Abstract
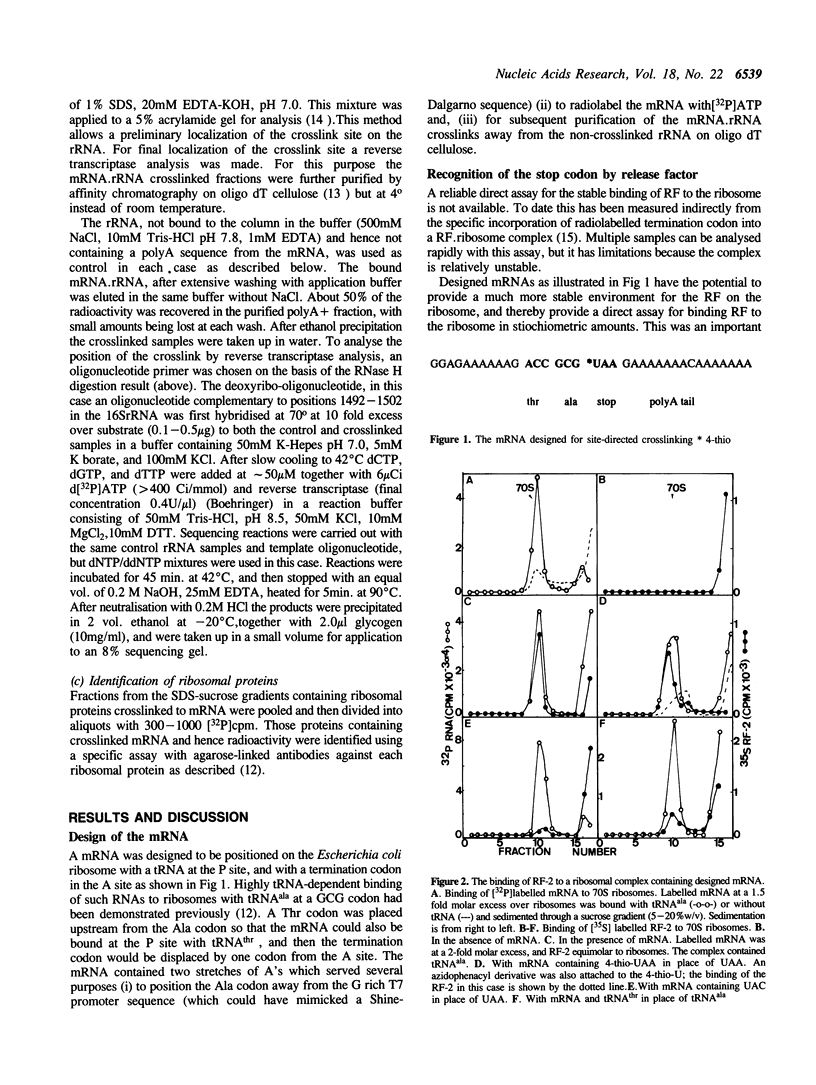
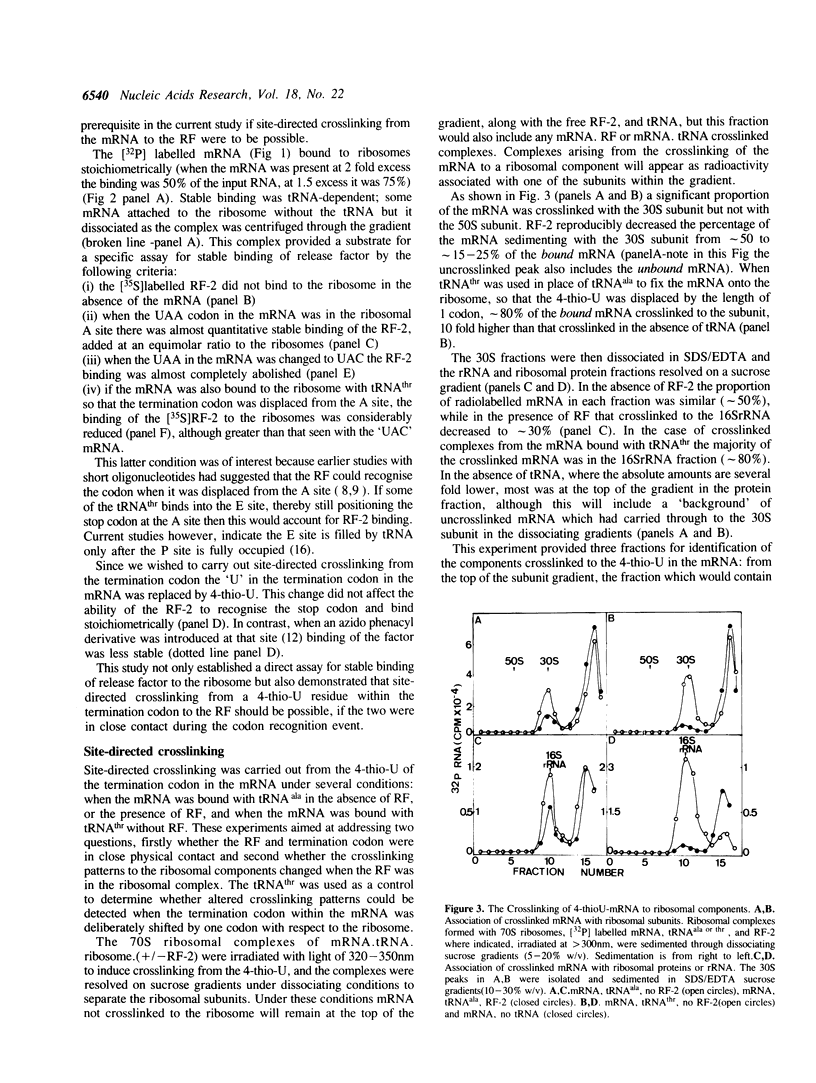
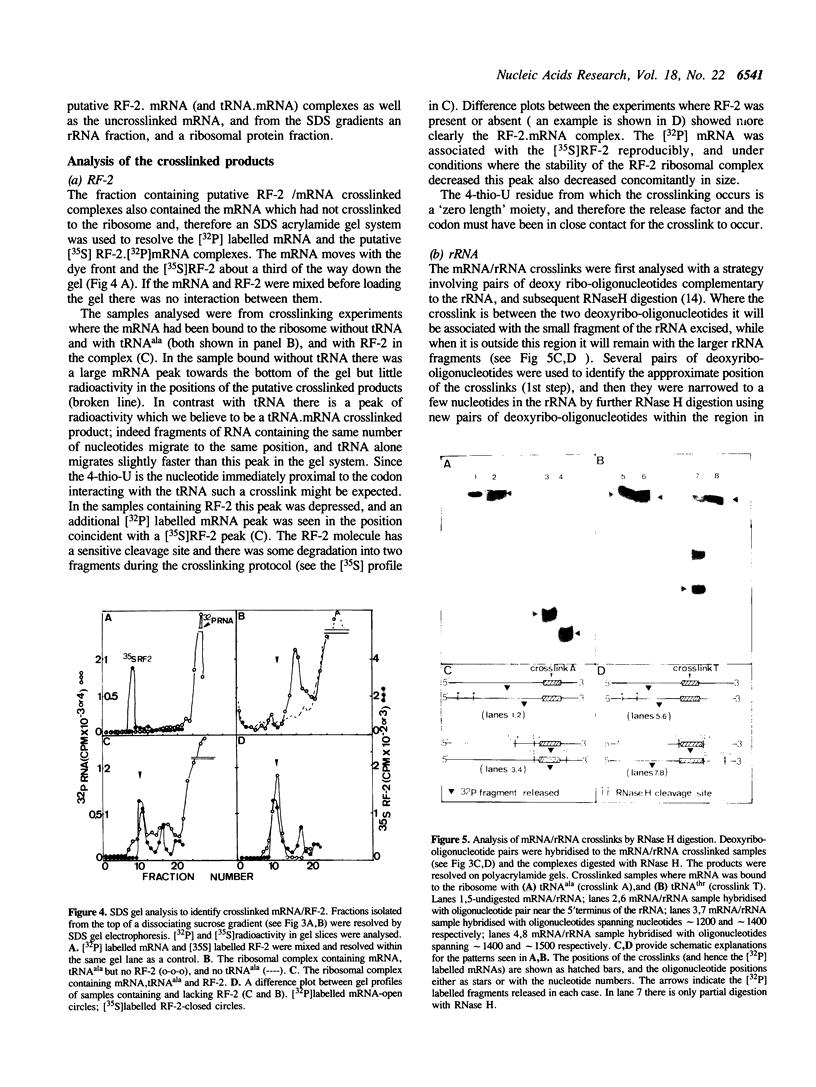
An RNA synthesized in vitro was positioned on the Escherichia coli ribosome at the P site with tRNAala, and with a termination codon, UAA, as the next codon in the A site. Such a complex bound stoichiometric amounts of release factor 2 (RF-2); a corresponding RNA with UAC in place of UAA was not a template for the factor. An RNA containing 4-thio-UAA in place of the UAA supported binding of RF-2, and this has allowed site-directed crosslinking from the first position of the termination codon to answer two long standing questions about the termination of protein biosynthesis, the position of the termination codon and its proximity to the release factor during codon recognition. An RF-2.mRNA crosslinked product was detected, indicating the release factor and the termination codon are in close physical contact during the codon recognition event of termination. The 4-thio-U crosslinked also to the ribosome but only to the 30S subunit, and the proteins and the rRNA site concerned were identified. RF-2 decreased significantly the crosslinking to the ribosomal components, but no new crosslink sites were found. If the stop codon was deliberately displaced from the decoding site by one codon's length then a different pattern of crosslinking in particular to the rRNA resulted. These observations are consistent with a model of codon recognition by RF-2 at the decoding site, without a major shift in position of the codon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brimacombe R. The emerging three-dimensional structure and function of 16S ribosomal RNA. Biochemistry. 1988 Jun 14;27(12):4207–4214. doi: 10.1021/bi00412a001. [DOI] [PubMed] [Google Scholar]
- Buckingham K., Chung D. G., Neilson T., Ganoza M. C. Recognition of translational termination signals. Biochim Biophys Acta. 1987 Jul 14;909(2):92–98. doi: 10.1016/0167-4781(87)90030-3. [DOI] [PubMed] [Google Scholar]
- Gnirke A., Geigenmüller U., Rheinberger H. J., Nierhaus L. H. The allosteric three-site model for the ribosomal elongation cycle. Analysis with a heteropolymeric mRNA. J Biol Chem. 1989 May 5;264(13):7291–7301. [PubMed] [Google Scholar]
- Gornicki P., Nurse K., Hellmann W., Boublik M., Ofengand J. High resolution localization of the tRNA anticodon interaction site on the Escherichia coli 30 S ribosomal subunit. J Biol Chem. 1984 Aug 25;259(16):10493–10498. [PubMed] [Google Scholar]
- Kastner B., Trotman C. N., Tate W. P. Localization of the release factor-2 binding site on 70 S ribosomes by immuno-electron microscopy. J Mol Biol. 1990 Mar 20;212(2):241–245. doi: 10.1016/0022-2836(90)90120-B. [DOI] [PubMed] [Google Scholar]
- Lang A., Friemert C., Gassen H. G. On the role of the termination factor RF-2 and the 16S RNA in protein synthesis. Eur J Biochem. 1989 Apr 1;180(3):547–554. doi: 10.1111/j.1432-1033.1989.tb14680.x. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Hijazi K. A., Göringer H. U., Dahlberg A. E. Mutant 16S ribosomal RNA: a codon-specific translational suppressor. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4162–4165. doi: 10.1073/pnas.85.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K., Rinke-Appel J., Brimacombe R. Site-directed cross-linking of mRNA analogues to the Escherichia coli ribosome; identification of 30S ribosomal components that can be cross-linked to the mRNA at various points 5' with respect to the decoding site. Nucleic Acids Res. 1989 Dec 11;17(23):9889–9908. doi: 10.1093/nar/17.23.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Weiser B., Noller H. F. Model for the three-dimensional folding of 16 S ribosomal RNA. J Mol Biol. 1988 Nov 20;204(2):447–481. doi: 10.1016/0022-2836(88)90588-8. [DOI] [PubMed] [Google Scholar]
- Stiege W., Stade K., Schüler D., Brimacombe R. Covalent cross-linking of poly(A) to Escherichia coli ribosomes, and localization of the cross-link site within the 16S RNA. Nucleic Acids Res. 1988 Mar 25;16(6):2369–2388. doi: 10.1093/nar/16.6.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler G., Tate W. P., Caskey C. T. Ribosomal proteins cross-linked to peptide chain termination release factor 2. J Biol Chem. 1982 Apr 25;257(8):4203–4206. [PubMed] [Google Scholar]
- Tate W. P., Hornig H., Lührmann R. Recognition of termination codon by release factor in the presence of a tRNA-occupied A site. Evidence for flexibility in accommodation of the release factor on the ribosome. J Biol Chem. 1983 Sep 10;258(17):10360–10365. [PubMed] [Google Scholar]





