Abstract
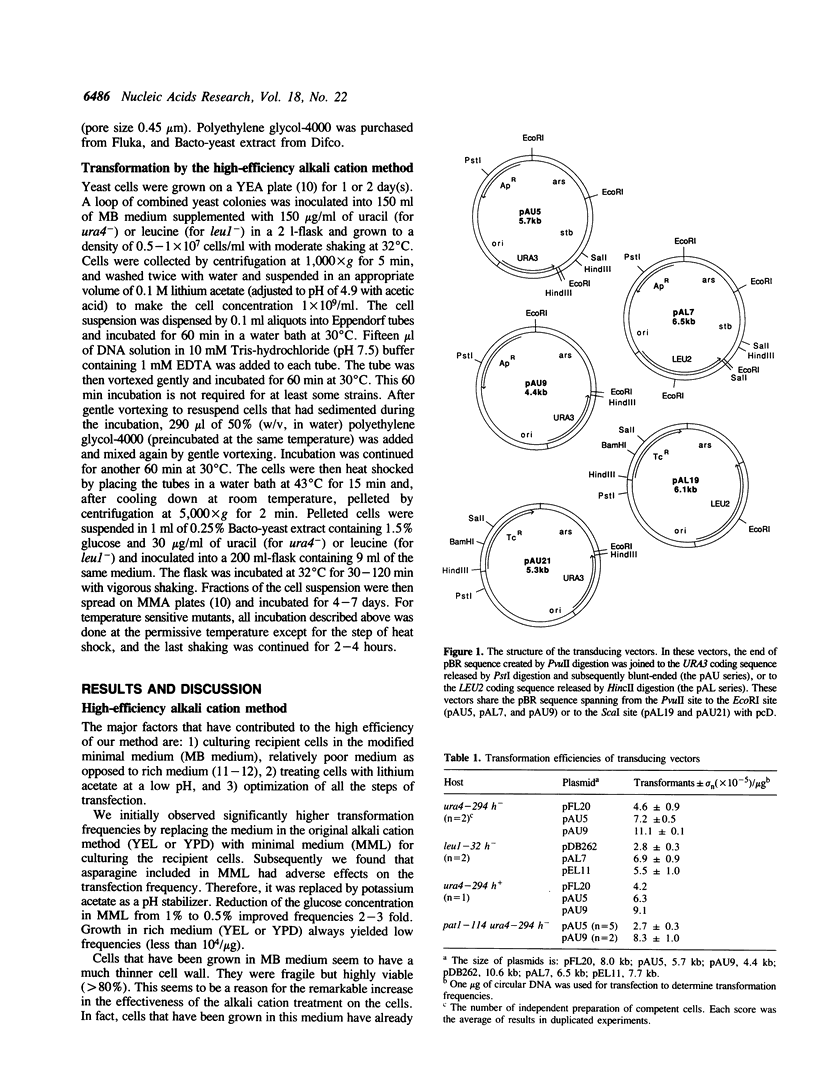
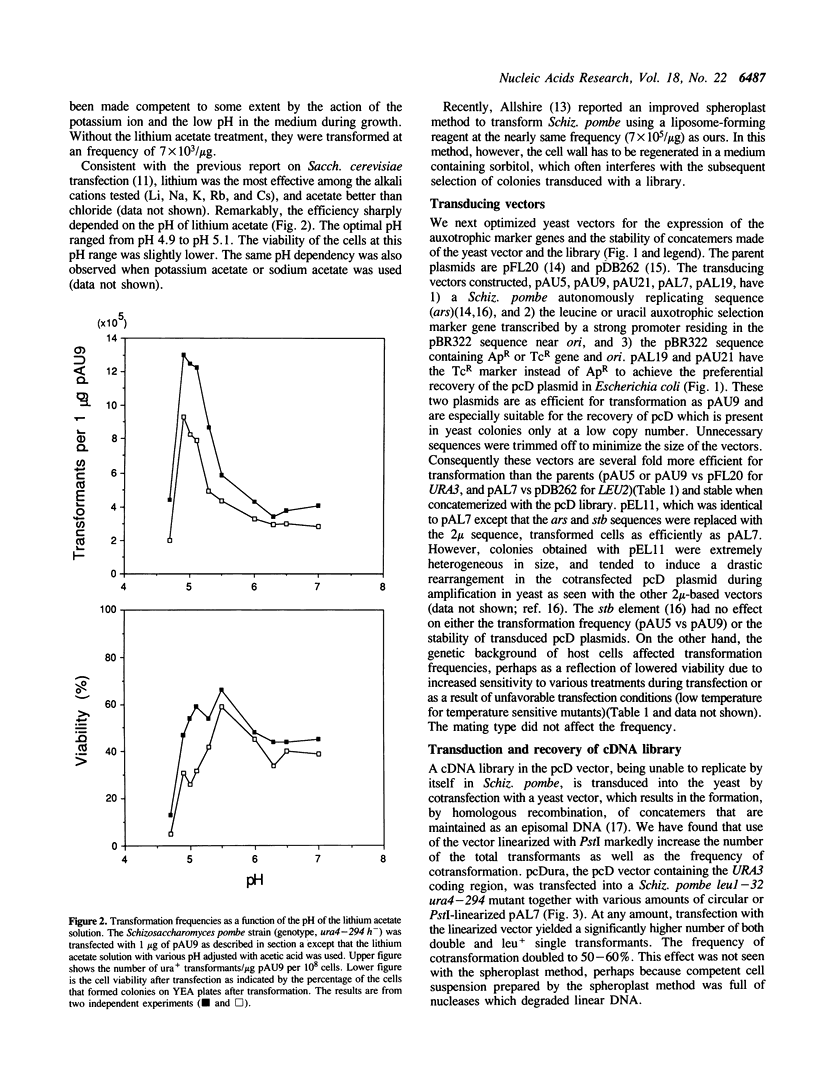
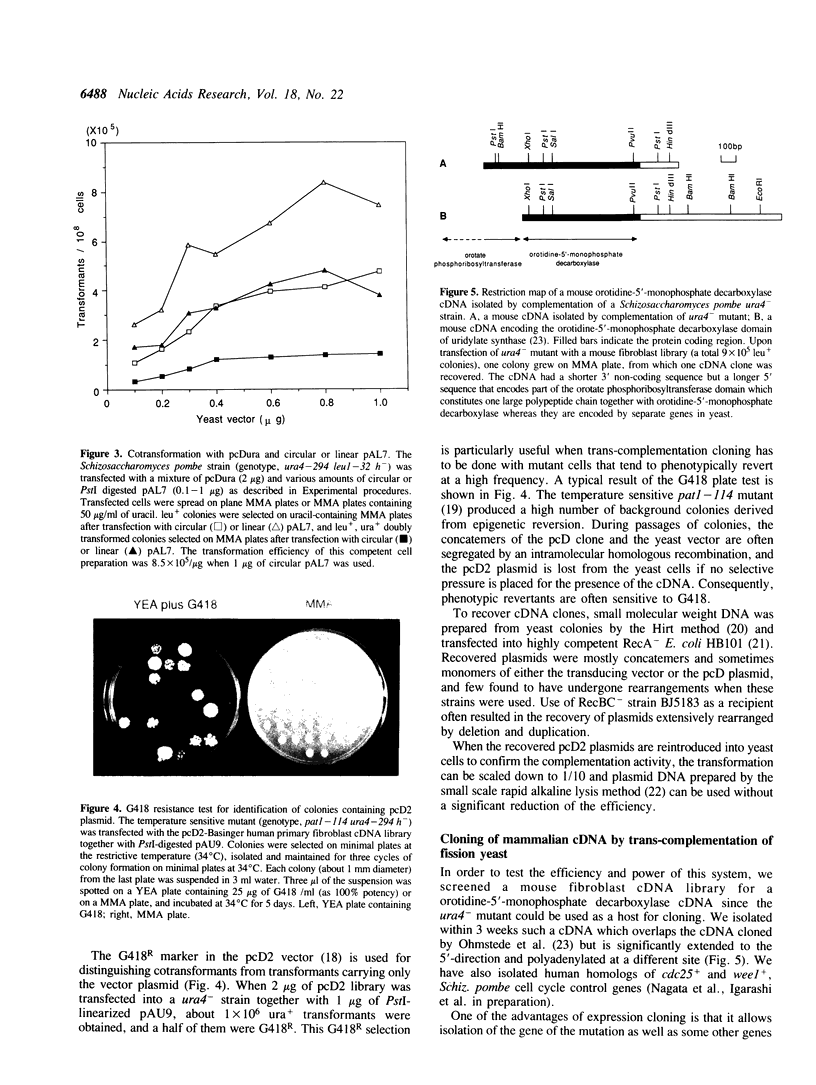
We describe a highly efficient alkali cation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of fission yeast Schizosaccharomyces pombe mutants. cDNA libraries constructed with the pcD or pcD2 vector are transduced into yeast by cotransfection with a linearized vector, which allows an enhanced homologous recombination between the yeast vector and the library plasmid leading to the efficient formation of concatemers containing pcD molecules. The transformation frequencies obtained by the method are 10(6) colonies per 10(8) cells transfected with 2 micrograms of library and 1 microgram of vector, 50-60% of which contain pcD molecules. The high-efficiency alkali cation method circumvents many of the shortcomings of the spheroplast method generally used for Schiz. pombe transfection. The vectors are maximized for the efficiency of library transduction and minimized for the rearrangements of pcD molecules during propagation in yeast. This system allows rapid screening of multi-million cDNA clone libraries for rare cDNAs in a routine scale of experiments. Using this system, various mammalian cDNAs that are extremely difficult, time-consuming, or unclonable to clone by other methods have been cloned.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C. Introduction of large linear minichromosomes into Schizosaccharomyces pombe by an improved transformation procedure. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4043–4047. doi: 10.1073/pnas.87.11.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol Gen Genet. 1982;187(2):326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer W. D., Sipiczki M., Kohli J. Replicating plasmids in Schizosaccharomyces pombe: improvement of symmetric segregation by a new genetic element. Mol Cell Biol. 1986 Jan;6(1):80–89. doi: 10.1128/mcb.6.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. H., Moreno S., Nurse P., Jones N. C. Expression of the SV40 promoter in fission yeast: identification and characterization of an AP-1-like factor. Cell. 1988 May 20;53(4):659–667. doi: 10.1016/0092-8674(88)90581-8. [DOI] [PubMed] [Google Scholar]
- Kohli J. Genetic nomenclature and gene list of the fission yeast Schizosaccharomyces pombe. Curr Genet. 1987;11(8):575–589. doi: 10.1007/BF00393919. [DOI] [PubMed] [Google Scholar]
- Käufer N. F., Simanis V., Nurse P. Fission yeast Schizosaccharomyces pombe correctly excises a mammalian RNA transcript intervening sequence. Nature. 1985 Nov 7;318(6041):78–80. doi: 10.1038/318078a0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Plasmids carrying the yeast OMP decarboxylase structural and regulatory genes: transcription regulation in a foreign environment. Cell. 1983 Feb;32(2):371–377. doi: 10.1016/0092-8674(83)90456-7. [DOI] [PubMed] [Google Scholar]
- Nasim A., Smith B. P. Genetic control of radiation sensitivity in Schizosaccharomyces pombe. Genetics. 1975 Apr;79(4):573–582. doi: 10.1093/genetics/79.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K., Nurse P. Cell division cycle mutants altered in DNA replication and mitosis in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1981;182(1):119–124. doi: 10.1007/BF00422777. [DOI] [PubMed] [Google Scholar]
- Noda M., Kitayama H., Matsuzaki T., Sugimoto Y., Okayama H., Bassin R. H., Ikawa Y. Detection of genes with a potential for suppressing the transformed phenotype associated with activated ras genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):162–166. doi: 10.1073/pnas.86.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Ohmstede C. A., Langdon S. D., Chae C. B., Jones M. E. Expression and sequence analysis of a cDNA encoding the orotidine-5'-monophosphate decarboxylase domain from Ehrlich ascites uridylate synthase. J Biol Chem. 1986 Mar 25;261(9):4276–4282. [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli A. S., Smith G. R. Meiotic recombination-deficient mutants of Schizosaccharomyces pombe. Genetics. 1989 Sep;123(1):45–54. doi: 10.1093/genetics/123.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potashkin J., Li R., Frendewey D. Pre-mRNA splicing mutants of Schizosaccharomyces pombe. EMBO J. 1989 Feb;8(2):551–559. doi: 10.1002/j.1460-2075.1989.tb03409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Sakaguchi J., Yamamoto M. High-frequency cotransformation by copolymerization of plasmids in the fission yeast Schizosaccharomyces pombe. Mol Cell Biol. 1984 Apr;4(4):651–656. doi: 10.1128/mcb.4.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach M. The isolation and genetic classification of UV-sensitive mutants of Schizosaccharomyces pombe. Mutat Res. 1971 Apr;11(4):361–371. [PubMed] [Google Scholar]
- Teitz T., Naiman T., Avissar S. S., Bar S., Okayama H., Canaani D. Complementation of the UV-sensitive phenotype of a xeroderma pigmentosum human cell line by transfection with a cDNA clone library. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8801–8804. doi: 10.1073/pnas.84.24.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama R., Okayama H. Human chorionic gonadotropin alpha and human cytomegalovirus promoters are extremely active in the fission yeast Schizosaccharomyces pombe. FEBS Lett. 1990 Jul 30;268(1):217–221. doi: 10.1016/0014-5793(90)81012-d. [DOI] [PubMed] [Google Scholar]
- Uzé G., Lutfalla G., Gresser I. Genetic transfer of a functional human interferon alpha receptor into mouse cells: cloning and expression of its cDNA. Cell. 1990 Jan 26;60(2):225–234. doi: 10.1016/0092-8674(90)90738-z. [DOI] [PubMed] [Google Scholar]
- Wright A., Maundrell K., Heyer W. D., Beach D., Nurse P. Vectors for the construction of gene banks and the integration of cloned genes in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Plasmid. 1986 Mar;15(2):156–158. doi: 10.1016/0147-619x(86)90051-x. [DOI] [PubMed] [Google Scholar]
- Yanagida M. Gene products required for chromosome separation. J Cell Sci Suppl. 1989;12:213–229. doi: 10.1242/jcs.1989.supplement_12.18. [DOI] [PubMed] [Google Scholar]



