Abstract
Purpose
Proteoglycans are important to the functioning of the intervertebral disc. In addition to aggrecan there are the small leucine-rich proteoglycans (SLRPs). These are less common but in other locations their functions include collagen organisation, sequestering growth factors and stimulating inflammation. We have performed a comparative analysis of the SLRP core protein species present in intervertebral discs with various pathologies.
Methods
Eighteen intervertebral discs from patients with scoliosis (n = 7, 19–53 years), degenerative disc disease (n = 6, 35–51 years) and herniations (n = 5, 33–58 years) were used in this study. Proteoglycans were dissociatively extracted from disc tissues and the SLRPs (biglycan, decorin, fibromodulin, keratocan and lumican) assessed by Western blotting following deglycosylation with chondroitinase ABC and keratanase.
Results
Intact SLRP core proteins and a number of core protein fragments were identified in most of the discs examined. Biglycan and fibromodulin were the most extensively fragmented. Keratocan generally occurred as two bands, one representing the intact core protein, the other a smaller fragment. The intact core protein of lumican was detected in all samples with fragmentation evident in only one of the older scoliotic discs. Decorin was less obvious in the disc samples and showed little fragmentation.
Conclusion
In this cohort of pathological intervertebral discs, fragmentation of certain SLRP core proteins was common, indicating that some SLRPs are extensively processed during the pathological process. Identification of specific SLRP fragments which correlate with disc pathology may not only help understand their aetiopathogeneses, but also provide biomarkers which can be used to monitor disease progression or to identify particular disc disorders.
Keywords: Small leucine-rich proteoglycans (SLRPs), Intervertebral disc, SLRP core protein fragmentation, Degenerative disc disease, Scoliosis
Introduction
The intervertebral disc (IVD) is an important weight bearing structure with unique viscoelastic and hydrodynamic functional properties conveyed by the interplay of connective tissue molecules of diverse structure and function. The outer region of the IVD, the annulus fibrosus (AF), is a collagen-rich tissue with the fibres being highly organised into bundles and concentric lamellae [34]. In contrast, the central nucleus pulposus (NP) is rich in the hylauronan-binding proteoglycan (PG), aggrecan, with more than a hundred glycosaminoglycan (GAG) side chains attached. This is responsible for the water retaining and hydrodynamic properties of the extracellular matrix, allowing the composite IVD to act as a weight bearing cushion [33]. Whilst aggrecan is the major IVD PG, there are also small leucine-rich proteoglycans (SLRPs) present, with decorin, biglycan, fibromodulin and lumican all having been reported in the disc [18, 29, 30].
The SLRPs are members of a large family of leucine-rich repeat (LRR) proteins [9] and have been categorised into a number of sub-families on the basis of gene organization, LRR number and GAG substitution patterns. Eight IVD SLRPs have been identified, including the chondroitin sulphate/dermatan sulphate-substituted decorin and biglycan and keratan sulphate-substituted lumican, fibromodulin and keratocan [16]. Non-glycanated disc SLRP members have also been identified including proline arginine-rich protein (Prolargin, PRELP), chondroadherin and asporin [16]. The function of the SLRPs depends on both their core proteins and GAG side chains. Their core proteins facilitate interaction with fibrillar collagens and regulate fibrillogenesis and sterically protect the fibrils from proteolysis in vitro [5], whilst the GAG side chains facilitate fibril–fibril interactions [28].
Decorin, biglycan, fibromodulin and lumican interact with many other matrix components other than the fibrillar collagens, including types VI, XII and XIV collagens, fibronectin, elastin, in addition to growth factors and cytokines such as EGF, TGFβ and TNFα [3, 13, 16, 23]. The GAG chains of the SLRPs provide them with their growth factor binding properties through which they can sequester these in the extracellular matrix. The SLRPs have diverse functions as modulators of tissue organization, cellular proliferation, matrix adhesion, and the cellular responses to growth factors and cytokines [13, 16, 23]. This family of proteoglycans have emerging roles in mammalian biology in health and disease and are now recognised as key signalling molecules with growth factors and a number of receptors which regulate cell growth, morphogenesis and immunity [13, 16, 23, 27].
Decorin [11], fibromodulin [8] and biglycan [15] are degraded by proteases such as matrix metalloproteinases (MMPs) or aggrecanases in vitro, and similar cleavages probably occur in vivo. Certainly, biglycan and fibromodulin are fragmented in models of IVD degeneration and osteoarthritis (OA) [19, 35] and in articular cartilage from total knee and hip replacement patients and from menisci of OA joints [17]. SLRPs are also extensively fragmented in pathological tendon and ligament [10, 24, 25]. Fragmentation of SLRPs may alter their function significantly, possibly rendering them more potent at stimulating inflammation.
In the present study we undertook a comparative evaluation of decorin, biglycan, fibromodulin, lumican and keratocan core protein species evident by Western blotting in extracts of human IVDs. We found SLRP core proteins to be present, some of them in fragmented form, in these discs from patients with various disc disorders.
Materials and methods
IVD samples
All tissues for this study were obtained with either informed consent under the authority of our institutional human ethics committee or from our existing Human Tissue Authority-licensed archive collection. Discs were removed from three groups of patients during anterior surgical procedures for scoliosis or degenerative disc disease (DDD) or via a posterior approach for herniation (detailed in Table 1). DDD samples were graded according to Thompson et al. [32]. Primarily annulus fibrosus tissue was used for biochemical analysis to provide consistent source location. This was clearly identifiable macroscopically in the scoliotic and DDD samples and dissected after excision from the patient. Herniated disc samples were more heterogeneous but AF tissue was defined by the operating surgeon and confirmed by their appearance microscopically.
Table 1.
Details of IVD samples
| Sample | Pathology | Grade | Level | Gender | Age |
|---|---|---|---|---|---|
| 1 | Scoliosis | T9–T10 | M | 19 | |
| 2 | Scoliosis | T11–T12 | M | 19 | |
| 3 | Scoliosis | L1–L2 | M | 19 | |
| 4 | Scoliosis | T5–T6 | F | 27 | |
| 5 | Scoliosis | T12–L1 | F | 34 | |
| 6 | Scoliosis | L2–L3 | F | 42 | |
| 7 | Scoliosis | L2–L3 | F | 53 | |
| 8 | DDD | I | L5–S1 | M | 35 |
| 9 | DDD | II | L5–S1 | F | 36 |
| 10 | DDD | III | L5–S1 | F | 40 |
| 11 | DDD | III | L4–L5 | M | 43 |
| 12 | DDD | IV | L5–S1 | F | 50 |
| 13 | DDD | IV | L5–S1 | M | 51 |
| 14 | Herniation | L5–S1 | F | 33 | |
| 15 | Herniation | L5–S1 | M | 44 | |
| 16 | Herniation | L4–L5 | M | 46 | |
| 17 | Herniation | L4–L5 | M | 48 | |
| 18 | Herniation | L5–S1 | M | 58 |
Extraction and preparation of tissue samples for SLRP analysis
SLRPs were extracted from finely diced disc tissues in 4 M GuHCl as previously described [17]. In brief the tissue extracts were dialysed (Mw cut-off 6–8 kDa), freeze dried and re-dissolved (2 mg dry wt/ml) in Tris acetate buffer before digesting with chondroitinase ABC (0.1 U) and keratanase (0.05 U) to remove the GAG chains.
Lithium dodecyl sulphate (LDS) PAGE and detection of SLRP fragments by Western blotting
Aliquots of the chondroitinase ABC, keratanase digested samples (0.1 ml) were mixed with 4× LDS PAGE application buffer (35 μl) and 500 mM dithiothreitol (15 μl). The samples were heated at 70°C for 30 min, cooled and 25 or 15 μl aliquots were applied to pre-poured NuPAGE 10 or 15 well Bis Tris 4–12% gradient gels and electrophoresed under reducing conditions in NuPAGE MOPS SDS running buffer at 200 V constant voltage for 35 min. The gels were electroblotted to nitrocellulose membranes (0.22 μm) either as previously described [17] using NuPAGE transfer buffer supplemented with 10% methanol at 30 V constant voltage for 1 h (Protocol 1) or transferred using an iBlot system and iBlot Gel Nitrocellulose Transfer Stacks (Invitrogen) and Program 3 (Protocol 2). SeeBlue-2 prestained protein molecular weight standards were also electrophoresed to enable calibration of molecular weights and to assess the blotting transfer efficiency.
For Protocol 1, blots were initially blocked for 3 h with 5% BSA in 50 mM Tris–HCl 0.15 M NaCl pH 7.2 (TBS) before primary antibodies diluted in 2% BSA in TBS were applied overnight at 4°C. Antibodies used were against keratocan (obtained from BC), biglycan, lumican and fibromodulin (from PR) and decorin (obtained from Developmental Studies Hybridoma Bank, Iowa) as detailed in Table 2. After a brief TBS rinse, goat anti-rabbit or anti-mouse IgG secondary antibodies conjugated to alkaline phosphatase (1:5,000 dilution) were added, as appropriate. After 1 h the blots were washed in TBS (3 × 10 min) and NBT/BCIP substrates were then added in alkaline phosphatase development buffer (0.1 M Tris–HCl pH 9.5 containing 5 mM MgCl2) for detection of immune complexes. Colour development was allowed to proceed for 20 min at room temperature then the blots were rinsed in distilled water and dried.
Table 2.
Summary of antibodies used in the study
| SLRP | Class | Antibody | Epitope | Dilution | Ref. |
|---|---|---|---|---|---|
| Decorin | I | mAb DS-1 | C-terminal of core protein | 1:500 | [21] |
| Keratocan | II | mAb Ker-1 | Epitope not identified | 1:100 | [4] |
| Biglycan | I | pAb PR-85 | C-terminus (CGG)TDRLAIQFGNYKK | 1:1,000 | [35] |
| Lumican | II | pAb PR-586 | C-terminus (CGG)LRVANEITVN | 1:1,000 | [31] |
| Fibromodulin | II | pAb PR-184 | C-terminus (CGG)LRLASLIEI | 1:1,000 | [35] |
For Protocol 2, following the transfer to nitrocellulose antibody detection was carried out using iBlot Western Detection Stacks and iBlot Western Detection Chromogenic Kits (Invitrogen). Briefly, primary antibodies were applied at double the concentration specified in Table 2 using an antibody matrix in the iBlot apparatus and Program 7. The appropriate secondary antibodies (anti-rabbit at 1:2,000 or anti mouse at 1:5,000) were also applied with a fresh antibody matrix within the iBlot apparatus using Program 7. The blots were then washed (4 × 5 min) with 1× wash solution (Invitrogen) and then washed with autoclaved distilled water (2 × 5 min). The water was autoclaved to remove any alkaline phosphatase activity. The chromogenic substrate was then applied to the membrane and colour development carried out for 1 h at room temperature.
Western blots were repeated a minimum of three times for each extract. Extracts of non-enzyme treated bovine nasal cartilage were run as positive controls for each of the SLRPs investigated; these produced bands in the region of expected molecular weight (data not shown). Control blots were also conducted omitting primary antibody to check that no IgG species were present in the tissue extracts which cross-reacted with the conjugated secondary detection antibodies.
Statistical analysis
Age differences between the members of the sample group were assessed using a two-tailed Student T test with statistical significance being taken at p ≤ 0.05.
Results
Intervertebral discs of the three pathological groups examined in this study were obtained from a mixture of male and female donors. The scoliotic group had four female and three male specimens; the DDD group had three female and three male specimens and the herniation group one female and four male subjects. The mean age of the subjects in the DDD group was 42.5 ± 6.8 years (mean ± SD) and 45.8 ± 9 years for the herniation group. The scoliotic patients (mean age 30.4 years ± 13.3) were significantly younger than the herniated patients (P < 0.05).
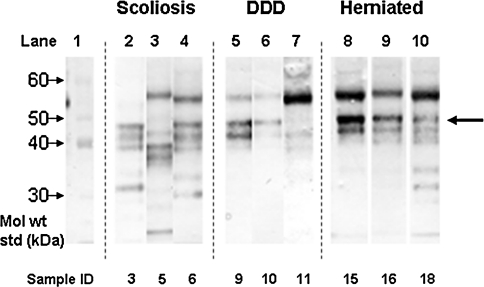
Western blotting for each individual SLRP showed different patterns of core protein presence and fragmentation. The intact biglycan core protein was detected as expected at ~46 kDa in most samples. All but one sample had biglycan core protein fragments with two core protein fragments at 39 and 42 kDa forming a predominant triplet of bands with the intact core protein in the majority of samples. Other core protein fragments were also detected down to molecular weights of 25 kDa (Fig. 1); sometimes as many as five clear bands could be seen. A decorin core protein of 45 kDa was detected but little fragmentation was evident in any of the specimens examined.
Fig. 1.
Identification of biglycan core protein in IVD via Western blotting. The intact core protein can be seen for most samples at 45 kDa (large arrow). Bands of lower molecular weight represent fragments of biglycan core protein down to 25 kDa in size, sometimes with multiple fragments. In some samples an intense band was observed at 55 kDa, which may be non-specific or cross-reactive with PRELP (see text). Details of individual samples can be seen in Table 1. DDD degenerative disc disease
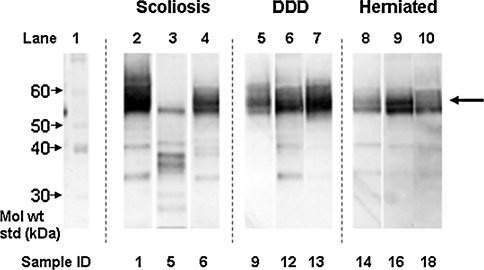
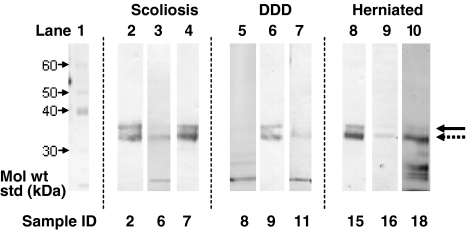
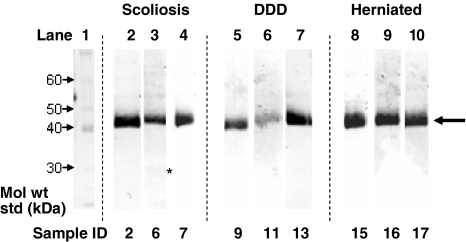
Full-length keratan sulphate-containing fibromodulin core protein was detected as a relatively polydisperse 56–65 kDa band (Fig. 2). Fibromodulin generally displayed a high degree of fragmentation in the size range 25–54 kDa, but with fewer bands evident in the DDD discs. Full length keratocan core protein was detected with a molecular weight of 35 kDa (Fig. 3) and usually co-existed with a keratocan core protein fragment of 33 kDa. Fragments were also detected at 31 or 29 kDa in some samples, with the 29 kDa band being more prevalent in the older IVD samples. Lumican displayed little fragmentation in any of the samples examined with a slightly polydisperse 42–48 kDa band corresponding to the full-length core protein with KS linkage regions (Fig. 4). A 23 kDa lumican fragment was observed in the 42-year-old scoliotic IVD specimen.
Fig. 2.
Identification of fibromodulin core protein in IVD via Western blotting. The intact core protein can be seen around the molecular weight of 59 kDa (large arrow). Several bands of lower molecular weight represent fragments of fibromodulin. Details of individual samples can be seen in Table 1. DDD degenerative disc disease
Fig. 3.
Identification of keratocan core protein in IVD via Western blotting. The intact core protein can often be seen at 35 kDa (solid arrow) in addition to a band at 33 kDa (dotted arrow). Details of individual samples can be seen in Table 1. DDD degenerative disc disease
Fig. 4.
Identification of lumican core protein in IVD via Western blotting. Bands for intact core protein can be seen at about 45 kDa (large arrow) with no evidence of fragmentation apart from a faint band (asterisk) at 23 kDa in sample 6 (42-year-old scoliotic). Details of other samples can be seen in Table 1. DDD degenerative disc disease
In summary, our results demonstrated intact SLRP core proteins and fragmentation, particularly of biglycan, fibromodulin and keratocan. A band at 55 kDa was seen in some of the blots especially when biglycan was the primary antibody. This may be due to non-specific binding with the heavy IgG chain as identified by [2] or alternatively may be due to the presence of PRELP which does interact with some IgG preparations (Roughley, personal communication); sometimes there was also a non-specific band at 21 kDa from the light IgG chain.
Discussion
It has long been known that SLRPs have a major influence on the size and organisation of collagen fibres and hence the structure of the extracellular matrix of tissues within the eye and in the IVD, but their role as signalling molecules, for example, in regulating inflammatory pathways, is a more recent recognition [26]. Biglycan has been shown to trigger cell-mediated inflammation via its effect on chemokines and B cell activation and has been described as a multireceptor signalling complex. When the complex is activated it can lead to many other effects including caspase activation and generation of the cytokines, TNFα and IL-1β [20]. There is some evidence that fragments may be more potent in cell signalling than intact molecules [27].
The presence of SLRPs within the IVD has been well-documented [1, 6, 12, 14, 18, 30], although as far as we are aware this is the first report of keratocan in human IVD. Whilst during aging and degeneration, the distribution of SLRPs within the disc extracellular matrix has been shown to vary, little has been reported on fragmentation, especially in pathological human discs.
In this study we have demonstrated fragmentation to some extent in all the SLRPs investigated in these pathological discs. Whilst this study is restricted by not having normal disc material for comparison, previous reports of fragmentation in human discs refer to ‘normal’ post-mortem tissue. Sztrolovics et al. [30] report little fragmentation of fibromodulin although a smaller fragment was observed in the AF tissue of older (>35 years) individuals. Two fragments of lumican have been reported to be present (38 and 32 kDa) in aged human disc [19]. In contrast, human adolescent discs (from scoliotics) showed no degradation of biglycan, decorin, fibromodulin and lumican [7]. Hence it appears that there may be a general increase in fragmentation in diseased IVDs studied here, compared to young or normal discs, similar to that seen in other degenerative joint diseases such as osteoarthritic cartilage [17].
Diffuse bands were seen for the intact core proteins of fibromodulin and, to some extent, lumican. This could be due to the enzyme treatment used to remove keratan sulphate in this study (keratanase, which is active predominantly against mono-sulphated GAG chains). These KS-containing SLRPs are likely to be over sulphated in the IVD so may not have been completely deglycosylated such as might have been achieved if keratanase II had been used. On the other hand, keratocan was detected as discrete bands which may indicate that this SLRP in these discs was either non-glycosylated or contained mono-sulphated GAG chains.
There was virtually no fragmentation seen of the lumican core protein, which was present in all the samples studied. This is consistent with the findings from an animal model of disc degeneration where no lumican core protein fragments were found in the injured ovine discs [19]. This suggests that unlike the other SLRPs investigated, the lumican core protein undergoes little if any enzymic cleavage with disc pathology.
Whilst degenerative disc disease has many similarities to osteoarthritic changes seen in articular cartilage, it appears that there may be subtle differences with respect to SLRP degradation. Unlike the little or no fragmentation of lumican seen here in IVD, Melrose et al. [17] found fragmentation of lumican to occur in osteoarthritic articular cartilage and meniscus. Similarly, whereas we report fragmentation of fibromodulin in the pathological IVDs, there was little evident in osteoarthritic cartilage. Differences such as these could prove very useful in developing tissue-specific biomarkers. There is currently a great deal of interest in this area with a paucity of accurate and specific markers for degenerative disc diseases.
SLRP fragmentation products may help to delineate different pathways of degradation. For example, in catabolism of tendon PGs, aggrecanase rather than MMPs has been identified as the predominant protease in degrading SLRPs and aggrecan [22]. Identification of the proteinases responsible for the generation of SLRP fragments in IVD degeneration may provide guidance to potential therapeutic targets for disorders of the IVD. In addition, isolation of the SLRP fragments we have identified in pathological IVDs and studying how they affect disc cells in vitro could identify pathways which they influence. This would likely help our understanding of the mechanisms and aetiopathogenesis of different disc disorders.
Conclusion
Overall, fragmentation of certain SLRP core proteins was common within these groups of pathological IVDs, suggesting that cleavage of core proteins occurs during the various pathological processes. Identification and isolation of these core protein fragments may provide insights to the cause and development of disc pathology in addition to possibly providing IVD-specific biomarkers.
Acknowledgments
Patients for their tissues, surgeons for their cooperation and the Medical Research Council Link Grant GO800248 (SR, SB & BC), Arthritis Research UK Grant 17933 (BC) and NHMRC Project Grant 512167 (JM) for funding.
Conflict of interest
None.
References
- 1.Cs-Szabo G, Juan DR-S, Turumella V, Masuda K, Thonar EJ-MA, An HS. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine. 2002;27:2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 2.Cs-Szabo G, Roughley PJ, Plaas AHK, Glant TT. Large and small proteoglycans of osteoarthritic and rheumatoid articular cartilage. Arthritis Rheum. 1995;38:660–668. doi: 10.1002/art.1780380514. [DOI] [PubMed] [Google Scholar]
- 3.Feng H, Danfelter M, Stromqvist B, Heinegard D. Extracellular matrix in disc degeneration. J Bone Jt Surg. 2006;88-A:25–29. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- 4.Gealy EC, Kerr BC, Young RD, Tudor D, Hayes AJ, Hughes CE, Caterson B, Quantock AJ, Ralphs JR. Differential expression of the keratan sulphate proteoglycan, keratocan, during chick corneal embryogenesis. Histochem Cell Biol. 2007;128:551–555. doi: 10.1007/s00418-007-0332-4. [DOI] [PubMed] [Google Scholar]
- 5.Geng Y, McQuillan D, Roughley PJ. SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol. 2006;25:484–491. doi: 10.1016/j.matbio.2006.08.259. [DOI] [PubMed] [Google Scholar]
- 6.Gotz W, Barnert S, Bertagnoli R, Miosge N, Kresse H, Herken R. Immunohistochemical localization of the small proteoglycans decorin and biglycan in human intervertebral discs. Cell Tissue Res. 1997;289:185–190. doi: 10.1007/s004410050864. [DOI] [PubMed] [Google Scholar]
- 7.Haglund L, Ouellet J, Roughley P. Variation in chondroadherin abundance and fragmentation in the human scoliotic disc. Spine. 2009;34:1513–1518. doi: 10.1097/BRS.0b013e3181a8d001. [DOI] [PubMed] [Google Scholar]
- 8.Heathfield TF, Onnerfjord P, Dahlberg L, Heinegard D. Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J Biol Chem. 2004;279:6286–6295. doi: 10.1074/jbc.M307765200. [DOI] [PubMed] [Google Scholar]
- 9.Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. doi: 10.1016/S0945-053X(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 10.Ilic MZ, Carter P, Tyndall A, Dudhia J, Handley CJ. Proteoglycans and catabolic products of proteoglycans present in ligament. Biochem J. 2005;385:381–388. doi: 10.1042/BJ20040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta 1 release. Biochem J. 1997;322:809–814. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inkinen RI, Lammi MJ, Lehmonen S, Puustjarvi K, Kaapa E, Tammi MI. Relative increase of biglycan and decorin and altered chondroitin sulfate epitopes in the degenerating human intervertebral disc. J Rheumatol. 1998;25:506–514. [PubMed] [Google Scholar]
- 13.Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–3875. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnstone B, Markopoulos M, Neame P, Caterson B. Identification and characterization of glycanated and non-glycanated forms of biglycan and decorin in the human intervertebral disc. Biochem J. 1993;292:661–666. doi: 10.1042/bj2920661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ. The cleavage of biglycan by aggrecanases. Osteoarthritis Cartil. 2006;14:1147–1154. doi: 10.1016/j.joca.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Melrose J, Roughley PJ. Proteoglycans of the intervertebral disc. In: Shapiro IM, Risbud MV, editors. The intervertebral disc. Berlin: Springer; 2012. [Google Scholar]
- 17.Melrose J, Fuller ES, Roughley PJ, Smith MM, Kerr B, Hughes CE, Caterson B, Little CB. Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthirits Res Ther. 2008;10:R79. doi: 10.1186/ar2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melrose J, Ghosh P, Taylor TKF. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat. 2001;198:3–15. doi: 10.1046/j.1469-7580.2001.19810003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melrose J, Smith SM, Fuller ES, Young AA, Roughley PJ, Dart A, Little CB. Biglycan and fibromodulin fragmentation correlates with temporal and spatial annular remodelling in experimentally injured ovine intervertebral discs. Eur Spine J. 2007;16:2193–2205. doi: 10.1007/s00586-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreth K, Brodbeck R, Babelova A, Gretz N, Spieker T, Zeng-Brouwers J, Pfeilschifter J, Young MF, Schaefer RM, Schaefer L. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest. 2010;120:4251–4272. doi: 10.1172/JCI42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole AR, Webber C, Pidoux I, Choi H, Rosenberg LC. Localization of a dermatan sulphate proteoglycan (DS-PGII) in cartilage and the presence of an immunologically related species in other tissues. J Histochem Cytochem. 1986;34:619–625. doi: 10.1177/34.5.3701029. [DOI] [PubMed] [Google Scholar]
- 22.Rees SG, Flannery CR, Little CB, Hughes CE, Caterson B, Dent CM. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem J. 2000;350:181–188. doi: 10.1042/0264-6021:3500181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mat. 2006;12:92–101. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- 24.Samiric T, Ilic MZ, Handley CJ. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004;23:127–140. doi: 10.1016/j.matbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Samiric T, Ilic MZ, Handley CJ. Large aggregating and small leucine-rich proteoglycans are degraded by different pathways and at different rates in tendon. Eur J Biochem. 2004;271:3612–3620. doi: 10.1111/j.0014-2956.2004.04307.x. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339:237–246. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 28.Scott JE. Extracellular matrix, supramolecular organisation and shape. J Anat. 1995;187(Pt 2):259–269. [PMC free article] [PubMed] [Google Scholar]
- 29.Singh K, Masuda K, Thonar EJ-MA, An HS, Cs-Szabo G. Age-related changes in the extracellular matrix of nucleus pulposus and annulus fibrosus of human intervertebral disc. Spine. 2008;34:10–16. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sztrolovics R, Alini M, Mort JS, Roughley PJ. Age-related changes in fibromodulin and lumican in human intervertebral discs. Spine. 1999;24:1765–1771. doi: 10.1097/00007632-199909010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Sztrolovics R, White RJ, Poole AR, Mort JS, Roughley PJ. Resistance of small leucine-rich repeat proteoglycans to proteolytic degradation during interleukin-1-stimulated cartilage catabolism. Biochem J. 1999;339:571–577. doi: 10.1042/0264-6021:3390571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IKY, Bishop PB. Preliminary evaluation of the scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22:145–157. doi: 10.3233/bir-1985-22205. [DOI] [PubMed] [Google Scholar]
- 34.Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young AA, Smith MM, Smith SM, Cake MA, Ghosh P, Read RA, Melrose J, Sonnabend DH, Roughley PJ, Little CB. Regional assessment of articular cartilage gene expression and small proteoglycan metabolism in an animal model of osteoarthritis. Arthritis Res Ther. 2005;7:R852–R861. doi: 10.1186/ar1756. [DOI] [PMC free article] [PubMed] [Google Scholar]