Abstract
Background
Approximately 40% of hospitalized older adults have cognitive impairment (CI) and are more prone to hospital-acquired complications. The Institute of Medicine suggests using health information technology to improve the overall safety and quality of the health care system.
Objective
Evaluate the efficacy of a clinical decision support system (CDSS) to improve the quality of care for hospitalized older adults with CI.
Design
A randomized controlled clinical trial.
Setting
A public hospital in Indianapolis.
Population
A total of 998 hospitalized older adults were screened for CI, and 424 patients (225 intervention, 199 control) with CI were enrolled in the trial with a mean age of 74.8, 59% African Americans, and 68% female.
Intervention
A CDSS alerts the physicians of the presence of CI, recommends early referral into a geriatric consult, and suggests discontinuation of the use of Foley catheterization, physical restraints, and anticholinergic drugs.
Measurements
Orders of a geriatric consult and discontinuation orders of Foley catheterization, physical restraints, or anticholinergic drugs.
Results
Using intent-to-treat analyses, there were no differences between the intervention and the control groups in geriatric consult orders (56% vs 49%, P = 0.21); discontinuation orders for Foley catheterization (61.7% vs 64.6%, P = 0.86); physical restraints (4.8% vs 0%, P = 0.86), or anticholinergic drugs (48.9% vs 31.2%, P = 0.11).
Conclusion
A simple screening program for CI followed by a CDSS did not change physician prescribing behaviors or improve the process of care for hospitalized older adults with CI.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-1994-8) contains supplementary material, which is available to authorized users.
KEY WORDS: cognitive impairment, clinical trial, decision support, hospitalized elders
INTRODUCTION
Every year, more than 4 million Americans aged 65 and older suffer from cognitive impairment (CI) during their hospital stay.1–5 These Americans are prone to hospital-acquired complications,1,6–8 which contribute to higher mortality, poorer functional status, limited rehabilitation, prolonged length of stay, increased institutionalization, and higher health care costs 1,2,9–12.
Evidence suggests that interdisciplinary geriatric inpatient services improve care for hospitalized elders without CI. However, their effectiveness among elders with CI is less clear.13–18 One reason for the limited effectiveness of geriatric services may be the ever-quickening pace of care in the hospital setting that limits the windows of opportunity for geriatric team input and implementation of geriatric recommendations. The Institute of Medicine recommends integrating information systems into health care as a promising route to improve the safety and quality of care.19 Such integration may provide patient-specific warnings to avoid potentially inappropriate care at the time of medical decision-making.
We report the results of the first randomized controlled clinical trial evaluating the efficacy of a screening program coupled with a clinical decision support system (CDSS) in enhancing hospital care for elders with CI. The primary hypothesis was that our CDSS would reduce the exposure to potentially inappropriate anticholinergic medications, urinary catheters, and physical restraints, as well as increase geriatric consultation referral.
METHOD
The study was approved by the Indiana University Purdue University at Indianapolis Institutional Review Board, and informed consent for identifiable chart review was obtained from subjects or their legally authorized representatives.
Population
The study was conducted at Wishard Memorial Hospital (WMH) between July 1, 2006 and March 30, 2008. WMH is a 340-bed, university-affiliated, public hospital serving between 1,500 and 2,000 hospitalized elders every year. The general medicine services at WMH are supported by a geriatric consultation service, the Acute Care for Elders (ACE). The ACE team includes a geriatrician, a geriatric nurse practitioner, a social worker, a pharmacist, a physical therapist, an occupational therapist, and an administrative assistant.3
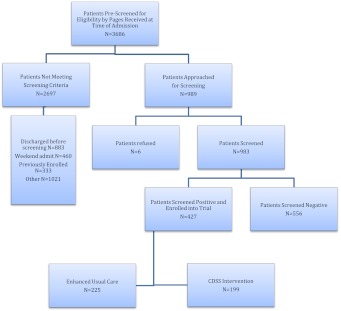
We enrolled patients that are: (1) at least 65 years of age; (2) hospitalized on a medical ward; (3) English-speaking; and (4) have CI at the time of hospital admission. Patients were excluded if they had previously been enrolled in the study, were aphasic, or unresponsive at the time of screening (Fig. 1).
Figure 1.
The randomized controlled trial enrollment flow chart.
The Regenstrief Medical Record System (RMRS)
RMRS processes data, and monitors patient and physician activity at WMH.20 It is composed of Registration and Scheduling, Laboratory, Pharmacy, and Database modules. RMRS contains retrievable observations generated during more than 600,000 outpatient visits and 60,000 inpatient stays per year.20 By linking with the Indiana Network for Patient Care, the RMRS captures data on hospitalization and emergency room visits from a network of hospital systems throughout the state of Indiana. Additionally, RMRS contains death certificate information transferred from the Indiana State Board of Health for all registered patients who die in or outside the state of Indiana. In addition, WMH uses the GOPHER Physician Order-Entry System. GOPHER captures all physician orders at WMH and is linked to the RMRS. Once orders are entered, the system sends them electronically to the nurses' workstation on the patient's ward, and requisitions are printed at appropriate locations. Less than 5% of orders are entered by nursing staff as verbal orders.20,21
Cognitive Screening
The leadership of the internal medicine physicians group approved screening for CI as part of the standard of care among their hospitalized patients aged 65 and older. The research assistant (RA) received the screening order via a page initiated by GOPHER from 8 a.m. to 5 p.m. on Monday through Friday. A delayed message was sent at 8 a.m. the following morning indicating any admissions that occurred between 5 p.m. and 8 a.m. from the previous evening/morning. CI was determined by the Short Portable Mental Status Questionnaire (SPMSQ).22,23 The SPMSQ is a ten-item test with a sensitivity of 86% and specificity 99.0% for dementia validated in both community-dwelling and hospitalized older adults.23 Patients having a score of 8 or less on the SPMSQ after adjusting for race and education were considered to have CI. At the time of cognitive assessment, delirium was assessed using the Confusion Assessment Method (CAM).24 At screening and every weekday, the CAM score was completed by the research assistant using data from the SPMSQ, subject’s direct observation, nursing staff or family interview, and medical record review to determine the presence of (1) acute and fluctuating changes in mental status, (2) inattention, (3) disorganized or incoherent thinking, and (4) an altered level of consciousness. A CAM score is considered to be positive if the patient displays both (1) and (2) with at least one of (3) or (4). The CAM has a sensitivity of 97% and a specificity of 92%.24 When feasible, the RA administered both the SPMSQ and the CAM within the first few hours of hospitalization, and more than 75% of screening occurred in the first 48 h of hospital admission. Once the RA enters the screening results into GOPHER, using a computer-generated process, cognitively impaired eligible patients were automatically randomized in a 1:1 ratio into a CDSS intervention group or usual care. The results of the cognitive screening were inserted into the written medical chart of all patients, including those randomized into the usual care group.
The Intervention (see Online Appendix)
An interdisciplinary team met monthly for an entire year and used available guidelines25–28 and two recently published systematic evidence reviews to develop the content and the format of the electronically delivered CDSS.3,29,30 The interdisciplinary team initially intended the CDSS to focus only on medication recommendations, which suggest anticholinergic medications should not be used as first-line treatments in patients with CI when equally effective alternatives exist.28 Additionally, the expert panel requested that, since the physician order entry system contained the capability to influence potentially harmful Foley catheter placement and physical restraints, the CDSS also included recommendations for removing these unnecessary tethers. The study investigators and the expert panel jointly selected the list of prohibited anticholinergic medications (along with suggestions for alternatives) and the process of eliminating physical restraints. The team selected only 18 medications with moderate to severe centrally acting anticholinergic properties as inappropriate for patients with CI and offered alternative treatments, changed doses of ordered medications, or discontinued medications. The use of the alternative medications was approved and authorized by the Pharmacy and Therapeutics Committee. The team also created an alternative to the use of physical restraints such as having a sitter and using a low dose of haloperidol or trazodone. Because the recommendations developed by the local group are appropriate for any form of CI, the CDSS was applied consistently regardless of the type of CI. The following steps outline the interaction between the physician and the CDSS:
Each time a physician enters an order for a patient randomized to the intervention arm, the physician received non-interruptive alerts of the presence of CI, Foley catheter, physical restraints, anticholinergic drugs, or the need for ACE services;
If the physician orders a urinary catheter, s/he will receive interruptive alerts to recommending discontinuing the catheter;
If the physician orders physical restraints, s/he will receive interruptive alerts recommending substituting physical restraints with the use of a professional sitter or low dose trazodone;
If the physician orders any of the 18 inappropriate anticholinergics, s/he will receive interruptive alerts recommending stopping the drug, suggesting an alternative, or recommending dose modification.
The physician was required to make a decision to accept, reject, or modify any of the interruptive alerts. However, when non-interruptive alerts were presented, physicians were able to quickly exit the screen by using the “F8” key.
The providers of the patients randomized into the usual care did not receive CDSS but had the opportunity to review the results of the cognitive screening in the medical record.
Primary Outcome Measures
The study primary outcomes were the orders of ACE consultation, the discontinuation orders of 18 potentially inappropriate anticholinergics medications, urinary catheter, and or physical restraints. The above outcomes were measured by the GOPHER database. In each of the above outcomes, we computed two different rates: (1) immediate: the percentage of patients receiving an order (or a discontinuation order) during the first 48 h of being admitted to the hospital and (2) hospital stay: the percentage of patients receiving the orders during the entire hospital stay.
Secondary Outcome Measures
The RMRS was used to determine the total number of hospital-acquired complications that may be related to CI. These complications included the number of patients with injuries (such as falls), urinary catheters, or pressure ulcers. In addition, complications included any patients with a new-onset delirium episode that developed during hospitalization as determined by a positive CAM score during any day of the hospital course if the initial CAM score on admission was negative.
Other Data Collection
Patient demographics such as age, gender, race, and education level were determined by the RMRS and by information obtained during the time of cognitive screening. Length of hospital stay, mortality, 30-day post-hospitalization mortality, 30-day readmission rates, discharge placement, ICD-9-based admission and discharge diagnosis codes, and hospital-acquired complications were all obtained from the RMRS. Comorbidity level was measured by reviewing the RMRS and determining each patient’s Charlson Comorbidity Index total score.31 This score was determined using ICD-9 codes gathered from 1 year prior to admission until the patient was discharged from the hospital.
Analysis
Descriptive statistics were calculated, including percentages for binary categorical variables, means and standard deviations for continuous variables. Comparisons between groups were based on intent to treat analysis and used Fisher’s exact tests for binary categorical variables and t-tests for continuous variables. Since the distributions of length of stay and Charlson Comorbidity Index were skewed, all statistical tests comparing them across groups were actually performed on their log-transformed values. To control for potential baseline imbalances across groups, further comparisons between groups were made using logistic regression for binary categorical outcome variables and multiple regression for continuous outcomes, with covariates that included age, gender, race, Charlson Comorbidity Index, and SPMSQ at screening.
RESULTS
Within 21 months, the study assessed the cognitive status of 998 patients aged 65 and older with a mean age of 74.8 years (SD 7.5), a mean education of 10.3 years (SD 2.8), a mean SPMSQ score of 7.7 (2.8), 67.8% female, 59.4% African Americans, and 42% cognitively impaired. Table 1 describes the baseline characteristics of the CDSS group (N = 199) and the usual care (N = 225). The usual care group were more likely to be female (71.1% vs 60.3%, p = 0.02) and have more chronic comorbidity (mean Charlson Comorbidity Index score of 2.4 vs 1.8, p < 0.001), but there were no differences between the two groups in other variables such as the prevalence of delirium at screening (intervention: 30.2% vs usual care: 31.1%, p = 0.83) and the level of CI (mean SPMSQ of intervention: 5.2 vs usual care: 5.1, p = 0.67).
Table 1.
Overall Baseline Characteristics of the CDSS and the Usual Care Groups
| Baseline variable | CDSS (N = 199) | Usual Care (N = 225) | P Value |
|---|---|---|---|
| Mean age (SD) | 76.8 (7.9) | 77.6 (8.3) | 0.32 |
| % Female | 60.3% | 71.1% | 0.02 |
| % African American | 61.8% | 57.3% | 0.37 |
| Mean Charlson Comorbidity Index (SD) | 1.8 (1.8) | 2.4 (2.1) | <0.001 |
| Mean SPMSQ (SD) | 5.2 (2.6) | 5.1(2.8) | 0.67 |
| % Delirium at screening | 30.2% | 31.1% | 0.83 |
CDSS: Clinical decision support system; SD: standard deviation; SPMSQ: short portable mental status questionnaire
As seen in Table 2, the CDSS did not increase physicians’ orders for ACE consults, physicians’ discontinuation of Foley catheterization, or discontinuation of physical restraints. Physicians receiving the CDSS issued more discontinuation orders of definite anticholinergics; however, this result was not statistically significant. The study found no differences in physician orders of benzodiazepines (intervention: 20.6% vs usual care: 15.6%, P 0.20) or atypical neuroleptics (intervention: 14.6% vs usual care: 11.6%, p = 0.74). However, patients randomized into the CDSS had more orders for typical neuroleptics (intervention: 17.6% vs usual care: 11.6%, p = 0.03). The CDSS did not specifically target the physician prescribing benzodiazepine or atypical neuroleptics; however, the CDSS alerted the physician of the presence of CI and recommended the use of low dose haloperidol as an alternative for physical restraints. Nevertheless, the study found no difference between the intervention and the usual group in the rate of recognizing CI at discharge (intervention: 54% vs usual care: 52%, p = 0.66).
Table 2.
The Differences Between the Intervention Group and the Control Group in Regard to Physicians’ Prescribing Behaviors
| CDSS (N = 199) | Usual care (N = 225) | P Value* | |
|---|---|---|---|
| ACE consult order | |||
| % with ACE consult order | |||
| First 48 h | 42% | 36% | 0.40 |
| Entire hospital stay | 56% | 49% | 0.28 |
| Foley catheterization | |||
| % FC order (N) | |||
| First 48 h | 20.1% (40) | 23.6% (53) | 0.50 |
| Entire hospital stay | 30.2% (60) | 35.1% (79) | 0.37 |
| % FC discontinuation order (N)† | |||
| First 48 h | 22.5% (9/40) | 18.9% (10/53) | 0.70 |
| Entire hospital stay | 61.7% (37/60) | 64.6% (51/79) | 0.86 |
| Physical restraints | |||
| % PR order (N) | |||
| First 48 h | 6.0% (12) | 3.6% (8) | 0.51 |
| Entire hospital stay | 10.5% (21) | 7.6% (17) | 0.66 |
| % PR discontinuation order (N)‡ | |||
| First 48 h | 0.0% (0/12) | 0.0% (0/8) | 1.00 |
| Entire hospital stay | 4.8% (1/21) | 0.0% (0/17) | 0.86 |
| AC order | |||
| % AC order (N) | |||
| First 48 h | 13.6% (27) | 14.7% (33) | 0.91 |
| Entire hospital stay | 23.6% (47) | 21.3% (48) | 0.33 |
| % AC discontinuation order (N)§ | |||
| First 48 h | 7.4% (2/27) | 3.0% (1/33) | 0.46 |
| Entire hospital stay | 48.9% (23/47) | 31.2% (15/48) | 0.11 |
*P value adjusted for baseline gender and Charlson Comorbidity Index score
†Denominator was the number of orders eligible for discontinuation
‡Denominator was the number of orders eligible for discontinuation
§Denominator was the number of orders eligible for discontinuation
ACE consult: Acute Care for Elderly Consultation; FC: Foley catheterization; PR: physical restraints; AC: anticholinergic medications; CDSS: clinical decision support system
Table 3 demonstrates that the CDSS had no statistically significant effects on health outcomes such as the mean days of hospital stay (intervention: 7.7 days vs usual care: 6.8, p = 0.12), 30-day mortality rate (intervention: 6% vs usual care: 5.8%, p = 0.69), home discharge (intervention: 43.2% vs usual care: 36.9%, p = 0.13), 30-day readmission rates (intervention: 18.6% vs usual care: 16.4%, p = 0.53), or hospital-acquired complications (intervention: 47.2% vs usual care: 44.9%, p = 0.94). Our hospital-acquired complications included incidence of delirium (intervention: 33.7% vs usual care: 31.1%, p = 0.78), the presence of ICD-9 codes of pressure ulcer at discharge (intervention: 12.1% vs usual care: 11.1%, p = 0.77), the presence of ICD-9 code for fall or injury at discharge (intervention: 4.5% vs usual care: 4.9%, p = 0.88), and orders for physical restraints or patients observed to be physically restrained (intervention: 11.1% vs usual care: 7.6%, p = 0.54).
Table 3.
The Differences Between the Intervention Group and the Control Group in Health Outcomes
| CDSS (N = 199) | Usual care (N = 225) | P Value | |
|---|---|---|---|
| Mean length of hospital stay | 7.7 (7.4) | 6.8 (5.4) | 0.12 |
| % Patients died within 30 days of hospitalization | 6% | 5.8% | 0.69 |
| % Patient discharged home | 43.2% | 36.9% | 0.13 |
| % Patients readmitted within 30 days of discharge | 18.6% | 16.4% | 0.53 |
| % Patients with at least one hospital complication | 47.2% | 44.9% | 0.94 |
P value adjusted for baseline differences in gender and Charlson Comorbidity Index score. Hospital complication included incidence of delirium, physical restraints, injury, or pressure ulcer. CDSS: clinical decision support system
We further tested the impact of our CDSS among the 130 patients who had delirium on admission and found similar results. We also found similar results for physicians prescribing and patient’s health outcomes among patients who did and those who did not receive an ACE consult.
DISCUSSION
A simple CDSS to alert the physicians of the presence of CI and provide recommendations to reduce the use of anticholinergic medications did not significantly change physician prescribing behavior. Additionally, the CDSS was not sufficient to increase the referral to geriatric consultation services or reduce potentially harmful procedures such as Foley catheterization and physical restraints. The CDSS also had no impact on health outcomes and did not improve recognition of CI at hospital discharge.
Numerous studies have provided evidence that CDSS can improve the process of care, lead to better patient outcomes, reduce medical errors, and decrease health care expenditures.32–35 However, our study is the first randomized trial targeting the efficacy of CDSS among elders with CI. A recent publication by Mattison and colleagues describes a reduction in orders for potentially inappropriate medications achieved by a similar CDSS targeting older adults admitted to a general medical ward.36 Their intervention notified prescribers of potentially inappropriate medications through a computerized alert at the time of order entry; however, the intervention did not suggest an alternative medication or treatment, and its efficacy was not evaluated in a randomized controlled trial design but in a “pre-post nonparallel” experimentation design. There were multiple factors that conspired to reduce the potential impact of our CDSS. For example, 51 patients were discharged from the hospital on the same day of cognitive screening, and 207 (49%) of the enrolled subjects did not receive any physical restraint, Foley catheterization, or definite anticholinergic medication orders and therefore could not have benefitted from the intervention. Additionally, contamination of the study intervention may have impacted the measured outcomes with close to 50% of the usual care group receiving geriatric consultation during their hospital stay.
Several controlled clinical trials evaluating the effectiveness of hospital-based models of geriatric care report conflicting results. Caring for hospitalized older adults in a specialized geriatric unit may lead to improvement in patients’ physical function and decrease the length of hospital stay without affecting overall mortality.14–17,37,38 Such positive results indicate that changing the system of care may improve patient outcomes. Unfortunately, geriatric wards will only reach a small number of older hospitalized patients. In contrast to the positive effect of geriatric-based hospital units, studies of geriatric consultation generally failed to demonstrate efficacy across a range of health outcomes.39–43 One exception is a study of patients undergoing surgical repair of hip fracture. In that study geriatric consultation decreased the incidence of delirium without affecting other outcomes such as length of stay, mortality, or functional status.18 These models of care have resulted in improved care in a variety of domains for hospitalized older adults;44–47 however our study is the first to attempt a computerized intervention exclusively in a population of older adults with CI.
Whereas we were unable to improve measures relating to the quality of care, previous interventions using CDSS methodology have successfully modified medication orders in various populations and practice environments.48–51 Our intervention targeted only anticholinergic medications and not sedative-hypnotics, which are also recognized to have potentially detrimental effects on cognition. We chose not to include sedative-hypnotics as targets for our intervention to avoid influencing sedation practices necessary for procedures in the acute care environment. Despite the success of CDSS tools in other populations and practice environments, our results suggest that human interaction may play a significant role in the acceptance of recommendations aimed at improving the care of hospitalized older adults with CI.
This study provided us with important lessons for the next step in enhancing the hospital care of elders with CI. First, our list of 18 anticholinergics needs to include two antipsychotics with definite anticholinergic activities: olanzapine and quetiapine. The FDA announcement of a “black box” warning against the use of neuroleptics in older adults with dementia suggests that reducing exposure to neuroleptic might improve outcomes in elders with dementia.52,53 Second, the non-interruptive alerts used to identify CI and recommend against the use of potentially harmful medications and procedures were ineffective; however, interruptive alerts suggesting the discontinuation of specific medications at the time of ordering followed by suggested alternatives might change physician’s prescribing behavior. Third, physicians did not respond to an order targeting nursing practice such as physical restraints or Foley catheterization. Fourth, despite our strong efforts to assess the cognitive status of patients as early as possible, at least 20% of the patients were screened after being in the hospital for 48 h, and thus had already received anticholinergic medications. As a result, our intervention may have missed the opportunity to interrupt medication orders at the time of admission. Therefore, the next generation CDSS will have a dedicated pharmacist who will assess the patient within 24 h of enrollment, discontinue inappropriate existing orders for anticholinergics, and suggest an alternative order. Fifth, similar to our research findings regarding enhancing the ambulatory care of vulnerable patients with CI, our study demonstrated that simply screening for CI in the hospital setting is not sufficient to improve the care of older adults. Enhancing care for elders with CI starts with improving recognition but requires more comprehensive subsequent interventions.54,55
Additionally, using previous literature rates, our sample was created with the assumptions that 45% of the population would be prescribed at least one inappropriate anticholinergic and 50% would receive orders for urinary catheters and/or physical restraints. These assumptions offered 85% power to detect a decrease in targeted interventions of 30% between groups. However, the rates of inappropriate anticholinergics, Foley catheter, and physical restraints were much lower than our estimates. These lower rates compromised the power of our study, which was substantially lower than planned.
In summary, we recommend development of a new and enhanced CDSS capable of integrating the strength of both the human and computer resources to deliver early, clinically relevant, validated, and easy to implement recommendations that may lead to reducing the exposure of the vulnerable hospitalized elders to potentially harmful anticholinergics and thus enhance their hospital care.
Electronic supplementary material
(DOC 53 kb)
ACKNOWLEDGMENTS
Manuscript design, methods, subject recruitment, data collections, analysis, and preparation were sponsored by grant K23-AG026770-01 and grant no. R01AG034205-01A1 from the National Institute on Aging.
Conflict of Interest
Dr. Boustani has work supported by grants from the NIA and AHRQ. He is also a member of the Pfizer speakers’ bureau.
Dr. Buckley has provided expert testimony for local law firms. Mr. Perkins owns stock in several pharmaceutical firms. If further information is needed, please notify our corresponding author.
Author Contributions
All authors had a role in the study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of the manuscript.
Funding Source
Dr. Boustani is supported by the NIA Paul B. Beeson K23 Career Development Award no. 1-K23-AG026770-01 and NIA award R01AG034205-01A1.
Footnotes
Author Contributions: All authors had a role in the study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of the manuscript.
REFERENCES
- 1.Boustani M, Baker MS, Campbell N, Munger S, Hui SL, Castelluccio P, et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5:69–75. doi: 10.1002/jhm.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 3.Boustani M, Munger S, Beck R, Campbell N, Weiner M. A gero-informatics tool to enhance the care of hospitalized older adults with cognitive impairment. Clin Interv Aging. 2007;2:1–7. doi: 10.2147/ciia.2007.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Hospital Discharge Survey 2005. Public use data file documentation. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics. Division of Health Care Statistics. Hospital Care Statistics Branch. http://www.cdc.gov/nchs/about/major/hdasd/nhds.htm (accessed January 10, 2012).
- 5.DeFrances CJ, Podgornik MN. 2004 National Hospital Discharge Survey. Advance data from vital and health statistics; no 371. Hyattsville, MD: National Center for Health Statistics 2006. [PubMed]
- 6.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13:234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106:565–573. doi: 10.1016/S0002-9343(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 8.Frels C, Williams P, Narayanan S, Gariballa SE. Iatrogenic causes of falls in hospitalised elderly patients: a case-control study. Postgrad Med J. 2002;78:487–489. doi: 10.1136/pmj.78.922.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.CCM.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 11.Saravay SM, Kaplowitz M, Kurek J, et al. How do delirium and dementia increase length of stay of elderly general medical inpatients? Psychosomatics. 2004;45:235–242. doi: 10.1176/appi.psy.45.3.235. [DOI] [PubMed] [Google Scholar]
- 12.Witlox J, Eurelings LSM, de Jonghe JFM, Kalaisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia. a meta-analysis. JAMA. 2010;304(4):443-451. [DOI] [PubMed]
- 13.Baztan JJ, Suarez-Garcia FM, Lopez-Arrieta J, Rodriguez-Manas L, Rodriguea-Artalejo F. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BMJ. 2009;338:b50. doi: 10.1136/bmj.b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Counsell SR, Holder CM, Liebenauer LL, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48:1572–1581. doi: 10.1111/j.1532-5415.2000.tb03866.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen HJ, Feussner JR, Weinberger M, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346:905–912. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]
- 16.Cole MG, McCusker J, Bellavance F, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167:753–759. [PMC free article] [PubMed] [Google Scholar]
- 17.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 18.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 19.Medicine, Institute of. Crossing the Quality Chasm. Washington, DC. Institute of Medicine Press 2001.
- 20.McDonald CJ, Overhage JM, Tierney WM, et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54:225–253. doi: 10.1016/S1386-5056(99)00009-X. [DOI] [PubMed] [Google Scholar]
- 21.Tierney WM, Miller ME, Overhage JM, McDonald CJ. Physician inpatient order writing on microcomputer workstations. Effects on resource utilization. JAMA. 1993;269:379–383. doi: 10.1001/jama.1993.03500030077036. [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 23.Erkinjuntti T, Sulkava R, Wikstrom J, Autio L. Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc. 1987;35:412–416. doi: 10.1111/j.1532-5415.1987.tb04662.x. [DOI] [PubMed] [Google Scholar]
- 24.Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Practice guidelines for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999 May;156(5 Suppl):1-20. [PubMed]
- 26.Chow TW, MacLean CH. Quality indicators for dementia in vulnerable community-dwelling and hospitalized elders. Ann Intern Med. 2001;135:668–676. doi: 10.7326/0003-4819-135-8_part_2-200110161-00005. [DOI] [PubMed] [Google Scholar]
- 27.Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405–1412. doi: 10.1001/archinte.164.13.1405. [DOI] [PubMed] [Google Scholar]
- 28.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 29.Campbell N, Boustani M, Ayub A, et al. Pharmacological management of delirium in hospitalized adults: a systematic evidence review. J Gen Intern Med. 2009;24:848–853. doi: 10.1007/s11606-009-0996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–233. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG., Jr Assessing illness severity: does clinical judgment work? J Chronic Dis. 1986;39:439–452. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 32.Osheroff JA, Teich JM, Middleton B, Steen EB, Wright A, Detmer DE. A roadmap for national action on clinical decision support. J Am Med Inform Assoc. 2007;14:141–145. doi: 10.1197/jamia.M2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudry B, Wang J, Wu S, et al. Systematic review: Impact of health information technology on quality, efficiency, and cost of medical care. Ann Int Med. 2006;144:742–749. doi: 10.7326/0003-4819-144-10-200605160-00125. [DOI] [PubMed] [Google Scholar]
- 34.Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138–147. doi: 10.1197/jamia.M1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terrell KM, Perkins AJ, Dexter PR, Hui SL, Callahan CM, Miller DK. Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: a randomized, controlled trial. J Am Geriatr Soc. 2009;57(8):1388–1394. doi: 10.1111/j.1532-5415.2009.02352.x. [DOI] [PubMed] [Google Scholar]
- 36.Mattison MLP, Afonso KA, Ngo LH, Mukamal KJ. Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336. doi: 10.1001/archinternmed.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–44. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- 38.Slaets JP, Kauffmann RH, Duivenvoorden HJ, Pelemans W, Schudel WJ. A randomized trial of geriatric liaison intervention in elderly medical inpatients. Psychosom Med. 1997;59:585–91. doi: 10.1097/00006842-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342:1032–6. doi: 10.1016/0140-6736(93)92884-V. [DOI] [PubMed] [Google Scholar]
- 40.Reuben DB, Borok GM, Wolde-Tsadik G, et al. A randomized trial of comprehensive geriatric assessment in the care of hospitalized patients. N Engl J Med. 1995;332:1345–50. doi: 10.1056/NEJM199505183322007. [DOI] [PubMed] [Google Scholar]
- 41.Campion EW, Jette A, Berkman B. An interdisciplinary geriatric consultation service: a controlled trial. J Am Geriatr Soc. 1983;31:792–6. doi: 10.1111/j.1532-5415.1983.tb03401.x. [DOI] [PubMed] [Google Scholar]
- 42.Becker PM, McVey LJ, Saltz CC, Feussner JR, Cohen HJ. Hospital-acquired complications in a randomized controlled clinical trial of a geriatric consultation team. JAMA. 1987;257:2313–7. doi: 10.1001/jama.1987.03390170069030. [DOI] [PubMed] [Google Scholar]
- 43.Gayton D, Wood-Dauphinee S, Lorimer M, Tousignant P, Hanley J. Trial of a geriatric consultation team in an acute care hospital. J Am Geriatr Soc. 1987;35:726–36. doi: 10.1111/j.1532-5415.1987.tb06350.x. [DOI] [PubMed] [Google Scholar]
- 44.Rubenstein LZ, Josephson KR, Wieland GD, English PA, Sayre JA, Kane RL. Effectiveness of a geriatric evaluation unit. A randomized clinical trial. N Engl J Med. 1984;311:1664–70. doi: 10.1056/NEJM198412273112604. [DOI] [PubMed] [Google Scholar]
- 45.Burns R, Nichols LO, Graney MJ, Cloar FT. Impact of continued geriatric outpatient management on health outcomes of older veterans. Arch Intern Med. 1995;155:1313–8. doi: 10.1001/archinte.1995.00430120103012. [DOI] [PubMed] [Google Scholar]
- 46.Owens NJ, Sherburne NJ, Silliman RA, Fretwell MD. The Senior Care Study. The optimal use of medications in acutely ill older patients. J Am Geriatr Soc. 1990;38:1082–7. doi: 10.1111/j.1532-5415.1990.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 47.Edwards RF, Harrison TM, Davis SM. Potentially inappropriate rescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine serice. Consult Pharm. 2003;18(37–42):47–49. [PubMed] [Google Scholar]
- 48.Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345:965. doi: 10.1056/NEJMsa010181. [DOI] [PubMed] [Google Scholar]
- 49.Pestotnik SL, Classen DC, Evans RS, Burke JP. Implementing antibiotic practice guidelines through computer-assisted decision support: clinical and financial outcomes. Ann Intern Med. 1996;124:884–90. doi: 10.7326/0003-4819-124-10-199605150-00004. [DOI] [PubMed] [Google Scholar]
- 50.Larsen RA, Evans RS, Burke JP, Pestotnik SL, Gardner RM, Classen DC. Improved perioperative antibiotic use and reduced surgical wound infections through use of computer decision analysis. Infect Control Hosp Epidemiol. 1989;10:316–20. doi: 10.1086/646035. [DOI] [PubMed] [Google Scholar]
- 51.Overhage JM, Tierney WM, Zhou XH, McDonald CJ. A randomized trial of "corollary orders" to prevent errors of omission. J Am Med Inform Assoc. 1997;4:364–75. doi: 10.1136/jamia.1997.0040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 53.Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- 54.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN. US Preventive Services Task Force. Screening for dementia in primary care: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2003;138(11):927–937. doi: 10.7326/0003-4819-138-11-200306030-00015. [DOI] [PubMed] [Google Scholar]
- 55.Callahan CM, Boustani M, Unverzagt FW, et al. Effectiveness of guideline-level care for older adults with Alzheimer's disease in primary care: a clinical trial. JAMA. 2006;295:2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 53 kb)