Abstract
Purpose
To identify pelvic rotation and/or distortion in able-bodied and untreated AIS girls with moderate and severe scoliosis and verify association of pelvic morphological changes with Cobb angle increase.
Methods
The 3D coordinates of nine anatomic bony landmarks were identified to estimate pelvic orientation using a Flock of Birds system. The distances between the first sacral vertebral body (S1) and each of the eight iliac spine landmarks in all three planes were calculated to identify pelvic distortion. Analysis of variance was used to assess pelvic orientation and determine pelvic distortion. Pearson coefficients of correlation were used to identify any relationships between Cobb angle and pelvic morphological parameters.
Results
Pelvic orientation was similar in able-bodied and scoliotic girls regardless of the severity of the spinal deformity. Significant differences were observed in pelvic morphology between AIS with severe untreated scoliosis and those with a moderate scoliosis for the right anterosuperior iliac spines (ASIS), the tip of the superior iliac crest (TSIC) and the widest tip of the iliac crest (WTIC) widths from S1. Statistically significant correlations were observed between the Cobb angles and the iliac crest distances measured from S1.
Conclusions
Differences in iliac spine geometries occurred in the transverse plane correlating to Cobb angles which suggest altered bone growth in AIS girls. Such findings could indicate right thoracic spinal deformity as a result of pelvic torsion.
Keywords: Adolescent idiopathic scoliosis, Moderate spinal deformity, Severe spinal deformity, Biomechanics
Introduction
Scoliosis is principally characterized by a spinal deformity and rib cage distortion [1]. As a result, the spatial orientation of other body segments are affected [2] which also allows body segment interdependency [3]. Mac-Thiong et al. [4] reported a strong sagittal spine and pelvic relationship in severe adolescent idiopathic scoliosis, while Legaye et al. [5] noted similar findings in scoliotic adults. Furthermore, structural changes in the pelvis and spine were observed also in severe scoliosis by Saji et al. [6]. Although altered pelvic geometry was documented in scoliosis, it is unclear whether pelvic orientation and geometry differ from age-matched nonscoliotic girls and how they are related to spinal deformity in moderate and severe untreated adolescent idiopathic scoliosis (AIS) girls.
Body posture is often measured by placing surface markers over anatomical landmarks to identify body segments and joints. Their three-dimensional (3D) coordinates are obtained by means of video-based systems [7, 8], or electromagnetic pointers such as the Flock of Birds [3]. Bony landmarks on the iliac crests and the spinous process of the first sacral vertebra (S1) served to identify pelvic spatial orientation [7, 9] or its relation to other body segments like the head or shoulders [8]. Abnormal bone growth distorts pelvic geometry and that could lead to erroneous pelvic spatial orientation estimations. From a radiographic analysis, iliac wing and crest appear wider on the side of the major curve [10]. However, changes in pelvic orientation could bias its morphology assessment when performing a planar radiographic analysis. Pelvic and iliac crest geometries are not reported in most studies where upright posture is assessed in AIS and a single group of scoliotic girls having a wide range of Cobb angles [3], severe scoliotic deformities [4] and several curve types [7] are often included. No study has described a 3D pelvic geometry in patients with selected types of scoliosis or distinguished its association with different degrees of spine curvature.
Skeletal deformity of the spine was linked with pelvic misalignment [11] and morphologic asymmetry [12] in patients with severe scoliosis. There is evidence suggesting an altered skeletal growth in AIS [13]. Not only scoliotic girls have a predisposition to be taller and slender [14, 15] than able-bodied girls, but growth spurt is also associated with a poorer prognosis [16, 17]. This is corroborated by a pelvic incidence that is closer to adult than adolescent suggesting a faster bone growth in AIS [4]. Nonetheless, there is no information on 3D pelvic geometry in AIS patients with a moderate scoliosis and how they compare with those with a severe one.
It is hypothesized that untreated AIS girls have morphologic pelvic abnormalities and that the extent of pelvic deformity is related to the Cobb angle. This would support that an asymmetrical bone growth could lead to the progression of scoliosis. The objective of this study was to determine if there is a rotation, a distortion, or both in the pelvis of able-bodied and untreated AIS scoliotic girls with moderate (less than 27° Cobb angle) and severe spinal deformity (more than 27° Cobb angle), and verify if pelvic morphological changes are associated with Cobb angle increase in the scoliotic groups.
Materials and methods
Seventy-four girls participated in this study. An orthopedic surgeon determined the diagnosis of scoliosis in 46 girls based on the definition given by Bunnell [18]. Their average age was 12.6 ± 1.6 years while their height and weight were 153.4 ± 9.6 cm and 43.3 ± 8.9 kg, respectively. Their average Cobb angle was 27.5° ± 11.3° and ranged between 11° and 52°. No patient was under active treatment although a body brace had been prescribed for 28 girls. All the spinal curves were to the right. Of the 44 thoracic curvatures 16 had a mean 28° ± 11° left lumbar compensatory curve. Two subjects had a 27° right thoracolumbar deviation and the remaining two girls had a 15° lumbar curve. The girls were arbitrarily divided into two groups according to the median Cobb angle to distinguish moderate (<27°) from severe (>27°) untreated scoliosis. This demarcation corresponds closely to the generally accepted notion that curves greater than 25° are often considered as severe spinal deformities [17, 18]. Table 1 summarizes the mean demographic characteristics of the untreated scoliotic groups. The nonscoliotic group consisted of 28 able-bodied girls who were also examined to ensure the absence of spinal deformity. Those wearing a foot orthosis, suffering from back pain or having a limb length discrepancy of more than 1 cm or had any other signs of postural orthopedic or neurological disorders were excluded from the study. No statistical difference was found between the three groups in terms of age (p ≥ 0.6311), height (p ≥ 0.1171) or mass (p ≥ 0.3247).
Table 1.
Mean values and standard deviations of the age, height and mass for the able-bodied girls and for the moderate and severe scoliosis groups as well as the mean Cobb angle and range when applicable
| Group | Number | Age (years) | Height (m) | Mass (kg) | Cobb angle (°) | Cobb angle range (°) |
|---|---|---|---|---|---|---|
| Able-body | 28 | 12.9 ± 1.4 | 1.56 ± 0.07 | 45.7 ± 7.6 | N/A | N/A |
| Moderate scoliosis | 23 | 12.3 ± 1.8 | 1.51 ± 0.09 | 41.8 ± 7.8 | 17.8 ± 4.7 | 11–26 |
| Severe scoliosis | 23 | 12.8 ± 1.4 | 1.54 ± 0.10 | 44.8 ± 9.0 | 37.2 ± 6.5 | 28–52 |
Measurements on the pelvis were performed with the subject standing barefoot in a standardized position [19]. The heels were aligned and spaced by about 23 cm and the midline of the feet pointing externally by 15°. Nine bony landmarks were identified on each pelvis and spine using a Flock of Birds system (Ascencion Technologies, Burlington, VT, USA). This system consists of a pen pointer that emits an electromagnetic signal to a receiver box located less than 1.5 m away. While subjects were asked to maintain a quiet upright stance, the operator had to touch lightly the skin lying over the anatomical landmarks with the tip of the pen and activate the signal to register their 3D coordinates. Landmarks visually hidden from the receiver by a body segment can be digitized because the signal is electromagnetic.
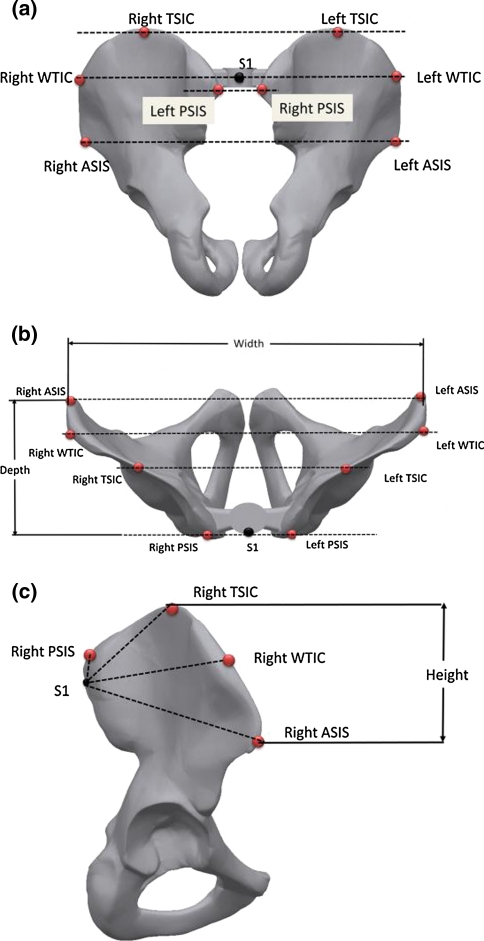
As shown in Fig. 1, the anatomic landmarks were the first sacral vertebral body (S1), the posterosuperior iliac spines (PSIS) and anterosuperior iliac spines (ASIS). The remaining four body landmarks were the right and left tip of the superior iliac crest (TSIC) and the widest tip of the iliac crest (WTIC). These correspond, respectively, to the highest and widest part of the pelvis. The Flock of Birds system was used by Leblanc et al. [14] to study body posture in adolescent idiopathic scoliosis and by Nault et al. [3] to determine the relationships between standing stability and body posture parameters in AIS as well. According to Bellefleur et al. [20], the Flock of Birds electromagnetic system has a resolution of 0.76 mm root mean square (RMS) in linear and 0.1° RMS in angular measures. Dao et al. [21] evaluated the intratester reproducibility of the Flock of Bird system by measuring 20 morphological parameters of 45 female subjects. No statistically significant difference was found between the two series of measurements and in 56% of these the difference of the means was less than 1° while the greatest was 1.8°. The 3D coordinates obtained by the Flock of Birds system were given with respect to S1 as the origin with positive axes to the right, anterior and upwards. These coordinates were used to calculate the distances between S1 and each of the eight iliac spine landmarks in all three planes. Differences in these 24 distances between the able-bodied and scoliotic groups are indicative of pelvic distortion.
Fig. 1.
S1, PSIS, ASIS, TSIC and WTIC positions on the pelvis as well as the angles calculated in the a transverse, b frontal and c sagittal planes
The orientation of the pelvis in the transverse (rotation) and frontal (tilt) planes was estimated by the angle sustained between the line joining the right and left PSIS, ASIS, TSIC and WTIC and the medio-lateral axis. It is assumed that the angles are constant between the four respective iliac spine landmarks and between groups if there is no rotation or tilt. Lateral tilt of the pelvis is estimated by the angle between the line joining the respective midpoint of the right and left PSIS, ASIS, TSIC and WTIC to S1 and the antero-posterior axis. Differences in these four angles between the able-bodied and scoliotic groups are considered as a deformed pelvic lateral tilt. This method of calculating pelvic rotation and tilts was preferred to that of Pasha et al. [9] because it relies on nine bony landmarks located on the pelvis and spine.
An analysis of variance (ANOVA) was performed on the 24 iliac spine distances to assess pelvic distortion and on 12 angles to determine pelvic orientation. Differences with a p value less than 0.05 between the able-bodied and scoliotic groups were considered significant and the number of subjects required in each group was based on preliminary analyses for statistical power of 80% or more [22]. The Bonferroni correction procedure was applied to control Type 1 error by adjusting the p values in the analysis of the aforementioned parameters [23]. Pearson coefficients of correlation were performed to identify any relationships between Cobb angles and 36 orientation and distortion parameters. If pelvic distortion is present, no correlation will be performed on the pelvic orientation parameters because changes in the iliac spine landmark’s positions could affect these correlations. Statistically significant correlations occurred with p value less than 0.05.
Results
Table 2 recapitulates the mean and standard deviation of pelvic rotation and tilts in all three planes for the able-bodied and scoliotic groups. No statistical difference was observed between groups or for all 12 angles describing pelvic rotation and tilts. In the transverse and frontal planes, it is expected that these angles should be close to zero since the bony landmarks should be side by side and leveled. Their overall mean was 1.2° ± 2.0°. The highest standard deviation was observed in the pelvic rotation given by the ASIS. Since the ASIS, PSIS, TSIC and WTIC are not at the same level from S1, their respective angles with the antero-posterior axis varied accordingly but no difference was detected between groups.
Table 2.
Mean and standard deviations (SD) of the angle between the PSIS, ASIS, TSIC and WTIC for the transverse and frontal planes and the angle sustained by the line joining the midpoint of the right and left ASIS, TSIC and WTIC and S1 in the sagittal plane for the able-bodied and subjects with a moderate and severe scoliosis groups
| Able-body girls | Moderate scoliosis | Severe scoliosis | ||||
|---|---|---|---|---|---|---|
| Mean (°) | SD | Mean (°) | SD | Mean (°) | SD | |
| Transverse plane (+left rotation) | ||||||
| ASIS | −1.6 | 13.2 | 0.9 | 15.1 | −7.7 | 15.4 |
| PSIS | 3.0 | 4.9 | 4.4 | 6.9 | 0.2 | 8.0 |
| TSIC | 2.1 | 4.4 | 2.5 | 4.6 | 0.8 | 6.0 |
| WTIC | 0.3 | 4.2 | 0.2 | 5.1 | −1.9 | 6.0 |
| Sagittal plane | ||||||
| ASIS | 1.8 | 8.6 | 6.7 | 9.3 | 6.0 | 7.5 |
| PSIS | 64.0 | 8.5 | 65.8 | 7.7 | 66.0 | 9.4 |
| TSIC | 48.0 | 9.1 | 45.7 | 11.0 | 47.0 | 10.8 |
| WTIC | 23.4 | 8.1 | 25.8 | 9.2 | 25.5 | 8.6 |
| Frontal plane (+left tilt) | ||||||
| ASIS | 1.8 | 1.3 | 1.9 | 3.2 | 1.4 | 3.0 |
| PSIS | 2.6 | 3.1 | 3.9 | 5.0 | 5.3 | 5.1 |
| TSIC | 2.0 | 2.5 | 1.7 | 3.8 | 1.0 | 3.9 |
| WTIC | 1.0 | 1.5 | 1.4 | 3.3 | 0.5 | 3.3 |
The mean and standard deviation of the medio-lateral, anterior and vertical distances measured from S1 to the right and left iliac spine landmarks for the able-bodied and scoliotic groups are given in Table 3. The severe scoliotic group displayed three statistically significant differences between the other groups. The right ASIS, TSIC and WTIC width from S1 were all longer by 10.3, 13.9 and 10.3 mm, respectively, when compared to the able-bodied girls (p ≤ 0.0284) and those of the moderate scoliosis group (p ≤ 0.0047). The left and right PSIS of the severe scoliosis group were, respectively, 5.2 mm in front (p = 0.0137) and 11.5 mm above (p = 0.0050) those of able-bodied girls. The moderate scoliosis group had 11.6 mm ASIS lesser depth (p = 0.0472) compared able-bodied girls. No statistical difference in the height of the iliac crest positions was found between the moderate and severe scoliosis groups.
Table 3.
Mean and standard deviations (SD) of the medio-lateral, anterior and vertical distances (mm) between S1 and the right and left ASIS, PSIS, TSIC and WTIC for the able-bodied girls and subjects with a moderate and severe scoliosis groups
| Able-body girls | Moderate scoliosis | Severe scoliosis | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Medio-lateral distance (width) | ||||||
| ASIS right | 105.6 | 16.4 | 100.9 | 12.3 | 115.9† | 9.1 |
| ASIS left | −112.4 | 17.0 | −112.0 | 17.3 | −109.5 | 22.3 |
| PSIS right | 35.7 | 5.3 | 33.6 | 9.2 | 36.0 | 6.8 |
| PSIS left | −36.1 | 6.3 | −38.6 | 9.5 | −41.2 | 7.1 |
| TSIC right | 107.4 | 13.3 | 106.5 | 13.5 | 121.3† | 18.6 |
| TSIC left | −112.0 | 15.7 | −115.4 | 13.9 | −112.8 | 20.3 |
| WTIC right | 120.2 | 13.7 | 116.3 | 12.9 | 129.6† | 10.7 |
| WTIC left | −129.0 | 14.9 | −126.6 | 14.0 | −124.2 | 20.1 |
| Antero-posterior distance (depth) | ||||||
| ASIS right | 155.7 | 16.4 | 148.7 | 20.7 | 149.4 | 19.7 |
| ASIS left | 158.2 | 18.4 | 146.6* | 16.4 | 160.6 | 14.4 |
| PSIS right | 12.8 | 6.7 | 16.6 | 7.7 | 13.9 | 6.7 |
| PSIS left | 8.7 | 6.0 | 11.0 | 6.1 | 13.9• | 7.1 |
| TSIC right | 62.5 | 24.4 | 73.7 | 19.7 | 71.1 | 19.8 |
| TSIC left | 53.9 | 19.9 | 63.9 | 16.0 | 66.6 | 20.9 |
| WTIC right | 122.2 | 22.7 | 115.9 | 18.4 | 116.4 | 19.0 |
| WTIC left | 121.0 | 24.3 | 115.5 | 15.8 | 123.6 | 19.9 |
| Vertical distance (height) | ||||||
| ASIS right | 9.3 | 25.1 | 20.4 | 21.7 | 19.2 | 20.6 |
| ASIS left | 2.1 | 25.4 | 13.8 | 25.4 | 13.9 | 22.2 |
| PSIS right | 25.1 | 14.0 | 33.6 | 11.7 | 36.6• | 11.3 |
| PSIS left | 21.9 | 14.9 | 28.6 | 10.9 | 29.4 | 8.2 |
| TSIC right | 69.0 | 22.1 | 74.4 | 18.3 | 76.6 | 21.2 |
| TSIC left | 61.1 | 22.2 | 67.5 | 23.2 | 73.1 | 22.2 |
| WTIC right | 54.9 | 19.8 | 59.7 | 18.8 | 58.6 | 21.0 |
| WTIC left | 50.5 | 20.8 | 53.5 | 24.5 | 56.9 | 22.9 |
†Statistical difference p < 0.05 between the severe scoliosis group and the able-bodied and moderate scoliosis groups
* Statistical difference p < 0.05 between the moderate scoliosis group and the other groups
•Statistical difference p < 0.05 between the severe scoliosis group and able-bodied group
Since there was no difference in the pelvic orientation parameters, Pearson coefficients of correlation were performed on the 24 distortion values only. Five statistically significant positive correlations were found between the Cobb angles and the iliac crest distances measured from S1 as shown in Table 4. As the right side ASIS (r = 0.601), TSIC (r = 0.372) and WTIC (r = 0.516) becomes wider and the left side ASIS (r = 0.311) and PSIS (r = 0.351) depth increase, the Cobb angle becomes more severe. No correlation was observed between the Cobb angles and iliac crest heights.
Table 4.
Pearson coefficients of correlation between the Cobb angle and the distortion pelvic parameters
| S1 to iliac crest landmark distance | Medio-lateral axis | Antero-posterior axis | Vertical axis |
|---|---|---|---|
| ASIS right | 0.601* | −0.094 | −0.140 |
| ASIS left | 0.158 | 0.317* | −0.120 |
| PSIS right | 0.120 | −0.154 | 0.028 |
| PSIS left | 0.013 | 0.366* | 0.079 |
| TSIC right | 0.372* | −0.053 | −0.048 |
| TSIC left | 0.072 | 0.218 | 0.024 |
| WTIC right | 0.516* | 0.029 | −0.168 |
| WTIC left | 0.129 | 0.208 | −0.093 |
* p ≤ 0.05
Discussion
This study evaluated if there was a rotation, distortion, or both in the pelvis of able-bodied and untreated scoliotic girls with different degree of spinal deformity severity. The 3D position of S1 and eight iliac spine positions were recorded by means of an electromagnetic system. This system was used in other studies [3, 20] because of its errors of less than 1 mm in translation and of 0.1° in angular measurements. This compares well to video-based systems where 10 mm in diameter surface markers are used to assess pelvis and trunk motions in standing [9] and for gait analysis [7] and where their accuracy has been analyzed and justified [24].
The 3D orientation of the pelvis is often based on the relative position of the ASIS and PSIS landmarks with respect to the centroid of the pelvis [9] or in reference to a specific plane [8] as in this study. Nault et al. [3] and Zabjek et al. [8] observed small pelvic rotations and frontal tilts in able-bodied and AIS girls but found no significant difference between these groups. In a retrospective study Mac-Thiong et al. [4] analyzed the pelvic sagittal alignment of 160 AIS subjects grouped into 5 curve types having a mean Cobb angle of 43° and found no statistical difference in sagittal pelvic tilt among the groups. This study not only confirms these observations using ASIS and PSIS landmarks, but also with two additional ones, namely the tip of the superior iliac crest (TSIC) and the widest tip of the iliac crest (WTIC) as shown in Fig. 1. All bony reference points essentially show no frontal and sagittal pelvic tilts as well as rotation. The standard deviation of the ASIS along the medio-lateral axis was the highest for all the groups. This could be the result of an altered pelvic morphology. A new finding is that the spatial orientations of the pelvis in scoliotic patients with moderated scoliosis are similar to those of able-bodied and severe scoliotic girls.
Abnormal bone growth is associated with scoliosis. Upper arm [25] and facial asymmetries [26] were reported in AIS, and skeletal disproportion is considered as a predisposition to scoliosis progression. Nicolopoulos et al. [13] were able to show that the relatively greater stature in a group of 143 girls with adolescent idiopathic scoliosis was due to changes in the pelvis and lower limbs. Although pelvic height was disproportionately increased in AIS, measures were not made directly on the pelvis. They estimated pelvic height by subtracting the subischial height from the mean of the subjects’ total leg lengths and where subischial height was calculated by subtracting the standing from sitting the height. Our findings do not support this observation. This can be explained in part by the fact that our classification is based on scoliotic severity rather than age (11–15 years, Nicolopoulos et al. [13]) and all their five groups corresponded to our severe scoliotic group.
Mahaudens et al. [7] showed that iliac crests were thicker in AIS than in control subjects, but there was no statistical difference in pelvic orientation. Our results are in agreement with their conclusion that geometry alterations occur without changes in pelvic 3D orientation. Gum et al. [10] also confirmed abnormal pelvic geometry in AIS. Their study which involved 239 AIS patients with average size Cobb angles of 61° had significant transverse plane pelvic left/right ratios. Although these results are not reported here, we noted right and left pelvic asymmetries even in able-bodied girls and even more so in the severe scoliotic group.
Burwell et al. [11] proposed a general theory of AIS etiology where a pelvic rotation-inducing system (dinner plate) transfers this rotation to the spine (flagpole). Our study is the first to report that there is very little pelvic distortion between able-bodied girls and girls with moderate scoliosis. Pelvic distortion essentially was noted between the severe scoliotic group and the others. It appears that the growth of right iliac wing crest increased more rapidly in the severe scoliosis group though the girls were essentially of the same age, height and mass as the other groups. This would result in a smaller mass moment of inertia about the vertical axis of the left pelvis compared to its right side. We postulate that an asymmetrical bone growth could create horizontal right hand torsion of the pelvis and it is part of the pelvic rotation-inducing system described by Burwell et al. [11].
Horizontal standing balance strategy was first reported by Dalleau et al. [27] using the free moment or the moment acting about the vertical axis and later confirmed later by Beaulieu et al. [28] when subjects stood on a single limb. This horizontal strategy adopted only by the untreated scoliotic girls was attributed to morphological changes due to the deformed spine and trunk as well as sensory and motor deficits in AIS. It could also be the result of a backward shift in distance between the scoliotic trunk’s center of mass to that of the whole body, which led to an increased medio-lateral imbalance in order to realign frontal plane balance [29]. Furthermore, it could explain in part why Bruyneel et al. [30] observed an increased asymmetry and variability in the AIS group, compared to the control group, during gait initiation in whatever the stepping direction was. We further propose that this rearward shift in the center of mass of the trunk is the result of an asymmetrical pelvic growth.
The second objective was to verify if the iliac spine morphological parameters are associated with Cobb angle in the scoliotic groups. Legaye et al. [5] reported significant correlations between kyphosis and lordosis and the sacropelvic morphologic parameters given by the pelvic incidence, sacral slope, and pelvic tilting in adults with severe scoliosis. Mac-Thiong et al. [4] found similar relationships in AIS with severe scoliosis. A number of articles relate trunk rotation with the Cobb angle (Goldberg et al. [2] and Amendt et al. [31]) and shoulder cosmesis (Qiu et al. [32]), but none reported any correlation with pelvic geometry. Gum et al. [10] found only a suggestive correlation of 0.3259 (p = 0.0736) between the largest Cobb angle and left/right pelvic width ratios for the AIS Lenke 1A1 thoracic sub-group. According to Phan et al. [33], few computer applications are routinely used in clinical applications to assist in the evaluation and treatment of adolescent idiopathic scoliosis because of huge amounts of clinical and geometrical data that need to be taken into consideration. The model reviewed in their survey focused primarily on the spine geometry. Since our study has shown several good correlations between the pelvic right side width and left side depth with the Cobb angle, these predictive models could be simplified and their performance accrued.
Our correlations in the sagittal plane were not significant and did not exceed 0.17 implying the implication of the transverse plane only. These results support the Burwell et al. [11] theory on a pelvic rotation-inducing system and our assumption of an altered right side pelvic growth as a predisposition to scoliosis progression. Conversely, pelvic deformity could represent a compensatory adaptation to increase spinal deformity. Nonetheless, altered transverse pelvic morphology could modify the loads acting on the spine and influence the progression of AIS as suggested by Mac-Thiong et al. [4].
This study is constrained as any cross-section study where measures are taken at a set instance. Therefore, we cannot ascertain the progression of AIS to altered pelvic growth over time. No definite conclusion can be drawn about the causal relationship between the Cobb angle and abnormal pelvic morphology. Only a longitudinal study involving both able-bodied and scoliotic girls is necessary to confirm the influence of the pelvic morphological changes on the Cobb angle. Even though these findings need to be corroborated by 3D imaging techniques, they provide a first attempt in quantifying pelvic distortion in AIS and indicate some reference parameters to be further investigated.
In conclusion, able-bodied girls and girls with moderate and severe scoliosis presented similar pelvic orientations when using S1 and eight iliac spine anatomical landmarks. Differences in iliac spine geometries were observed between scoliotic girls with a severe spinal deformity and those with a moderate Cobb angle or no scoliosis. These principally occurred in the transverse plane and suggest an altered bone growth in AIS. Girls with a severe scoliosis have a larger left pelvis depth and a wider right pelvis while no statistical difference was observed in pelvis height dimensions. Transverse plane pelvic geometry was correlated with Cobb angles. These observations support the assumption that a horizontal asymmetrical bone growth could result in pelvic torsion leading to right thoracic spinal deformity. The prognosis of AIS is difficult to make at the time of the diagnosis since spinal deformity can progress, stabilize or regress over time. A clinical significance regarding the changes in pelvic geometry could be used in addition to other clinical observations as a risk factor in the progression of scoliosis. Understanding the characteristics of pelvic morphology and spinal compensations that occur in AIS patients may help to improve their care.
Acknowledgments
Partial funding for this project was obtained from Natural Science and Engineering Council of Canada (NSERC). The authors wish to express their gratitude to Jaisuy Bourrai for his technical assistance.
Conflict of interest
None.
References
- 1.Stokes IAF. Three-dimensional terminology of spinal deformity: a report presented to the Scoliosis Research Society by the Scoliosis Research Society Working Group on 3-D terminology of spinal deformity. Spine. 1994;19:236–248. doi: 10.1097/00007632-199401001-00020. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg CJ, Kaliszer M, Moore DP, et al. Surface topography, Cobb angles, and cosmetic change in scoliosis. Spine. 2001;26:E55–E63. doi: 10.1097/00007632-200102150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Nault ML, Allard P, Hinse S, et al. Relationships between standing stability and body posture parameters in adolescent idiopathic scoliosis. Spine. 2002;27:1911–1917. doi: 10.1097/00007632-200209010-00018. [DOI] [PubMed] [Google Scholar]
- 4.Mac-Thiong J-M, Labelle H, Charlesbois M, et al. Sagittal plane analysis of the spine and pelvis in adolescent idiopathic scoliosis according to the coronal curve type. Spine. 2003;13:1404–1409. doi: 10.1097/01.BRS.0000067118.60199.D1. [DOI] [PubMed] [Google Scholar]
- 5.Legaye J, Duval-Beaupere G, Hecquet J, et al. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7:99–103. doi: 10.1007/s005860050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saji M, Upadhyay S, Leong J. Increased femoral neck–shaft angles in adolescent idiopathic scoliosis. Spine. 1995;20:303–311. doi: 10.1097/00007632-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Mahaudens P, Thonnard J-L, Detrembleur C. Influence of structural pelvic disorders during standing and walking in adolescents with idiopathic scoliosis. Spine J. 2005;5:427–433. doi: 10.1016/j.spinee.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Zabjek KF, Leroux MA, Coillard C, et al. Evaluation of segmental postural characteristics during quiet standing in control and idiopathic scoliosis patients. Gait Posture. 2005;20:483–490. doi: 10.1016/j.clinbiomech.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Pasha S, Sangole AP, Aubin C-É, et al. Characterizing pelvis dynamics in adolescent with idiopathic scoliosis. Spine. 2010;35:E820–E826. doi: 10.1097/BRS.0b013e3181e6856d. [DOI] [PubMed] [Google Scholar]
- 10.Gum JL, Asher MA, Burton DC, et al. Transverse plane pelvic rotation in adolescent idiopathic scoliosis: primary or compensatory? Eur Spine J. 2007;16:1579–1586. doi: 10.1007/s00586-007-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burwell RG, Cole AA, Cook TA, et al. Pathogenesis of idiopathic scoliosis: the Nottingham Concept. Acta Orthop Belg. 1992;58(Suppl.1):33–58. [PubMed] [Google Scholar]
- 12.Burwell RG, Freeman BJ, Dangerfield PH, et al. Etiologic theories of idiopathic scoliosis: neurodevelopmental concepts of maturational delay of the CNS body schema (“body-in-the-brain”) Stud Health Technol Inform. 2006;123:72–79. [PubMed] [Google Scholar]
- 13.Nicolopoulos KS, Burwell RG, Webb JK. Stature and its component in adolescent idiopathic scoliosis: cephalocaudal disproportion in the trunk of girls. J Bone Jt Surg. 1985;67B:594–601. doi: 10.1302/0301-620X.67B4.4030857. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc R, Labelle H, Rivard C-H, et al. Relation between adolescent idiopathic scoliosis and morphologic somatotypes. Spine. 1997;22:2532–2536. doi: 10.1097/00007632-199711010-00013. [DOI] [PubMed] [Google Scholar]
- 15.Lowe TG, Edgar M, Margulies JY, et al. Etiology of idiopathic scoliosis: current trends in research. J Bone Jt Surg Am. 2000;82:1157–1168. doi: 10.2106/00004623-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Dickson RA, Sevitt EA. Growth and idiopathic scoliosis: a longitudinal cohort study. J Bone Jt Surg Br. 1982;64:385. [Google Scholar]
- 17.Lonstein JE, Carlson M. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Jt Surg. 1984;66:1061–1071. [PubMed] [Google Scholar]
- 18.Bunnell WP. The natural history of idiopathic scoliosis before skeletal maturity. Spine. 1986;11:773–776. doi: 10.1097/00007632-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 19.McIlroy WE, Maki BE. Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clin Biomech. 1997;12:66–70. doi: 10.1016/S0268-0033(96)00040-X. [DOI] [PubMed] [Google Scholar]
- 20.Bellefleur C, Labelle H, Dansereau J, et al. Évaluation tridimensionnelle per-opératoire de la procédure Cotrel-Dubousset pour le traitement de la scoliose idiopathique. Ann Chir. 1994;48:723–730. [PubMed] [Google Scholar]
- 21.Dao TV, Labelle H, LeBlanc R. Intra-observer variability of measurement of posture with three-dimensional digitization. Ann Chir. 1997;51:848–853. [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences. Mahwah: Lawrence Erlbaum; 1988. [Google Scholar]
- 23.Holland BS, Copenhaver M. Improved Bonferroni-type multiple testing procedures. Psychol Bull. 1988;104:145–149. doi: 10.1037/0033-2909.104.1.145. [DOI] [Google Scholar]
- 24.Chockalingam N, Danderfield PH, Giakas G, et al. Study of marker placement in the back for opto-electronic motion analysis. Stud Health Technol Inform. 2002;88:105–109. [PubMed] [Google Scholar]
- 25.Burwell RG, Dangerfield PH, Vernon CL. Bone asymmetry and joint laxity in the upper limbs of children with adolescent idiopathic scoliosis. Ann R Coll Surg Eng. 1981;63:209. [Google Scholar]
- 26.Mehta MH (1981) Moire topography and associated asymmetries in scoliosis. In: Moreland MS, Pope MH, Armstrong GWD (eds) Moire fringe topography and spinal deformity: proceedings of an international symposium. Pergamon Press, New York, 186–189
- 27.Dalleau G, Allard M, Beaulieu M, et al. Free moment contribution to quiet standing in able-bodied and scoliotic girls. Eur Spine J. 2007;16:1593–1599. doi: 10.1007/s00586-007-0404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaulieu M, Allard P, Simoneau M, et al. Relationship between axial rotation and center of pressure displacements in single and double leg upright stance. J Phys Med Rehabil. 2010;89:809–816. doi: 10.1097/PHM.0b013e3181f1b4af. [DOI] [PubMed] [Google Scholar]
- 29.Dalleau G, Damavandi M, Leroyer P, Verkindt C, Rivard CH, Allard P. Horizontal body and trunk center of mass offset and standing balance in scoliotic girls. Eur Spine J. 2011;20(1):123–128. doi: 10.1007/s00586-010-1554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruyneel AV, Chavet P, Bollini G, Allard P, Berton E, Mesure S. Dynamical asymmetries in idiopathic scoliosis during forward and lateral initiation step. Eur Spine J. 2009;18(2):188–195. doi: 10.1007/s00586-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amendt LE, Ause-Ellias KL, Lundahl-Eybers J, et al. Validity and reliability testing of the scoliometer. Phys Ther. 1990;70:108–117. doi: 10.1093/ptj/70.2.108. [DOI] [PubMed] [Google Scholar]
- 32.Qiu XS, Ma WW, Li WG, Wang B, Yu Y, Zhu ZZ, Qian BP, Zhu F, Sun X, Ng BK, Cheng JC, Qiu Y. Discrepancy between radiographic shoulder balance and cosmetic shoulder balance in adolescent idiopathic scoliosis patients with double thoracic curve. Eur Spine J. 2009;18(1):45–51. doi: 10.1007/s00586-008-0833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan P, Mezghani N, Aubin CE, Guise JA, Labelle H. Computer algorithms and applications used to assist the evaluation and treatment of adolescent idiopathic scoliosis: a review of published articles 2000–2009. Eur Spine J. 2011;20(7):1058–1068. doi: 10.1007/s00586-011-1699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]