prevention” strategy [46]. Future research could also investigate whether “test and treat” strategies could be economically sustainable. As results of the present paper suggest, the answer would notably depend on the ability of such a strategy to reduce inter-individual transmission, patients' mortality and control viral load.
Of course, there are two obvious limitations when extrapolating our results to the current situation of HIV funding. Our estimations are limited to only three countries and may not be valid for other African contexts. However, the three selected countries are quite representative of the heterogeneity of the epidemiological and economic situations in sub-Saharan Africa. Moreover, the reality of HIV funding in the next future may be less dramatic than the extreme “freezing” scenario which we selected. Despite the financial crisis, OECD figures showed continuing progress in total net official development assistance (ODA) in both 2008 and 2009 [47]. Significant efficiency gains in the use of already available funding may be obtained through improved synergies with other Millennium Development Goals-related programs and health systems strengthening [48] and through the use of innovations in care delivery to promote broader healthcare reforms [49]. However, recent trends, such as the limited 20% increase in donor pledges for the next three years for the GFATM, show that the threat of a significant delay in the scaling up of universal access to ART is a very real one.
In addition, a number of methodological limitations should be acknowledged. For example, individual's behaviors included in our modeling are quite static. We do not consider questions of adherence or therapeutic failure in an in-depth manner: a “delivered” treatment in our model is one which operates with a given percentage of efficacy (without consideration for age, illness duration or treatment duration) and the probabilities of survival of cohorts of agents benefitting from ART are estimated on the basis of published results in the literature. Another limitation is that our “net cost” approach does not really tackle the programs' feasibility by countries' healthcare systems [50]: if a nation experiences a shortage in the health workforce, Universal Access may be unattainable despite being budgeted and economically sustainable. It could therefore be quite interesting to examine whether the large procurement of treatment to the population, besides generating GDP gains, could enhance the public-health sector. It can also be noticed that we do not take into account the “value” of human life or the quality of added surviving years. A large improvement on the current analysis could be provided by incorporating these dimensions, mainly for countries in which the “net cost” remains positive (such as Tanzania). Concerning this latter point, the model used for this analysis has the strong advantage of being applicable to any country covered by the DHS program (26 surveys were conducted, including 20 in Sub-Saharan Africa). There is, then, the possibility to create a list of countries in which programs are unambiguously cost saving, and (symmetrically) a list of countries in which we must add an ethical “value of human life” argument in order to advocate in favor of universal ART access.
In spite of these limitations, the general message underlined by our results is that scaling-up strategies for universal access to ART, which are more costly in the short term, remain the best economic choice in the long term. Renouncing or significantly delaying the achievement of this goal, due to “legitimate” short term budgetary constraints would be a misguided choice.
Supporting Information
Detailed methodology of the Micro-simulation Model.
(DOCX)
(TIFF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This paper is a common project between the UMR912 Economics & Social Health and Medical Information Processing Research Unit (French National Institute of Health & Medical Research - French Research Institute on Development - Aix-Marseille University) and The Joint Nations Programme on HIV and AIDS (UNAIDS) (UNAIDS Contract EMP/AFE/EDA/2212). Colleagues from UNAIDS interacted with the authors in the analysis of data and in the preparation of the manuscript; they are authors. The authors would also like to thank the French Agence Nationale de Recherche sur le Sida for its financial and intellectual support. Except this, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS Getting to zero: UNAIDS 2011–2015 strategy. 2010;2 Geneva Available: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2010/JC2034_UNAIDS_Strategy_en.pdf. Accessed 2012 March. [Google Scholar]
- 2.UNAIDS Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva. 2010;2 Available: http://www.unaids.org/globalreport/Global_report.htm. Accessed 2012 March. [Google Scholar]
- 3.WHO, UNAIDS, UNICEF Toward universal access. Scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. 2010;2 Geneva Available: http://www.who.int/hiv/pub/2010progressreport/report/en/index.html. Accessed 2012 March. [Google Scholar]
- 4.Katzenstein D, Koulla-Shiro S, Laga M, Moatti JP. Learning and doing: operational research and access to HIV treatment in Africa. AIDS. 2010;24(Suppl 1):S1–4. doi: 10.1097/01.aids.0000366077.37827.0a. [DOI] [PubMed] [Google Scholar]
- 5.Walensky RP, Kuritzkes DR. The impact of the President's Emergency Plan for AIDS Relief (PEPfAR) beyond HIV and why it remains essential. Clin Infect Dis. 2010;50:272–275. doi: 10.1086/649214. [DOI] [PubMed] [Google Scholar]
- 6.Crowley S, Rollins N, Shaffer N, Guerma T, Vitoria M, et al. New WHO HIV treatment and prevention guidelines. Lancet. 2010;375:874–875. doi: 10.1016/S0140-6736(09)62064-X. [DOI] [PubMed] [Google Scholar]
- 7.Orsi F, Carrieri MP, Coriat B, Delaporte E, Moatti JP, et al. Call for action to secure universal access to ART in developing countries. Lancet. 2010;375:1693–1694. doi: 10.1016/S0140-6736(10)60737-4. [DOI] [PubMed] [Google Scholar]
- 8.GFATM Funding of round 10 and timing and resource scenarios for future funding opportunities. 2010. GF/B22/18. Twenty-Second Board Meeting 2010 Dec 13–15. Sofia; Bulgaria.
- 9.GFATM Report of the Executive Director. 2010. GF/ B22/3. Twenty-Second Board Meeting Dec 13–15. Sofia; Bulgaria.
- 10.Gostin LO, Kim SC. Ethical allocation of preexposure HIV prophylaxis. JAMA. 2011;305:191–192. doi: 10.1001/jama.2010.1975. [DOI] [PubMed] [Google Scholar]
- 11.Bongaarts J, Over M. Public health. Global HIV/AIDS policy in transition. Science. 2010;328:1359–1360. doi: 10.1126/science.1191804. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS Making sense of the money. Where does the money for AIDS go? 2010 Geneva Available: http://data.unaids.org/pub/Outlook/2010/20100713_outlook_money_en.pdf. Accessed 2012 March 2. [Google Scholar]
- 13.Izazola-Licea JA, Wiegelmann J, Aran C, Guthrie T, De Lay P, et al. Financing the response to HIV in low-income and middle-income countries. J Acquir Immune Defic Syndr. 2009;52(Suppl 2):S119–126. doi: 10.1097/QAI.0b013e3181baeeda. [DOI] [PubMed] [Google Scholar]
- 14.Hecht R, Stover J, Bollinger L, Muhib F, Case K, et al. Financing of HIV/AIDS programme scale-up in low-income and middle-income countries, 2009-31. Lancet. 2010;376:1254–1260. doi: 10.1016/S0140-6736(10)61255-X. [DOI] [PubMed] [Google Scholar]
- 15.Young A. The Gift of the Dying: The Tragedy of AIDS and the Welfare of Future African Generations. The Quarterly Journal of Economics. 2005;120:423–466. [Google Scholar]
- 16.Bell C, Devarajan S, Gersbach H. The Long-Run Economic Costs of aids: A Model with an Application to South Africa. The World Bank Economic Review. 2006;20:55–89. [Google Scholar]
- 17.Goenka A, Liu L. Infectious diseases and endogenous fluctuations. Economic Theory. 2010:1–25. [Google Scholar]
- 18.Ventelou B, Moatti JP, Videau Y, Kazatchkine M. 'Time is costly': modelling the macroeconomic impact of scaling-up antiretroviral treatment in sub-Saharan Africa. AIDS. 2008;22:107–113. doi: 10.1097/QAD.0b013e3282f1d49f. [DOI] [PubMed] [Google Scholar]
- 19.Leclerc PM, Matthews AP, Garenne ML. Fitting the HIV epidemic in Zambia: a two-sex micro-simulation model. PLoS One. 2009;4:e5439. doi: 10.1371/journal.pone.0005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United Nations Statistics Division. National Accounts Estimates of Main Aggregates. 2010 Available: http://unstats.un.org/unsd/snaama/introduction.asp. Accessed 2012 March 2. [Google Scholar]
- 21.Swaziland Central Statistical Office, ORC Macro. The 2006–2007 Swaziland Demographic and Health Survey; Final Report. 2008;2 Available from: http://www.measuredhs.com/pubs/pdf/FR202/FR202.pdf. Accessed 2012 March. [Google Scholar]
- 22.Tanzania National Bureau of Statistics, ORC Macro The 2004–2005 Tanzania Demographic and Health Survey; Final Report. 2005 Available from: http://www.measuredhs.com/publications/publication-AIS1-AIS-Final-Reports.cfm. Accessed 2012 March 2. [Google Scholar]
- 23.Institut National de la Statistique du Cameroon, ORC Macro. Enquête Démographique et–de Santé Cameroon 2004. 2005 Available: http://www.measuredhs.com/pubs/pdf/FR163/FR163-CM04.pdf. Accessed 2012 March 2. [Google Scholar]
- 24.UNPD World Population Prospects. 2010 Available: http://esa.un.org/unpd/wpp/unpp/panel_population.htm. Accessed 2012 March 2. [Google Scholar]
- 25.WHO Antiretroviral therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. 2006 Revision. 2006 Geneva Available: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. Accessed 2012 March 2. [PubMed] [Google Scholar]
- 26.WHO Antiretroviral therapy for HIV Infection in Adults and Adolescents: Towards Universal Access. Recommendations for a Public Health Approach. 2010 Revision. Geneva. 2010 Available: http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. Accessed 2012 March 2. [PubMed] [Google Scholar]
- 27.UNAIDS Cost estimates. AIDS Financing and Economics. Geneva. 2009 Available: http://www.who.int/hiv/topics/treatment/ART_cost_estimates.pdf. Accessed 2012 March 2. [Google Scholar]
- 28.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 29.Fox MP, Rosen S, MacLeod WB, Wasunna M, Bii M, et al. The impact of HIV/AIDS on labour productivity in Kenya. Tropical Medicine & International Health. 2004;9:318–324. doi: 10.1111/j.1365-3156.2004.01207.x. [DOI] [PubMed] [Google Scholar]
- 30.Habyarimana J, Mbakile B, Pop-Eleches C. The Impact of HIV/AIDS and ARV Treatment on Worker Absenteeism. Journal of Human Resources. 2010;45:809–839. [Google Scholar]
- 31.Thirumurthy H, Zivin JG, Goldstein M. The Economic Impact of AIDS Treatment. Journal of Human Resources. 2008;43:511–552. [PMC free article] [PubMed] [Google Scholar]
- 32.Kyereh K, Hoffman D. 5th Post Graduate Conference on Construction Industry Development. Bloemfontein, South Africa; 2008. The Impact of HIV/AIDS on Skills availability in South African Coal Mines. [Google Scholar]
- 33.UNITAID New Prices Reductions for key drugs. Geneva. 2009 Available: http://www.unitaid.eu/fr/20090417198/ACTUALITES/UNITAID-and-the-Clinton-HIV/AIDS-Initiative-Announce-New-Price-Reductions-for-key-drugs.html. Accessed 2012 March 2. [Google Scholar]
- 34.Stover J, Bollinger L, Avila C. AIDS Res Treat 2011: 738271; 2011. Estimating the Impact and Cost of the WHO 2010 Recommendations for Antiretroviral Therapy.738271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stover J, Korenromp EL, Blakley M, Komatsu R, Viisainen K, et al. Long-Term Costs and Health Impact of Continued Global Fund Support for Antiretroviral Therapy. PLoS One. 2011;6:e21048. doi: 10.1371/journal.pone.0021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dongmo Nguimfack B. Transaction prices for Antiretroviral Medicines and HIV Diagnostics from 2008 to October 2010. Geneva: WHO.Global Price Reporting Mechanism. 2010 Available: http://www.who.int/hiv/pub/amds/gprm_summary_report_dec2010.pdf. Accessed 2012 March 2. [Google Scholar]
- 37.Over M, Marseille E, Sudhakar K, Gold J, Gupta I, et al. Antiretroviral therapy and HIV prevention in India: modeling costs and consequences of policy options. Sex Transm Dis. 2006;33:S145–152. doi: 10.1097/01.olq.0000238457.93426.0d. [DOI] [PubMed] [Google Scholar]
- 38.Cleary SM, McIntyre D, Boulle AM. The cost-effectiveness of antiretroviral treatment in Khayelitsha, South Africa–a primary data analysis. Cost Eff Resour Alloc. 2006;4:20. doi: 10.1186/1478-7547-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badri M, Cleary S, Maartens G, Pitt J, Bekker LG, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther. 2006;11:63–72. [PubMed] [Google Scholar]
- 40.Bachmann MO. Effectiveness and cost effectiveness of early and late prevention of HIV/AIDS progression with antiretrovirals or antibiotics in Southern African adults. AIDS Care. 2006;18:109–120. doi: 10.1080/09540120500159334. [DOI] [PubMed] [Google Scholar]
- 41.Bendavid E, Grant P, Talbot A, Owens DK, Zolopa A. Cost-effectiveness of antiretroviral regimens in the World Health Organization's treatment guidelines: a South African analysis. AIDS. 2011;25:211–220. doi: 10.1097/QAD.0b013e328340fdf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moatti JP, Marlink R, Luchini S, Kazatchkine M. Universal access to HIV treatment in developing countries: going beyond the misinterpretations of the 'cost-effectiveness' algorithm. AIDS. 2008;22(Suppl 1):S59–66. doi: 10.1097/01.aids.0000327624.69974.41. [DOI] [PubMed] [Google Scholar]
- 43.Resch S, Korenromp E, Stover J, Blakley M, Krubiner C, et al. Economic Returns to Investment in AIDS Treatment in Low and Middle Income Countries. PLoS One. 2011;6:e25310. doi: 10.1371/journal.pone.0025310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walensky RP, Wood R, Ciaranello AL, Paltiel AD, Lorenzana SB, et al. Scaling up the 2010 World Health Organization HIV Treatment Guidelines in resource-limited settings: a model-based analysis. PLoS Med. 2010;7:e1000382. doi: 10.1371/journal.pmed.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Sadr WM, Affrunti M, Gamble T, Zerbe A. Antiretroviral therapy: a promising HIV prevention strategy? J Acquir Immune Defic Syndr. 2010;55(Suppl 2):S116–121. doi: 10.1097/QAI.0b013e3181fbca6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dabis F, Newell ML, Hirschel B. HIV drugs for treatment, and for prevention. Lancet. 2010;375:2056–2057. doi: 10.1016/S0140-6736(10)60838-0. [DOI] [PubMed] [Google Scholar]
- 47.OECD Aid Statistics. 2010 Available: http://www.oecd.org/dac/stats/data . Accessed 2012 March 2. [Google Scholar]
- 48.Samb B, Evans T, Dybul M, Atun R, Moatti JP, et al. An assessment of interactions between global health initiatives and country health systems. Lancet. 2009;373:2137–2169. doi: 10.1016/S0140-6736(09)60919-3. [DOI] [PubMed] [Google Scholar]
- 49.Bennett S, Ozawa S, Rao KD. Which path to universal health coverage? Perspectives on the World Health Report 2010. PLoS Med. 2010;7:e1001001. doi: 10.1371/journal.pmed.1001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.USAID . Health Systems 2020. United States Agency of International Development 2010; 2010. Using HAPSAT for HIV Program Sustainability Analysis: an Introductory Guide. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed methodology of the Micro-simulation Model.
(DOCX)
(TIFF)