Abstract
Summary of background data
The sagittal profile of lumbar endplates is discrepant from current simplified disc replacement and fusion device design. Endplate concavity is symmetrical in the coronal plane but shows considerable variability in the sagittal plane, which may lead to implant–endplate mismatch.
Objective
The aim of this investigation is to provide further analysis of the sagittal endplate morphology of the mid to lower lumbar spine study (L3–S1), thereby identifying the presence of common endplate shape patterns across these levels and providing morphological reference values complementing the findings of previous studies.
Study design
Observational study
Methods
A total of 174 magnetic resonance imaging (MRI) scans of the adult lumbar spine from the digital archive of our centre, which met the inclusion criteria, were studied. Superior (SEP) and inferior (IEP) endplate shape was divided into flat (no concavity), oblong (homogeneous concavity) and ex-centric (inhomogeneous concavity). The concavity depth (ECD) and location of concavity apex (ECA) relative to endplate diameter of the vertebrae L3–S1 were determined.
Results
Flat endplates were only predominant at the sacrum SEP (84.5%). The L5 SEP was flat in 24.7% and all other endplates in less than 10%. The majority of endplates were concave with a clear trend of endplate shape becoming more ex-centric from L3 IEP (56.9% oblong vs. 37.4% ex-centric) to L5 IEP (4% oblong vs. 94.3% ex-centric). Ex-centric ECA were always found in the posterior half of the lumbar endplates. Both the oblong and ex-centric ECD was 2–3 mm on average with the IEP of a motion segment regularly possessing the greater depth. A sex- or age-related difference could not be found.
Conclusion
The majority of lumbar endplates are concave, while the majority of sacral endplates are flat. An oblong and an ex-centric endplate shape can be distinguished, whereby the latter is more common at the lower lumbar levels. The apex of the concavity of ex-centric discs is located in the posterior half of the endplate and the concavity of the inferior endplate is deeper than that of the superior endplate. Based on the above, the current TDR and ALIF implant design does not sufficiently match the morphology of lumbar endplates in the sagittal plane.
Keywords: Vertebral endplate anatomy, Lumbar spine, Total disc replacement, Sagittal profile
Introduction
The vertebral endplates of the lower human lumbar spine are remarkable in possessing the largest known endplate surface area in relation to body size amongst terrestrial mammals [1]. While surgical procedures replacing the intervertebral disc (total disc replacement—TDR) and fusing the intervertebral space (anterior lumbar interbody fusion—ALIF) have become routine techniques, the endplate designs of these devices are significantly simplified in contrast to the morphological variability and biomechanical demand in vivo. While a reasonable range of endplate surface sizes has been developed, the sagittal profile of TDR devices is limited to flat endplates or at best minor convexity with some modularity of the slope angle. Recent CT-based investigations have, however, confirmed an even surface shape to be present in only a minority of vertebrae of the thoracolumbar [2] and lumbar spine [3]. Sagittal plane morphology in particular is significantly variable, and common morphological patterns have not been clearly identified.
The aim of this investigation is to provide further analysis of the sagittal endplate morphology of the mid to lower lumbar spine study (L3–S1), thereby identifying the presence of common endplate shape patterns across these levels and providing morphological reference values complementing the findings of previous studies.
Materials and methods
Digitally archived magnetic resonance imaging (MRI) scans of the lumbar spine performed at our institution for reasons unrelated to this study were reviewed with regard to suitability for assessment of endplate morphology. As no additional examination of patients or clinical correlation of findings was conducted, the study was deemed exempt from formal ethics committee approval. The mid-sagittal T2-weighted MRI Images of adult patients under the age of 60 years without significant degenerative disease, i.e. Pfirrmann grades 1 and 2 were included [4]. Scans with evidence of trauma, tumour, infection or metabolic conditions of the lower lumbar spine were excluded.
In total, 174 suitable MRI scans of the lumbar spine were identified. The mean age of the patients (105 female and 69 male) was 43.5 years (range 28–60); 59 patients (33.9%) were under the age of 40 years, 81 patients (46.6%) between 40 and 50 years and 34 (19.5%) between 50 and 60 years.
The mid-sagittal images of the superior (SEP) and inferior (IEP) endplates between L3 (IEP only) and S1 were analysed and 1,044 endplates in total were included in the analysis.
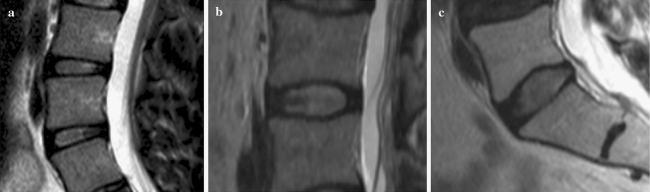
Determination of endplate shape (Fig. 1a–c)
Fig. 1.
Types of endplate morphology as seen in mid-sagittal T2 MRI. a Flat endplate devoid of curvature. b Oblong endplate with homogenous curvature towards apex. c Ex-centric endplate with a clear inhomogeneous curvature towards the apex
The shape of the endplate was as follows:
flat, if the endplate did not demonstrate a measurable concavity (<1 mm) (Fig. 1a);
oblong, if the endplate concavity was uniform starting from the anterior margin and finishing at the posterior margin (Fig. 1b);
ex-centric, if the endplate concavity clearly started after a less curved or flat portion with an ex-centric apex (Fig. 1c).
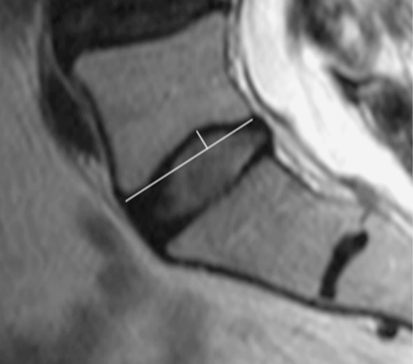
Endplate measurements (Fig. 2)
Fig. 2.
Endplate concavity depth (ECD) measured in millimetre from the concavity apex to the line connecting the anterior and posterior margins of the endplate. Endplate concavity apex location (ECA) was expressed as the percentage of the mid-sagittal anterior–posterior endplate diameter (total endplate diameter divided by distance of apex from the anterior cortex)
The endplate concavity depth (ECD) was measured in millimetres from the concavity apex to the line connecting the anterior and posterior margins of the endplate.
The location of the endplate concavity apex (ECA) was expressed as the percentage of the mid-sagittal anterior–posterior endplate diameter, whereby 50% indicates central apex location, 0–50% an apex in the anterior half of the endplate and 50–100% an apex in the posterior half of the endplate.
Results
Endplate shape
Overall, 22.3% of endplates across all levels were flat, 24.7% were oblong and 53% were ex-centric in shape (Fig. 1a–c; Table 1). No significant difference in endplate morphology was noted between sexes (Table 2) and between age groups.
Table 1.
Distribution of endplate morphology in 174 adult lumbar spines L3–S1 (1,044 endplates)
| Level/morphology | Ex-centric (%) | Flat (%) | Oblong (%) |
|---|---|---|---|
| L3 IEP | 65 (37.4) | 10 (5.7) | 99 (56.9) |
| L4 SEP | 82 (47.1) | 17 (9.8) | 75 (43.1) |
| L4 IEP | 109 (62.6) | 13 (7.5) | 52 (29.9) |
| L5 SEP | 107 (61.5) | 43 (24.7) | 24 (13.8) |
| L5 IEP | 164 (94.3) | 3 (1.7) | 7 (4.0) |
| S1 SEP | 26 (14.9) | 147 (84.5) | 1 (0.6) |
| Total (overall average) | 553 (53) | 233 (22.3) | 258 (24.7) |
Average percentage of total in brackets
IEP inferior endplate, SEP superior endplate
Table 2.
Sex distribution of endplate morphology and vertebral level
| Ex-centric | Flat | Oblong | ||||
|---|---|---|---|---|---|---|
| Male (%) | Female (%) | Male (%) | Female (%) | Male (%) | Female (%) | |
| L3 IEP | 26 (37.7) | 39 (37.1) | 4 (5.8) | 6 (5.7) | 39 (56.5) | 60 (57.2) |
| L4 SEP | 33 (47.8) | 49 (46.7) | 6 (8.7) | 11 (10.5) | 30 (43.5) | 45 (42.8) |
| L4 IEP | 47 (68.1) | 62 (59.0) | 3 (4.3) | 10 (9.5) | 19 (27.6) | 33 (31.5) |
| L5 SEP | 42 (60.9) | 65 (61.9) | 19 (27.5) | 24 (22.9) | 8 (11.6) | 16 (15.2) |
| L5 IEP | 66 (95.7) | 98 (93.3) | 1 (1.4) | 2 (1.9) | 2 (2.9) | 5 (4.8) |
| S1 SEP | 12 (17.4) | 14 (13.3) | 56 (81.2) | 91 (86.7) | 1 (1.4) | 0 |
Averages of total in brackets
Flat endplates (Fig. 1a): The SEP of S1 showed 84.5% flat morphology, followed by L5 SEP with 24.7%. At all other levels, flat endplates were present in under 10%.
Oblong (Fig. 1b): Oblong endplates were most common at L3 IEP (56.9%). All other levels revealed this shape in under 50% with a decline towards the sacrum S1 SEP with (0.6%).
Ex-centric endplates: (Fig. 1c) The ex-centric shape was seen most predominantly at the L5 IEP (94.3%) followed by the L5 SEP and L4 IEP (61.5 and 62.6% respectively); L4 SEP and L3 IEP were ex-centric in less than 50%.
ECD
The greatest average ECD was found in the L5 IEP with both the oblong and the ex-centric group measuring an average of 2.9 mm (Table 3). L5 SEP had the minimum average ECD with 1.52 mm. The SEP ECD was found to be less than the IEP ECD for any given disc space.
Table 3.
Average endplate concavity depth (ECD) in millimetre for ex-centric and oblong endplate types
| Ex-centric | Oblong | |
|---|---|---|
| L3 IEP | 2.747 | 2.479 |
| L4 SEP | 2.046 | 1.965 |
| L4 IEP | 2.505 | 2.547 |
| L5 SEP | 1.699 | 1.529 |
| L5 IEP | 2.943 | 2.942 |
| S1 SEP | 1.616 | 1.9 |
Location of ECA
The average location of the ECA was determined for the ex-centric and oblong endplate shapes (Table 4). The ex-centric ECA was always located in the posterior half of the endplate (at 54–60% endplate diameter). The oblong ECA was shown to be in a similar position to the ex-centric ECA from L3 IEP to L4 IEP (51–55%), but located in the anterior half of the disc space at L5 SEP (46%) and at the midpoint for L5 IEP (50%). A minority of 27 S1 SEPs were concave with the average ex-centric ECA at 60% (n = 26) and the single oblong ECA 58%.
Table 4.
Average endplate concavity apex location (ECA) expressed as the percentage of the endplate diameter
| Ex-centric (%) | Oblong (%) | |
|---|---|---|
| L3 IEP | 56.24 | 55.30 |
| L4 SEP | 54.23 | 51.10 |
| L4 IEP | 56.92 | 54.18 |
| L5 SEP | 54.30 | 46.00 |
| L5 IEP | 59.89 | 50.83 |
| S1 SEP | 60.21 | 58.09 |
Central apex location is indicated by 50%; 0–50% indicates an apex in the anterior half of the endplate and 50–100% indicates an apex in the posterior half of the endplate
Discussion
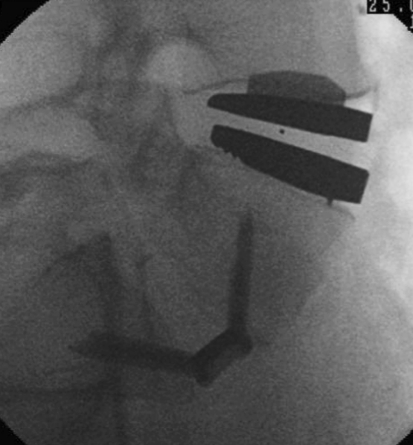
Total disc replacement has recently become popular in the management of degenerative disease of the lumbar spine. Subsidence, however, is a significant cause of poor outcome following TDR [5, 6]. The footprints of the currently available TDR are simplified and can have a significant mismatch to the vertebral endplates. This has been well demonstrated by Gstoettner et al. [7], who measured the dimensions of the lumbar vertebrae on CT scans and assessed the accuracy of match in currently available lumbar disc prosthesis. In 220 endplates, only 10 (3%) matched the AP diameter of the prosthesis. While the constraints of matching endplate shapes to implants in the transverse plane is well recognised and sufficient anatomical data exists to optimise endplate geometry, far less attention has been paid to the endplate morphology in the sagittal plane. This has recently been pointed out by Chen et al. [2], who analysed endplate geometry of the thoracolumbar junction for the optimisation of vertebral body replacement devices. Figure 3 is an intraoperative example of how an ex-centric endplate shape can lead to a reduced surface contact area with a TDR implant, which in turn can be a cause of subsidence or later malalignment. It is reasonable to assume that similar mismatch can occur in ALIF devices which, even though this has also not been formally proven, may contribute to failure of fusion.
Fig. 3.
Intraoperative lateral fluoroscopy view demonstrating mismatch of flat TDR endplate with ex-centric superior endplate
Our investigation on 1,044 endplates revealed three principal sagittal endplate shapes: oblong, ex-centric and flat (Fig. 1a–c). Remarkably—and contrary to what current implant design suggests—flat endplates were the exception rather than the rule at all levels except the sacrum SEP, which was flat in 84.5%. The L5 SEP was flat in 24.7% and all other endplates in less than 10%. The majority of endplates therefore were concave with a clear trend of endplate shape becoming more ex-centric from L3 IEP (56.9% oblong vs. 37.4% ex-centric) to L5 IEP (4% oblong vs. 94.3% ex-centric). Ex-centric ECA were always found in the posterior half of the lumbar endplates, located at approximately 55–60% of the AP diameter (Table 4). The data by van der Houwen et al. [3] and Chen et al. [2] indicate that the endplates towards T12 also show a tendency towards a posterior ECA. Interestingly, the oblong ECA in this investigation was located at 46% at the L5 SEP. This was not formally described in the investigation by van der Houwen et al. [3] on 77 spines and may be due to the greater number of endplates investigated in this study, possibly enabling this group to be distinguished as a separate entity. Both the oblong and ex-centric ECD was 2–3 mm on average (Table 3) with the IEP of a motion segment regularly possessing the greater depth. This confirms the CT-based findings by van der Houwen et al. [3], who found similar averages with a maximum depth of 5.3 mm. A sex- or age-related difference could not be found (Table 2).
No explanation was derived from the gathered data for the location of the ECA or the difference in ECD between SEP and IEP. It would be of interest to investigate any correlation of this finding—which relates to the location of the nucleus pulposus—with lumbar lordosis and pelvic incidence in future studies.
Use of MRI instead of CT scans for studying the bony endplate anatomy may be considered as a limitation of this study. However, Ravi et al. [8] performed a study of patients with lateral radiographs, CT and MRI scan of the spine. They found that the difference between the mean anteroposterior vertebral body dimensions as measured on CT and MRI was <0.1 mm. Further, to choose patients with only early degenerative changes and to exclude those with severe degenerative changes on MRI scans is more reliable and, hence, we utilised MRI scans for this study rather than CT scans. While flat endplate shapes are easily distinguished, the differentiation of ex-centric to oblong disc shapes is less distinct in some cases and remains subjective in the absence of surface reconstruction methodology, more readily available for CT [3]. It is a limitation of our MRI data that the parasagittal planes were predetermined at the time of the investigation and did not adhere to a common protocol (the investigations were not obtained for the purpose of this study). It was therefore not possible to reliably analyse the lateral extent of the endplate concavity patterns. As endplate morphology has been shown to be symmetrical in the coronal plane [3], we assume but cannot prove that the concavity resolves gradually towards the lateral border of the endplate without further variation. Our investigation only included discs with minimal degeneration to define the true anatomical shape of the endplate. It is recognised that endplate shape changes with advanced degeneration and that the incidence of endplate concavity may be less in surgical cohorts—especially patients with loss of ≥50% of disc height considered for ALIF. The findings may therefore be more relevant to TDR cohorts where implantation more commonly takes place prior to excessive loss of disc height. A further limitation is that we considered the dome-shaped S1 endplates as flat in some cases as the summit height was less than 1 mm. For practical purposes, it is usually possible to surgically flatten the endplate by this minor amount, so that these endplates can probably be considered as flat as far as implant design is concerned.
Conclusion
The majority of lumbar endplates are concave, while the majority of sacral endplates are flat. An oblong and an ex-centric endplate shape can be distinguished; the latter is more common at the lower lumbar levels. The apex of the concavity of ex-centric discs is located in the posterior half of the endplate and the concavity of the inferior endplate is deeper than that of the superior endplate.
As yet, these variations in sagittal endplate shape have not been identified conclusively as a factor which contributes to implant subsidence in published literature series on TDR or ALIF. This may be due to the lack of awareness of distinctions in this particular morphology. Investigations into implant subsidence should consider the described sagittal endplate morphology to determine if alterations in design are warranted.
Acknowledgments
Conflict of interest
None.
Contributor Information
Palaniappan Lakshmanan, Email: lakunns@gmail.com.
Bronek Maximilian Boszczyk, Phone: +44-115-9249924, FAX: +44-115-9709991, Email: bronek.boszczyk@nuh.nhs.uk.
References
- 1.Boszczyk BM, Boszczyk AA, Putz R. Comparative and functional anatomy of the mammalian lumbar spine. Anat Rec. 2001;264:157–168. doi: 10.1002/ar.1156. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Jiang D, Ou Y, Zhong J, Lv F. Geometry of thoracolumbar vertebral endplates of the human spine. Eur Spine J. 2011;20:1814–1820. doi: 10.1007/s00586-011-1787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houwen EB, Baron P, Veldhuizen AG, et al. Geometry of the intervertebral volume and vertebral endplates of the human spine. Ann Biomed Eng. 2010;38:33–40. doi: 10.1007/s10439-009-9827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfirrmann C, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charite disc. J Spinal Disord Tech. 2003;16:369–383. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Punt IM, Visser VM, Rhijn LW, Kurtz SM, et al. Complications and reoperations of the SB Charite lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008;17:36–43. doi: 10.1007/s00586-007-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gstoettner M, Heider D, Liebensteiner M, Bach CM. Footprint mismatch in lumbar total disc arthroplasty. Eur Spine J. 2008;11:1470–1475. doi: 10.1007/s00586-008-0780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravi B, Rampersaud R. Clinical magnification error in lateral spinal digital radiographs. Spine. 2008;33:E311–E316. doi: 10.1097/BRS.0b013e31816f6c3f. [DOI] [PubMed] [Google Scholar]