Abstract
We know very little about how soil-borne pollutants such as selenium (Se) can impact pollinators, even though Se has contaminated soils and plants in areas where insect pollination can be critical to the functioning of both agricultural and natural ecosystems. Se can be biotransferred throughout the food web, but few studies have examined its effects on the insects that feed on Se-accumulating plants, particularly pollinators. In laboratory bioassays, we used proboscis extension reflex (PER) and taste perception to determine if the presence of Se affected the gustatory response of honey bee (Apis mellifera L., Hymenoptera: Apidae) foragers. Antennae and proboscises were stimulated with both organic (selenomethionine) and inorganic (selenate) forms of Se that commonly occur in Se-accumulating plants. Methionine was also tested. Each compound was dissolved in 1 M sucrose at 5 concentrations, with sucrose alone as a control. Antennal stimulation with selenomethionine and methionine reduced PER at higher concentrations. Selenate did not reduce gustatory behaviors. Two hours after being fed the treatments, bees were tested for sucrose response threshold. Bees fed selenate responded less to sucrose stimulation. Mortality was higher in bees chronically dosed with selenate compared with a single dose. Selenomethionine did not increase mortality except at the highest concentration. Methionine did not significantly impact survival. Our study has shown that bees fed selenate were less responsive to sucrose, which may lead to a reduction in incoming floral resources needed to support coworkers and larvae in the field. If honey bees forage on nectar containing Se (particularly selenate), reductions in population numbers may occur due to direct toxicity. Given that honey bees are willing to consume food resources containing Se and may not avoid Se compounds in the plant tissues on which they are foraging, they may suffer similar adverse effects as seen in other insect guilds.
Introduction
Over 60% of the world's crop species are animal pollinated, with honey bees constituting a large component [1], [2]. The value of the honey bee (Apis mellifera L., Hymenoptera: Apidae) as managed pollination services in the United States is estimated to be up to 14 billion dollars per year [3]–[5]. Declines in honey bee populations due to pesticide poisoning have been a focus of recent research [6], but the role of soil-borne pollutants on honey bee survival has not been examined. Few studies have focused on the toxicological effects of metal or metalloid pollutants on bee behaviors and survival.
Honey bees forage over very large areas and bring plant materials (nectar, pollen and propolis) back to their hives, and thus may collect significant amounts of toxic contaminants. Plant pollinators such as honey bees and their honey products have been investigated as potential bioindicators of metal and metalloid pollutants [7], [8]. Varying amounts of contaminants that are toxic to insects have been found in honey, propolis, and pollen from honey bee hives located in close proximity to polluted sites around the world [9]–[15]. With regards to the soil-borne pollutant, selenium (Se), pollen collected by bees from plants growing in fly ash from coal-burning electrical power plants contained 14 mg Se kg−1 [16]. In an urban, uncontaminated area of Poland, honey bee foragers collected from stationary hives contained 7.03 mg Se kg−1 [17]. Honey collected from different regions of Turkey contained 38 to 113 µg kg−1 [18]. Honey collected from hives located in seleniferous areas of Colorado contained up to 0.73 mg Se kg−1 [19]. These findings raise the following issues: 1) Do nectar and pollen from plants growing in high metal or metalloid soils contain levels of these elements that, when collected, are toxic to brood or workers? 2) What is the potential for adverse effects on pollinator health of widespread contamination of selenium? Although there has been some interest in using honey bees and their products as bioindicators of pollution, few studies have examined the effects of foraged plant tissues containing soil-borne pollutants such as Se on pollinator health.
Selenium (Se) is a metalloid that occurs naturally in certain alkaline soils from shale deposits of prehistoric inland seas [20]. Agricultural water drainage dissolves Se from these naturally seleniferous soils and has caused the buildup of selenate (SeO4 2−), the predominant and bioavailable form of Se. One of the worst cases of Se pollution occurred at the Kesterson Reservoir in the San Joaquin Valley (Merced County, California, USA), a major drainage site for many agricultural regions of California [21]. The EPA maximum contaminant level (MCL) of 0.05 mg L−1 for Se in drinking water was based on evidence from this well-documented case of Se poisoning as well as 96 hour acute and chronic toxicity testing of aquatic animals. However, the MCL does not consider bioaccumulation or biomagnification of Se. Studies have demonstrated the biomagnification of Se throughout the food web [22], but few studies have examined the effects of plants and the insects that feed on them in Se-contaminated sites. However, in several studies examining Se levels in arthropods collected from accumulating plants, various floral visitors contained up to 75 µg Se g−1 dry weight (dw) [23], honey bees contained 14.8 µg Se g−1 dw and bumble bees contained 251 µg Se g−1 dw [19]. Thus, there is the possibility for biotransfer of Se from plant to pollinator.
Despite its toxic properties, selenium is also a micronutrient that is essential to many organisms, including mammals, fish, and bacteria [24], but slightly higher levels can cause toxic effects. Selenium's toxicity is attributed to its similarity to sulfur. Se replaces sulfur in amino acids such as cysteine and methionine and can change protein folding, disrupt cell metabolism [25], [26], and alter the activity of enzymes if the Se replaces S near the active site [27]. Inorganic forms of Se can also cause oxidative stress [28] and DNA damage [29]. Although Se is a micronutrient for many living organisms, a surplus of the element can cause developmental deformities and toxicity.
There is good evidence that Se accumulation can have negative effects on plant growth, insect herbivores, their predators and parasites, and the detritivores that feed on decaying plant and animal tissues [30], [31], yet we know very little about how pollutants such as Se impact pollinators. Herbivores fed plant tissues containing high levels of metals, metalloids (such as Se), or other accumulated elements have shown reduced development and survival [32], and several studies have shown some insect species can not detect detrimental levels of Se [33], [34], but there are no studies to date examining the effects of Se-containing floral tissues on insect pollinator behaviors and survival.
Our overall objective was to determine whether the two main forms of Se commonly found in accumulating plants, selenate and selenomethionine [35]–[37], can have sublethal or lethal effects on the honey bee (Apis mellifera L., Hymenoptera: Apidae). Our first objective examined whether the presence of Se affected honey bee gustatory behavior via two different chemosensory organs (antenna or proboscis). Our second objective was to examine whether Se has sublethal effects on the honey bee's feeding behaviors, particularly if it can alter the bee's responsiveness to sucrose. Our third objective tested whether increasing concentrations of Se can cause mortality when administered as a single or chronic dose to honey bee foragers. If pollinators cannot detect and avoid Se compounds in the pollen and nectar on which they are foraging and collecting for their progeny, they may suffer similar adverse effects as seen in other insect guilds.
Results
Antennal response assays
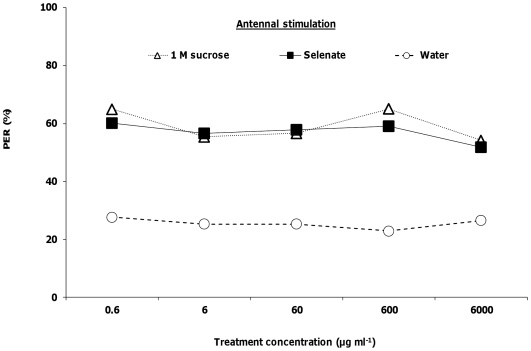
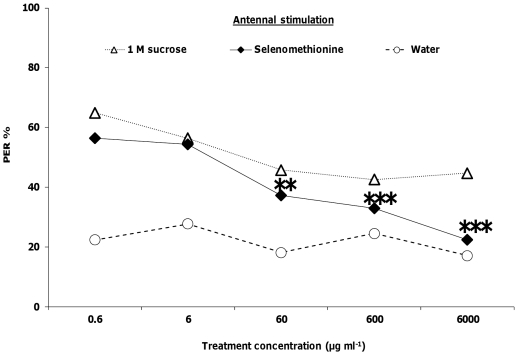
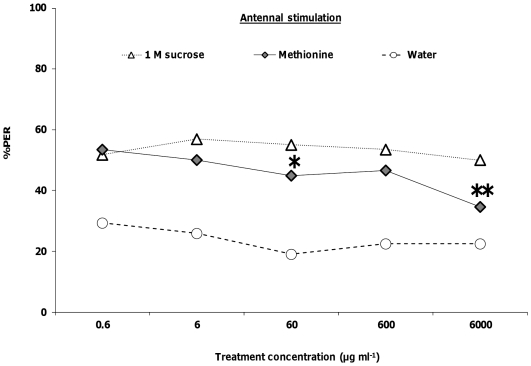
The proboscis extension reflex (PER) involves stimulating a honey bee's antennae with a sucrose solution. The bee will then reflexively extend its proboscis in response to the stimulation. We examined whether honey bees exhibited a reduced PER response to sucrose solutions that contained selenate, selenomethionine or methionine over a range of concentrations spanning five orders of magnitude, from 0.6 to 6000 µg ml−1. Honey bee foragers' PER responses to antennal stimulation by selenate were not significantly different than the responses to 1 M sucrose at any of the 5 concentrations (logistic regression, Χ6 2<3.43, p>0.06 for all; Figure 1). Responses to selenomethionine plus sucrose were significantly lower than the 1 M sucrose control at 60 µg ml−1 (Χ1 2 = 11.80, p<0.001), 600 µg ml−1 (Χ1 2 = 22.40, p<0.0001) and 6000 µg ml−1 (Χ1 2 = 46.51, p<0.0001; Figure 2). For methionine, responses were only significantly lower than the 1 M sucrose control at the 60 µg ml−1 (Χ1 2 = 4.19, p<0.05) and 6000 µg ml−1 treatments (Χ1 2 = 8.15, p<0.001; Figure 3). PER responses to antennal stimulation with solutions containing 1 M sucrose plus selenate (Figure 1) or methionine (Figure 3) were significantly higher than responses to water at all 5 concentrations (Χ6 2>6.75, p<0.01 for all). Responses to antennal stimulation by sucrose plus selenomethionine were significantly higher than responses to water at the 4 lowest concentrations (Χ5 2>11.42, p<0.001 for all; Figure 2). However, at the 6000 µg ml−1 concentration, the response (22%) was not significantly different from that for water (17%, Χ1 2 = 0.02, p = 0.88).
Figure 1. Honey bee behavioral responses to antennal stimulation with selenate.
Honey bees were stimulated with 1 M sucrose, water, and selenate in 1 M sucrose (N = 83). Asterisks indicate significance of *P<0.05, **P<0.001, ***P<0.0001 (Logistic regression with multiple comparisons) between 1 M sucrose and treatment lines.
Figure 2. Honey bee behavioral responses to antennal stimulation with selenomethionine.
Honey bees were stimulated with 1 M sucrose, water, and selenomethionine in 1 M sucrose (N = 94). Asterisks indicate significance of *P<0.05, **P<0.001, ***P<0.0001 (Logistic regression with multiple comparisons) between 1 M sucrose and treatment lines.
Figure 3. Honey bee behavioral responses to antennal stimulation with methionine.
Honey bees were stimulated with 1 M sucrose, water, and methionine in 1 M sucrose (N = 58). Asterisks indicate significance of *P<0.05, **P<0.001, ***P<0.0001 (Logistic regression with multiple comparisons) between 1 M sucrose and treatment lines.
Proboscis response assays
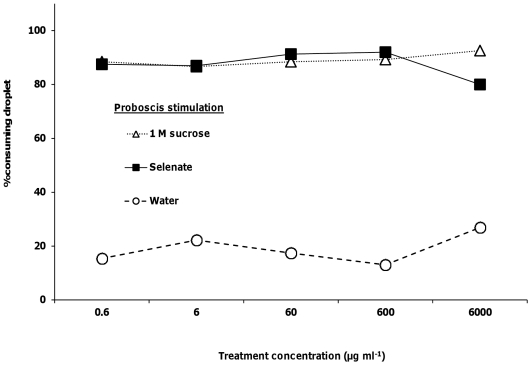
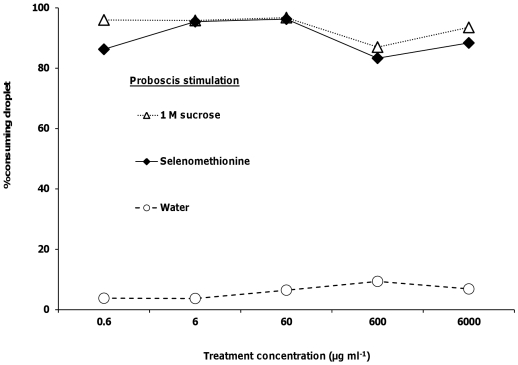
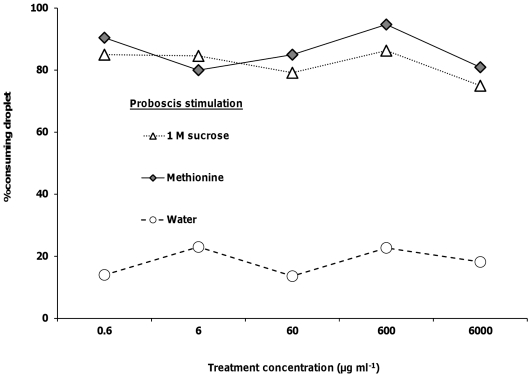
As well as receptors on the antennae, honeybees also have gustatory receptors on the proboscis. We examined if the presence of selenate, selenomethionine or methionine, at the same 5 concentrations, affected the willingness of bees to actually consume 1 M sucrose solutions. Bees that were given sucrose solutions containing selenate showed no significant differences in consumption of the droplet between 1 M sucrose and any of the 5 selenate concentrations (logistic regression, Χ5 2<1.45, p>0.23 for all; Figure 4). Proboscis stimulation with the water treatment elicited a significantly lower response than 1 M sucrose or any of the 5 selenate concentrations (Χ6 2>13.99, p<0.002 for all; Figure 4). There were no significance differences in the percent of bees consuming the droplet between 1 M sucrose and any of the selenomethionine (Χ5 2<0.79, p>0.37 for all; Figure 5) or methionine (Χ5 2<0.76, p>0.38 for all concentrations; Figure 6) treatments. Consumption responses to proboscis stimulation with water were significantly lower than responses to 1 M sucrose and selenomethionine (Χ6 2>13.99, p<0.002 for all concentrations) or methionine (Χ6 2>14.46, p<0.0001 for all concentrations).
Figure 4. Honey bee behavioral responses to proboscis stimulation with selenate.
Honey bees' proboscises were stimulated with 1 M sucrose, water, and selenate in 1 M sucrose (N = 23–30).
Figure 5. Honey bee behavioral responses to proboscis stimulation with selenomethionine.
Honey bees' proboscises were stimulated with 1 M sucrose, water, and selenomethionine in 1 M sucrose (N = 22–31).
Figure 6. Honey bee behavioral responses to proboscis stimulation with methionine.
Honey bees' proboscises were stimulated with 1 M sucrose, water, and methionine in 1 M sucrose (N = 19–26).
Sucrose response threshold assays
The effects of selenate, selenomethionine, and methionine consumption on the responsiveness of honey bee foragers to sugars were determined using sucrose response thresholds (SRT), or the lowest sucrose concentration that elicits a PER response. Bees from all selenate treatment groups showed a dose-dependent change in PER to increasing concentrations of sucrose (logistic regression, Χ6 2 = 58.09, p<0.0001, Table S1). The sucrose response threshold occurred between 3 and 10%, except for the group of bees fed 60 µg selenate ml−1, whose response to sucrose never significantly differed from that of water. Selenate feeding treatment had a significant effect on proboscis extension response (Χ5 2 = 13.34, p<0.02), resulting in a decrease in overall average PER for all selenate feeding treatments (Table S1). The percentage of bees responding with proboscis extension dropped from 48% in bees fed the control (1 M sucrose) to as low as 17% in the 6000 µg ml−1 selenate-fed bees. However, there was no significant interaction between the sucrose antennal treatment and the selenate feeding treatment (Χ30 2 = 37.30, p = 0.17), indicating that selenate feeding did not alter the sucrose response threshold of 3 to 10% (Table S1).
All selenomethionine treatment groups showed a dose-dependent change in PER to increasing concentrations of sucrose (Χ6 2 = 40.08, p<0.0001). The sucrose response threshold occurred between 3 and 10%, except for the 0.6 and 6 µg ml−1 treatment groups whose sucrose response thresholds were as high as 30% (Table S1). Selenomethionine feeding treatment did not have a significant effect on sucrose response threshold (Χ5 2 = 4.41, p = 0.49). In addition, the interaction of selenomethionine feeding treatment and the sucrose antennal treatment was not significant (Χ30 2 = 41.15, p = 0.09).
All methionine treatment groups showed a dose-dependent change in PER to increasing concentrations of sucrose (Χ6 2 = 57.93, p<0.0001). The sucrose response threshold occurred between 1 and 10% (Table S1). Methionine feeding treatment did not have a significant effect on sucrose response threshold (Χ5 2 = 7.98, p = 0.16). The interaction of methionine feeding treatment and sucrose antennal treatment was also not significant (Χ30 2 = 24.94, p = 0.73).
Total consumption and single dose mortality
Honey bee foragers were fed a single dose of Se or sulfur as selenate, selenomethionine, or methionine plus sucrose at 5 concentrations, and then mortality was scored for 5 days. Treatments were compared to bees fed 1 M sucrose as the control. There was no significant difference in total consumption of selenate (ANOVA, F5,232 = 0.79, p = 0.56), selenomethionine (F5,108 = 1.26, p = 0.29) or methionine (F5,129 = 2.19, p = 0.06) at the 5 concentrations. Bees ingested an overall average of 21.94±0.47 µl of selenate in 1 M sucrose (N = 18–21), 21.83±0.97 µl of selenomethionine in 1 M sucrose (N = 18–20), and 20.51±0.63 µl of methionine in 1 M sucrose (N = 21–24) across all concentrations.
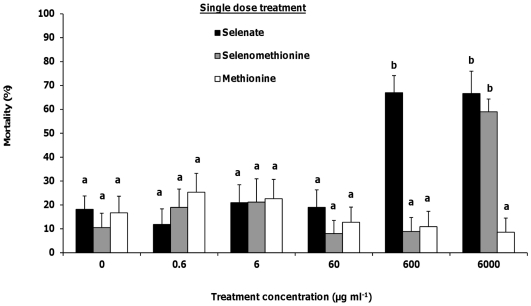
Single dosage with selenate significantly increased final percent mortality in honey bee foragers at the 600 µg ml−1 (Kruskal-Wallis, Χ1 2 = 29.83, p<0.0001) and 6000 µg ml−1 (Χ1 2 = 37.31, p<0.0001) treatment levels compared to 1 M sucrose (Figure 7). Mortality reached as high as 67% at the 6000 µg ml−1 selenate concentration. Selenomethionine consumption also had a significant effect on mortality (Figure 7), and increased mortality to 59% at the highest concentration (Χ1 2 = 24.22, p<0.0001). Methionine consumption had no significant effect on mortality at all concentrations (Figure 7). Overall mortality across all methionine concentrations ranged from 9 to 23%.
Figure 7. Honey bee forager mortality from a single dose of selenium.
Percentages of honey bee mortality after a single dosage of selenate (N = 20–22), selenomethionine (N = 17–20) or methionine (N = 21–24) in 1 M sucrose at 6 concentrations. Control bees received 0 µg ml−1, or 1 M sucrose only. Mortality was recorded for 5 subsequent days. Final percent mortality is shown. Letters above the means indicate statistically significant differences between groups (α = 0.05) using the Mann-Whitney U test. Values are means ± standard error (SE).
Chronic dose mortality
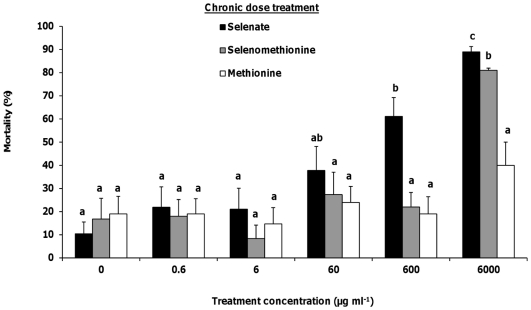
Honey bee foragers were fed Se or sulfur as selenate, selenomethionine, or methionine plus sucrose at 5 concentrations for 5 days, and then mortality was scored on each day. Treatments were compared to bees fed 1 M sucrose as the control. Chronic dosing with selenate significantly increased mortality (Figure 8) at the 60 µg ml−1 (Χ1 2 = 5.40, p<0.02), 600 µg ml−1 (Χ1 2 = 17.81, p<0.0001) and 6000 µg ml−1 (Χ1 2 = 32.84, p<0.0001) concentrations compared with bees fed 1 M sucrose. Selenate consumption for 5 days increased mortality to as high as 89% in the 6000 µg ml−1 concentration. Similar to single dose mortality, chronic doses of selenomethionine increased mortality only at the highest concentration (Χ1 2 = 24.70, p<0.0001; Figure 8), although more bees died with a chronic dose (81%) compared to the single dose (59%). Chronic dosing with methionine at all concentrations did not have a significant effect on mortality (Χ1 2<3.19, p>0.07), although mortality was higher for chronic dosing compared to single dosing in the 6000 µg ml−1 treatment group (13% vs. 40%).
Figure 8. Honey bee forager mortality from chronic doses of selenium.
Percentages of honey bee mortality after chronic dosage of selenate (N = 18–21), selenomethionine (N = 19–20) or methionine (N = 19–20) in 1 M sucrose at 6 concentrations. Control bees received 0 µg ml−1, or 1 M sucrose only. Bees were fed 20 µl of each treatment for 6 days. Mortality was recorded for 5 subsequent days after the first dosage. Final percent mortality is shown. Letters above the means indicate statistically significant differences between groups (α = 0.05) using the Mann-Whitney U test. Values are means ± standard error (SE).
Discussion
Our first objective examined whether the presence of Se affected the gustatory behaviors of honey bees via two different chemosensory organs (antenna or proboscis). Honey bee sensillae used to taste sugars and salts have been found on mouthparts associated with the proboscis [38]–[40]. Taste sensilla on the antennae respond to sugars [41] and salt solutions [42]. Se deters feeding in certain insect [30], [43], [44] and mammalian [45], [46] herbivores, and may reduce feeding behaviors such as PER in honey bees. However, some insects cannot detect Se and will ingest it in laboratory feeding studies [47], [34]. In our study, the presence of selenate in sucrose did not reduce the responses of honey bees to stimulation of the antennae or proboscis. However, antennal stimulation with selenomethionine significantly reduced PER at 600 µg ml−1 and higher, indicating that there was some decrease in response. Antennal stimulation with methionine also reduced PER at higher concentrations, suggesting that deterrence may be due to the methionine portion of the selenomethionine molecule. Methionine causes behavioral deterrence in the leaf-chewing herbivores Spodoptera litura F. (Lepidoptera: Noctuidae) [48], Grammia geneura Strecker (Lepidoptera: Arctiidae) [49] and Mamestra brassicae L. (Lepidoptera: Noctuidae) [50] under experimental conditions. Selenomethionine and methionine may interfere with the honey bee's perception of the sucrose reward when antennae are stimulated, thus reducing PER. In a study by de Brito Sanchez et al. [42], antennal stimulation with solutions containing sucrose and the bitter substance quinine inhibited PER and reduced electrophysiological responses to sucrose in the honey bee. Alternatively, honey bees may respond less to the amino acid depending on the amount of amino acid already in their hemolymph prior to capture [51]. Honey bees that have recently fed on a protein rich plant source may be less responsive to it in subsequent feeding stimulations.
In the proboscis response assays, the bees could choose to drink a small droplet of Se or sulfur containing sucrose solution. There was no significant difference between consumption of the 1 M sucrose alone (control) and selenomethionine or methionine in 1 M sucrose treatment droplets, indicating that the decrease in response was mediated by the antennae and not the proboscis. Honey bee foragers prefer to feed upon sugar solutions containing certain amino acids [51], [52], [53]. Methionine is an essential amino acid for honey bee development [54], although higher concentrations in nectar may act as a deterrent. In our study, deterrence was specific to antennal stimulation, suggesting that receptors detecting either methionine or selenomethionine may not be present on the proboscis.
Our second objective examined the effects of Se ingestion on the sucrose responsiveness of honey bees. Foraging honey bees evaluate floral resources based on the sugar concentrations in nectar, and adjust their foraging and recruitment behaviors accordingly [55]. The sucrose response threshold is an important benchmark for bees to recruit to a floral resource. In our study, the sucrose response threshold, or the point when the probability of responding to sucrose was significantly greater than water, was not significantly altered by feeding honey bees with Se compounds or methionine prior to testing for sucrose responsiveness. However, selenate did significantly reduce the overall responsiveness of the foragers to sucrose as fewer bees fed selenate responded to any sucrose concentration compared to bees fed 1 M sucrose alone. Selenate may lower the honey bee's overall level of responsiveness and arousal, reducing its ability to evaluate relevant stimuli such as a rich floral resource. Honey bees fed toxins such as ethanol [56], the pesticides fipronil [57], or thiamethoxam [58] showed reduced responsiveness to sucrose. If honey bee foragers ingest nectar containing selenate, foraging behaviors may be altered and bees may be less responsive to floral resources.
Our third objective examined the lethal effects of Se ingestion in honey bee foragers when applied at single or chronic dosages. Se as a micronutrient is essential for survival, but higher concentrations can be toxic to insects [31]. Se ingestion increased mortality and development time in Cotesia marginiventris Cresson (Hymenoptera: Braconidae) [59], Heliothis virescens F. (Lepidoptera: Noctuidae) [60], P. maculiventris [61] and S. exigua [30], [33], [34]. In our study, selenate was more toxic than selenomethionine or methionine when fed to honey bee foragers as either a single or chronic dose. Selenomethionine was toxic only at the highest dosage. In other insect plant-feeders, selenomethionine was as toxic as selenate in S. exigua [33], but more toxic than selenate in H. virescens [60]. In the detritivore Megaselia scalaris Loew (Diptera: Phoridae), selenomethionine was more toxic than selenate [47]. In insects fed various forms of Se, selenocompounds concentrated in the hindgut of the Se-tolerant Plutella xylostella L. (Lepidoptera: Plutellidae) [62], whereas Se concentrated in the Malpighian tubules of the Se-intolerant Tenebrio molitor L. (Coleoptera: Tenebrionidae) [63], suggesting these are the sites of sequestration and detoxification. Se detoxification in tolerant insects has been attributed to their ability to sequester Se as methylated forms of selenocompounds [62], which can prevent their misincorporation into proteins. In addition, trimethylselenonium-like species were found in the parasitoid C. marginiventris, suggesting they may detoxify the selenium accumulated from contaminated hosts by using methylation and volatilization [59]. Honey bees may employ similar mechanisms of detoxification by methylating or even volatilizing the Se.
Bees chronically fed 60 µg ml−1 selenate and higher experienced a significant decrease in survival. Greenhouse studies irrigating Brassica juncea (Indian mustard) [64] and Raphanus sativus (radish, unpublished data) with selenate treatment levels comparable to contaminated water in the western San Joaquin Valley of California revealed flowers accumulated up to 60 µg Se ml−1 Se in the nectar of both plant species. In the field, plants growing in soils containing 5 to 10 mg Se kg−1 accumulated approximately 1800 mg Se kg−1 dw in their flowers [65], and insect floral visitors to hyperaccumulator and non-accumulator plants contained an average of 44 and 10 µg Se g−1 dw respectively [23]. For the hyperaccumulator plant Stanleya pinnata, flowers accumulated 2323 mg Se kg−1 dw, with nectar containing 244 µl Se ml−1 fw [19]. Pollen collected by bees from New England aster growing in fly ash from coal-burning electrical power plants contained 14 mg Se kg−1 [16]. Floral visitors on Se-accumulating plants contained up to 75 µg Se g−1 dw [23], honey bees contained 14.8 µg Se g−1 dw and bumble bees contained 251 µg Se g−1 dw [19] when collected from seleniferous field sites in Colorado. Several greenhouse and field studies suggest there is the potential for honey bee foragers to acquire toxic levels of Se from certain species of plants growing in Se-contaminated areas.
Se in plant tissue or artificial diet has been shown to have negative effects on several insect species, yet we know very little about how soil-borne pollutants can impact pollinators. Insect herbivores fed plant tissues containing high levels of metals, metalloids (such as Se), or other accumulated elements have shown reduced development and survival [32], and several studies have shown some insect species cannot detect detrimental levels of Se [33], [34]. If nectar contains Se in the form of selenate, honey bees may not avoid these plants. If the foraging honey bees feed on nectar containing Se (particularly selenate), reductions in population numbers may occur due to direct toxicity. The older, foraging population of workers may be reduced, and younger workers may need to precociously forage to maintain the constant flow of resources into the colony. On the other hand, if the nectar contains selenomethionine, bees may detect and avoid these flowers. Additionally, our study has shown that fewer bees respond to sucrose when fed selenate. If a forager bee does survive the ingestion of selenate, she may be less responsive, forage and recruit less, and not properly evaluate valuable floral resources. Fewer responsive foragers may reduce the incoming floral resources needed to support coworkers and larvae. Taken together, effects on survival and foraging behaviors may significantly reduce the productivity and longevity of the colony. Our study is the first to examine the sublethal and lethal effects of a plant-accumulated pollutant on honey bee feeding preference, sucrose response threshold and mortality.
Materials and Methods
Compounds tested
Sodium selenate (henceforth, selenate, Na2SeO4, 98% purity), seleno-DL-methionine (henceforth, selenomethionine, C5H11NO2Se, 99% purity) and DL-methionine (henceforth, methionine, C5H11NO2S, 99% purity) were all purchased from Sigma-Aldrich (St. Louis, MO). These forms of Se were chosen for comparison to toxicity assays using Spodoptera exigua Hübner (Lepidoptera: Noctuidae) [33], [30], [34]. Compounds were prepared at 5 treatment levels so that each treatment contained the following concentrations of Se or sulfur: 0.6 µg ml−1, 6 µg ml−1, 60 µg ml−1, 600 µg ml−1, and 6000 µg ml−1. A control containing 0 µg ml−1 (1 M sucrose alone) was also included. In previous experiments, two non-hyperaccumulator plant species, Brassica juncea L. (Indian mustard) [64] and Raphanus sativus L. (radish) (unpublished data), accumulated up to 60 µg ml−1 total Se in the nectar when irrigated with selenate in the greenhouse. Therefore treatments included this concentration and two orders of magnitude higher and two lower. Stock solutions were prepared in 1 M sucrose solution (99.9% purity, Fisher Scientific, Pittsburg, PA). Sucrose solution alone and deionized water alone were used for control treatments.
Animals
Tests were performed from June 2010 until January 2011 at the University of California – Riverside (UCR, Riverside, CA) using honey bee (A. mellifera) foragers collected at the entrance of a hive maintained at Agricultural Operations at UCR. The queen was not changed during the course of these experiments to minimize genetic variation. Bees were captured in small glass scintillation vials and chilled briefly at 4°C until immobile. Each individual was restrained in a harness comprised of a 3.8 cm long piece of drinking straw with a diameter of 7 mm. A thin strip of duct tape secured between the head and thorax permitted movement of the antennae and proboscis. Each bee was fed ad libitum with 1 M sucrose solution after harnessing. Bees were then left for 24 hours in a humid box at room temperature within the laboratory before use in experiments.
Antennal response assays
Honey bee taste sensillae have been found on mouthparts associated with the proboscis [38], [39], [40], as well as on the antennae [41], therefore we tested the bee's response to stimulation of both. Honey bee foragers were tested with a range of Se concentrations to determine whether they would respond with PER to antennal stimulation with Se. Assays were based on methodology from de Brito Sanchez et al. [42], and delivered the test compound dissolved in 1 M sucrose to the antennae, eliciting PER. PER responses were scored as (+), proboscis extended upon antennae stimulation, or (−), proboscis retained after antennae stimulation. Bees that did not extend their proboscis even when their antennae were stimulated with sucrose were recorded as non-responsive. We determined the response thresholds for 2 Se compounds (selenate and selenomethionine) and 1 sulfur compound (methionine) dissolved in 1 M sucrose at 5 concentrations (0.6 µg ml−1, 6 µg ml−1, 60 µg ml−1, 600 µg ml−1, and 6000 µg ml−1). In addition, 1 M sucrose only and water only touched to the antennae served as controls. Bees were stimulated with solution contained within a Gilmont micrometer glass syringe (Gilmont Instruments, Barrington, IL). Immediately before the assay, each honey bee was tested for their motivation to feed by touching the antennae with a droplet of 1 M sucrose solution and observing the proboscis extension. Only bees extending their proboscis were chosen for subsequent trials. Antennal stimulation with water in between each treatment stimulation served as a control for sensitization, with an intertrial time of about 3 minutes.
Proboscis response assays
Proboscis response assays were based on methods used in Wright et al. [40]. Each bee's antenna was stimulated with a 1 M sucrose droplet to elicit the PER [66], then each bee was fed 0.6 µl of the treatment solution. The proboscis stimulation treatment involved exposing the proboscis to selenate, selenomethionine, or methionine dissolved in 1 M sucrose solution in a 0.6 µl droplet administered with a Gilmont syringe. The small volume used to stimulate proboscises ensured that bees would not feed enough to reach satiation and become less responsive. Groups of bees were tested with either selenate, selenomethionine or methionine dissolved in 1 M sucrose at 5 treatment concentrations (0.6 µg ml−1, 6 µg ml−1, 60 µg ml−1, 600 µg ml−1, and 6000 µg ml−1 as Se or sulfur). Proboscis exposure to a droplet of 1 M sucrose or water acted as positive and negative controls, respectively. Bees were scored as (+), bee consumed entire 0.6 µl droplet, or (−), bee did not consume droplet.
Sucrose response threshold assays
To examine the effects of the consumption of selenium on the responsiveness to sugars, bees were fed an acute dose of selenate, selenomethionine or methionine and then their sucrose response thresholds were determined. The sucrose response threshold assays were based on methods from Mustard et al. [56] and Page et al. [55]. Honey bees were captured and harnessed as described above. Twenty four hours later, bees were fed 20 µl solutions of 1 M sucrose containing 0 (control), 0.6 µg ml−1, 6 µg ml−1, 60 µg ml−1, 600 µg ml−1, and 6000 µg ml−1 of Se or sulfur in the forms of selenate, selenomethionine or methionine. Two hours after the bees had consumed the treatment, they were assayed for sucrose response threshold. Each bee's antennae were stimulated with sucrose solutions at increasing concentrations of 0.1%, 0.3%, 1%, 3%, 10% and 30%, interspersed with antennal stimulation with water. Water stimulations were interspersed between sucrose stimulations to serve as a control for increased sensitization or habituation on subsequent responses from repeated sucrose stimulation. After antennae were stimulated, proboscis extension (+) or retention (−) was recorded. Intertrial times were 3 minutes.
Total consumption and single dose mortality assays
Bees were captured and harnessed as described above and fed 1 M sucrose only ad libitum. Twenty four hours later, bees were fed treatments of Se or sulfur as selenate, selenomethionine, or methionine dissolved in 1 M sucrose at 6 concentrations (0, 0.6, 6, 60, 600, and 6000 µg ml−1) for a total of 18 treatment groups. Bees were fed using a Gilmont syringe. The total volume consumed from each treatment was calculated. Bees remained harnessed for 5 days after the single dosage and mortality per day was scored in control and treated groups and has been presented as final mortality after 5 days. Surviving bees were fed 1 M sucrose ad libitum on each of the 5 subsequent days.
Chronic dose mortality assays
Based on the average volume of treatment solution consumed in each treatment in the single dose assay, bees were fed 20 µl for each control and treated group on day 0, and were fed an additional 20 µl of treatment solution on each of the 5 subsequent days. Treatments consisted of selenate, selenomethionine, or methionine dissolved in 1 M sucrose at 6 concentrations (0, 0.6, 6, 60, 600, and 6000 µg ml−1 as Se or S) for a total of 18 treatment groups. Throughout the assay, bees were evaluated in control and treated groups for mortality per day.
Statistical analysis
Antennal response, proboscis response, and sucrose threshold response probabilities were analyzed as a binary variable using repeated-measures logistic regression with each bee as a unit of replication. Data were analyzed using the GENMOD procedure in SAS (version 9.2, SAS Institute, Cary, NC) with post hoc multiple comparisons. Antennal and proboscis response compared PER probabilities in the 1 M sucrose control group to the treated groups unless otherwise noted. Sucrose response threshold assays compared response probabilities between the water trials and each sucrose concentration. Total consumption was analyzed for each treatment group using ANOVA (GLM procedure) and post hoc Tukey's HSD test. For mortality assays, as recommended in the EPA Ecological Effects Test Guidelines (OPPTS 850.3020), mortality was 20% or less in all control groups. Based on preliminary studies feeding harnessed foragers with 1 M sucrose, mortality increased above 20% by day 6, therefore we concluded the toxicity bioassays at day 5. Each honey bee represented a unit of replication. Pairwise comparisons were made of mortality in the 1 M sucrose (control) group to each treatment level and within each Se form. Se forms were not compared to each other. Mortality data was not normally distributed; therefore comparisons were made using the nonparametric Kruskal-Wallis test with post hoc separations using the Mann-Whitney U test (NPAIR1WAY procedure).
Supporting Information
Honey bee sucrose response thresholds after selenium feeding treatments.
(XLS)
Acknowledgments
The authors thank Casey Butler, William Carson, John Diaz-Montano, Greg Kund, Christina Mogren, David Parker, and Beatriz Vindiola for their review of this manuscript. Thank you to P. Kirk Visscher for providing us with the honey bee colony used in this study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Department of Entomology at University of California – Riverside and an Environmental Protection Agency Science to Achieve Results (EPA STAR) fellowship to KRH. This publication was developed under a STAR Research Assistance Agreement no. F08F20896 awarded by the United States Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kearns CA, Inouye DW, Waser NM. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu Rev Ecol Syst. 1998;29:83–112. [Google Scholar]
- 2.Klein AM, Vassière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, et al. Importance of crop pollinators in changing landscapes for world crops. Proc of the Royal Soc of London Series B – Biol Sci. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Southwick EE, Southwick L. Estimating the economic value of honeybees (Hymenoptera: Apidae) as agricultural pollinators in the United States. J Econ Entomol. 1992;85:621–633. [Google Scholar]
- 4.Delaplane KS, Mayer DF. Crop Pollination by Bees. New York: CABI Publishing; 2000. 352 [Google Scholar]
- 5.Morse RA, Calderone NW. The value of honey bees as pollinators of U.S. crops in 2000. Bee Culture. 2002;128:1–15. [Google Scholar]
- 6.Desneux N, Decourtye A, Delpuech J. The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol. 2007;52:81–106. doi: 10.1146/annurev.ento.52.110405.091440. [DOI] [PubMed] [Google Scholar]
- 7.Celli G, Maccagnani B. Honey bees as bioindicators of environmental pollution. Bull of Insectology. 2003;56:137–139. [Google Scholar]
- 8.Kevan PG. Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agriculture Ecosyst and Environ. 1999;74:373–393. [Google Scholar]
- 9.Achudume AC, Nwafor BN. The ecological assessment of metals in local brands of honey in Southwest Nigeria. African J of Agric Res. 2010;5:2608–2610. [Google Scholar]
- 10.Bibi S, Husain SZ, Malik RN. Pollen analysis and heavy metals detection in honey samples from seven selected countries. Pak J Bot. 2008;40:507–516. [Google Scholar]
- 11.Bogdanov S. Contaminants of bee products. Apidologie. 2006;37:1–18. [Google Scholar]
- 12.Bromenshenk JJ, Carlson SR, Simpson JC, Thomas JM. Pollution monitoring of Puget Sound with honey bees. Science. 1985;22:632–634. doi: 10.1126/science.227.4687.632. [DOI] [PubMed] [Google Scholar]
- 13.Jones KC. Honey as an indicator of heavy metal contamination. Water Air and Soil Poll. 1987;33:179–189. [Google Scholar]
- 14.Leita L, Muhlbachova G, Cesco S, Barbattini R, Mondini C. Investigation of the use of honey bees and honey bee products to assess heavy metals contamination. Environ Monit and Assess. 1996;43:1–9. doi: 10.1007/BF00399566. [DOI] [PubMed] [Google Scholar]
- 15.Yarsan E, Karacal F, Ibrahim IG, Dikmen B, Koksal A, et al. Contents of some metals in honeys from different regions in Turkey. Bull Environ Contam Toxicol. 2007;79:255–258. doi: 10.1007/s00128-007-9034-9. [DOI] [PubMed] [Google Scholar]
- 16.De Jong D, Morse RA, Gutenmann WH, Lisk DJ. Selenium in pollen gathered by bees foraging on fly ash-grown plants. Bull Environ Contam and Toxicol. 1977;18:442–444. doi: 10.1007/BF01683714. [DOI] [PubMed] [Google Scholar]
- 17.Roman A. Levels of copper, selenium, lead and cadmium in forager bees. Polish J Environ Stud. 2010;19:663–669. [Google Scholar]
- 18.Tuzen M, Silici S, Mendil D, Soylak M. Trace element levels in honeys from different regions of Turkey. Food Chem. 2007;103:325–330. [Google Scholar]
- 19.Quinn CF, Prins CN, Freeman JL, Gross AM, Hantzis LJ, et al. Selenium accumulation in flowers and its effects on pollination. New Phytol. 2011;192:727–737. doi: 10.1111/j.1469-8137.2011.03832.x. [DOI] [PubMed] [Google Scholar]
- 20.Emmons SF, Cross W, Eldridge GH. Geology of the Denver basin in Colorado. US Geol Survey Monogr. 1896;27:527. [Google Scholar]
- 21.Wu L. Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotox and Environ Safety. 2004;57:257–269. doi: 10.1016/S0147-6513(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 22.Ohlendorf HM. Ecotoxicology of Selenium. In: Hoffman DJ, Rattner BA, Burton GA, Cairns J, editors. Handbook of Ecotoxicology. Boca Raton: Lewis Publishers; 2003. pp. 465–500. [Google Scholar]
- 23.Galeas ML, Klamper EM, Bennett LE, Freeman JL, Kondratieff BC, et al. Selenium hyperaccumulation reduces plant arthropod loads in the field. New Phytol. 2008;177:715–724. doi: 10.1111/j.1469-8137.2007.02285.x. [DOI] [PubMed] [Google Scholar]
- 24.Burau RG. Environmental chemistry of selenium. California Agric. 1985;39:16–18. [Google Scholar]
- 25.Daniels LA. Selenium metabolism and bioavailability. Biol Trace Elem Res. 1996;54:185–199. doi: 10.1007/BF02784430. [DOI] [PubMed] [Google Scholar]
- 26.Lemly AD. Environmental implications of excessive selenium: A review. Biomed Environ Sci. 1997;10:415–435. [PubMed] [Google Scholar]
- 27.Schrauzer GN. Selenomethionine: A review of its nutritional significance, metabolism and toxicity. Recent Adv Nutr Sci. 2000;130:1653–1656. doi: 10.1093/jn/130.7.1653. [DOI] [PubMed] [Google Scholar]
- 28.Spallholz JE. Free radical generation by selenium compounds and their prooxidant toxicity. Biomed Environ Sci. 1997;10:260–270. [PubMed] [Google Scholar]
- 29.Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998;79:179–192. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 30.Vickerman DB, Trumble JT. Feeding preferences of Spodoptera exigua in response to form and concentration of selenium. Arch Insect Biochem and Physiol. 1999;42:64–73. doi: 10.1002/(SICI)1520-6327(199909)42:1<64::AID-ARCH7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 31.Jensen PD, Trumble JT. Ecological consequences of bioavailability of metals and metalloids in insects. Recent Res Dev Entomol. 2003;42:1–17. [Google Scholar]
- 32.Boyd RS. The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions. Plant and Soil. 2007;293:153–176. [Google Scholar]
- 33.Trumble JT, Kund GS, White KK. Influence of form and quantity of selenium on the development and survival of an insect herbivore. Environ Pollut. 1998;101:175–182. doi: 10.1016/s0269-7491(98)00086-4. [DOI] [PubMed] [Google Scholar]
- 34.Vickerman DB, Young JK, Trumble JT. Effect of selenium-treated alfalfa on development, survival, feeding and oviposition preferences of Spodoptera exigua (Lepidoptera: Noctuidae). Env Entomol. 2002;31:953–959. [Google Scholar]
- 35.de Souza MP, Pilon-Smits EAH, Lytle CM, Hwang S, Tai J, et al. Rate-limiting steps in selenium assimilation and volatilization by Indian mustard. Plant Physiol. 1998;117:1487–1494. doi: 10.1104/pp.117.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahakachchi C, Boakye TH, Uden PC, Tyson JF. Chromatographic speciation of anionic and neutral selenium compounds in Se-accumulating Brassica juncea (Indian mustard) and in selenized yeast. J Chromatogr. 2004;1054:303–312. [PubMed] [Google Scholar]
- 37.Pedrero Z, Madrid Y, Cámara C. Selenium species bioaccessibility in enriched radish (Raphanus sativus): A potential dietary source of selenium. J Agric Food Chem. 2006;54:2412–2417. doi: 10.1021/jf052500n. [DOI] [PubMed] [Google Scholar]
- 38.Whitehead AT. Electrophysiological responses of honey bee labial palp contact chemoreceptors to sugars and electrolytes. Physiol Entomol. 1978;3:241–248. [Google Scholar]
- 39.Whitehead AT, Larsen JR. Electrophysiological responses of galeal contact chemoreceptors of Apis mellifera to selected sugars and electrolytes. J Insect Physiol. 1976;22:1609–1616. doi: 10.1016/0022-1910(76)90052-4. [DOI] [PubMed] [Google Scholar]
- 40.Wright GA, Mustard JA, Simcock NK, Ross-Taylor AAR, McNicholas LD, et al. Parallel reinforcement pathways for conditioned food aversions in the honey bee. Curr Biol. 2010;20:1–7. doi: 10.1016/j.cub.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haupt SS. Antennal sucrose perception in the honey bee (Apis mellifera L.): behaviour and electrophysiology. J Comp Physiol A. 2004;190:735–745. doi: 10.1007/s00359-004-0532-5. [DOI] [PubMed] [Google Scholar]
- 42.de Brito Sanchez MG, Giurfa M, de Paula Mota TR, Gauthier M. Electrophysiological and behavioural characterization of gustatory responses to antennal ‘bitter’ taste in honey bees. Europ J Neurosci. 2005;22:3161–3170. doi: 10.1111/j.1460-9568.2005.04516.x. [DOI] [PubMed] [Google Scholar]
- 43.Hanson B, Garifullina GF, Lindblom SD, Wangeline A, Ackley A, et al. Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol. 2003;159:461–469. doi: 10.1046/j.1469-8137.2003.00786.x. [DOI] [PubMed] [Google Scholar]
- 44.Hanson B, Lindblom SD, Loeffler ML, Pilon-Smits EAH. Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytol. 2004;162:655–662. doi: 10.1111/j.1469-8137.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- 45.Franke KW, Potter VR. The ability of rats to discriminate between diets of varying degrees of toxicity. Science. 1936;83:330–332. doi: 10.1126/science.83.2153.330-a. [DOI] [PubMed] [Google Scholar]
- 46.Quinn CF, Freeman JL, Galeas ML, Klamper EM, Pilon-Smits EAH. The role of selenium in protecting plants against prairie dog herbivory: implications for the evolution of selenium hyperaccumulation. Oecologia. 2008;155:267–275. doi: 10.1007/s00442-007-0907-8. [DOI] [PubMed] [Google Scholar]
- 47.Jensen PD, Johnson LR, Trumble JT. Individual and joint actions of selenate and methylmercury on the development and survival of insect detritivores Megaselia scalaris (Diptera: Phoridae). Arch Env Contam and Toxicol. 2006;50:523–530. doi: 10.1007/s00244-005-0111-y. [DOI] [PubMed] [Google Scholar]
- 48.Hirao T, Arai N. On the role of gustatory recognition in host-plant selection by the silkworm, Bombyx mori L. Jpn J Appl Entomol Zool. 1991;35:197–206. [Google Scholar]
- 49.Bernays EA, Chapman RF. Taste cell responses in the polyphagous arctiid, Grammia geneura: towards a general pattern for caterpillars. J Insect Physiol. 2001;47:1029–1043. doi: 10.1016/s0022-1910(01)00079-8. [DOI] [PubMed] [Google Scholar]
- 50.Wieczorek H. The glycoside receptor of the larvae of Mamestra brassicae L. (Lepidoptera, Noctuidae). J Comp Physiol. 1976;106:153–176. [Google Scholar]
- 51.Kim YS, Smith BH. Effect of amino acid on feeding preferences and learning behavior in the honey bee, Apis mellifera. J Insect Physiol. 2000;46:793–801. doi: 10.1016/s0022-1910(99)00168-7. [DOI] [PubMed] [Google Scholar]
- 52.Alm JD, Simpson SJ. Preference of cabbage white butterflies and honey bees for nectar that contains amino acids. Oecologia. 1990;84:53–57. doi: 10.1007/BF00665594. [DOI] [PubMed] [Google Scholar]
- 53.Inouye DW, Waller GD. Responses of honey bees Apis mellifera to amino acid solutions mimicking nectars. Ecology. 1984;65:618–625. [Google Scholar]
- 54.de Groot AP. Protein and amino acid requirements of the honey bee. Physiol Comp Oecol. 1953;3:1–90. [Google Scholar]
- 55.Page RE, Erber J, Fondrk MK. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A. 1998;182:489–500. doi: 10.1007/s003590050196. [DOI] [PubMed] [Google Scholar]
- 56.Mustard JA, Edgar EA, Mazade RE, Wu C, Lillvis JL, et al. Acute ethanol ingestion impairs appetitive olfactory learning and odor discrimination in the honey bee. Neurobiol Learn Mem. 2008;90:633–643. doi: 10.1016/j.nlm.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El Hassani AK, Dacher M, Gauthier M, Armengaud C. Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera). Pharmacol Biochem Behav. 2005;82:30–39. doi: 10.1016/j.pbb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Aliquane Y, El Hassani AK, Gary V, Armengaud C, Lambin M, et al. Subchronic exposure of honeybees to sublethal doses of pesticides: effects on behavior. Environ Toxicol and Chem. 2009;28:113–122. doi: 10.1897/08-110.1. [DOI] [PubMed] [Google Scholar]
- 59.Vickerman DB, Trumble JT, George GN, Pickering IJ, Nichol H. Selenium biotransformations in an insect ecosystem: Effects of insects on phytoremediation. Environ Sci Technol. 2004;38:3581–3586. doi: 10.1021/es049941s. [DOI] [PubMed] [Google Scholar]
- 60.Popham HJR, Shelby KS. Effect of inorganic and organic forms of selenium supplementation on development of larval Heliothis virescens. Entomol Exp et Appl. 2007;125:171–178. [Google Scholar]
- 61.Vickerman DB, Trumble JT. Biotransfer of selenium: Effects on an insect predator, Podisus maculiventris. Ecotoxicol. 2003;12:497–504. doi: 10.1023/b:ectx.0000003036.81351.31. [DOI] [PubMed] [Google Scholar]
- 62.Freeman JL, Quinn QF, Marcus MA, Fakra S, Pilon-Smits EAH. Selenium-tolerant diamondback moth disarms hyperaccumulator plant defense. Curr Biol. 2006;16:2181–2192. doi: 10.1016/j.cub.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Hogan GR, Razniak HG. Selenium-induced mortality and tissue distribution studies in Tenebrio molitor (Coleoptera: Tenebrionidae). Environ Entomol. 1991;20:790–794. [Google Scholar]
- 64.Hladun KR, Parker DP, Trumble JT. Selenium accumulation in the floral tissues of two Brassicaceae species and its impact on floral traits and plant performance. Environ and Exp Bot. 2011;74:90–97. [Google Scholar]
- 65.Galeas ML, Zhang LH, Freeman JL, Wegner M, Pilon-Smits EAH. Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related nonaccumulators. New Phytol. 2007;173:517–525. doi: 10.1111/j.1469-8137.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 66.Kuwabara M. Formation of the conditioned reflex of Pavlov's type in the honey bee, Apis mellifera. J Fac Sci Hokkaido Univ Zool. 1957;13:458–464. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Honey bee sucrose response thresholds after selenium feeding treatments.
(XLS)