Abstract
Introduction
The conventional open pedicle screw fusion (PSF) requires an extensive detachment of the paraspinal muscle from the posterior aspect of the lumbar spine, which can cause muscle injury and subsequently lead to “approach-related morbidity”. The spinous process-splitting (SPS) approach for decompression, unilateral laminotomy for bilateral decompression, and the Wiltse approach for pedicle screw insertion are considered to be less invasive to the paraspinal musculature. We investigated whether SPS open PSF combined with the abovementioned techniques attenuates the paraspinal muscle damage and yields favorable clinical results, including alleviation in the low back discomfort, in comparison to the conventional open PSF.
Methods
We studied 53 patients who underwent single-level PSF for the treatment of degenerative spondylolisthesis (27 patients underwent SPS open PSF and the other 26 underwent the conventional open PSF). The clinical outcomes were assessed using the Japanese Orthopedic Association (JOA) score, the Roland–Morris disability questionnaire (RDQ), and the visual analog scale (VAS) for low back pain and low back discomfort (heavy feeling or stiffness). Postoperative multifidus (MF) atrophy was evaluated using MRI. Follow-up examinations were performed at 1 and 3 years after the surgery.
Results
Although there was no significant difference in the JOA and RDQ score between the two groups, the VAS score for low back pain and discomfort after the surgery were significantly lower in the SPS open PSF group than in the conventional open PSF group. The extent of MF atrophy after SPS open PSF was reduced more significantly than after the conventional open PSF during the follow-up. The MF atrophy ratio was found to correlate with low back discomfort at the 1-year follow-up examination.
Conclusion
In conclusion, SPS open PSF was less damaging to the paraspinal muscle than the conventional open PSF and had a significant clinical effect, reducing low back discomfort over 1 year after the surgery.
Keywords: Posterior lumbar fusion, Multifidus muscle, Wiltse approach, Minimally invasive, Conventionally open
Introduction
Lumbar spinal fusion using pedicle screws (PSs) is a common procedure for the management of various of spinal disorders requiring spinal stabilization. However, the approach-related morbidity, that results from iatrogenic soft-tissue injury, which includes paraspinal muscle injury, has become a major problem [1, 2]. Several procedures, including minimally invasive techniques, have been developed as potential solutions to this problem [3–6]. Lumbar spinous process-splitting laminectomy, in which the muscular attachment is left intact, has been reported to decrease the degree of postoperative paraspinal muscular atrophy [7]. Moreover, unilateral laminotomy for bilateral decompression of lumbar spinal stenosis is expected to preserve the paraspinal muscles on the contralateral side of the approach [8]. The Wiltse et al. [9] approach for insertion of pedicle screws (PSs) is also considered a less invasive technique that helps preserve paraspinal musculature [10]. A major advantage of all these techniques is the reduction in iatrogenic paraspinal muscular injury, because damage to these muscles, can lead to denervation and atrophy and thus increased the risk of “fusion disease” [1, 11]. There are, however, still relatively few studies reporting the clinical effects of paraspinal preservation in the late postoperative stages [12].
We investigated whether spinous process-splitting (SPS) open pedicle screw fusion (PSF) using the Wiltse et al. approach for PS insertion combined with unilateral laminotomy for bilateral decompression is superior to conventional open PSF for a single-level instrumented posterior lumbar decompression and fusion. The two procedures were compared by investigating the perioperative data and the degree of paraspinal muscle injury as assessed by the level of atrophy in the multifidus (MF) muscle and an increase in T2-signal intensity on magnetic resonance imaging (MRI). Postoperative serum creatinine kinase (CK) levels were also compared for both procedures. Furthermore, we evaluated the efficacy of SPS open PSF on clinical outcomes using the visual analog scale (VAS) for low back pain and discomfort over 1 year after surgery.
Subjects and methods
Patient population
In all, 65 patients with no history of lumbar surgery underwent a single-level posterolateral lumbar fusion (PLF) or transforaminal lumbar interbody fusion (TLIF) for the treatment of degenerative spondylolisthesis between May 2006 and August 2007. Thirty patients were treated with SPS open PSF, and 35 patients were treated with the conventional open PSF. Patients who visited our hospital initially on Monday and Tuesday were assigned to the conventional open PSF group, and patients on Wednesday and Thursday were assigned to SPS open PSF group. Patients were not informed to be involved in the study and were thus blinded to the different surgical procedures. The Ethical Committee of our institution approved the study. Of all the patients, 53 patients were followed up for more than 3 years and were examined. Of the 53 patients, 26 were men and 27 were women with a mean age of 63.5 years (range 44–79 years). The mean duration of follow-up was 3 years and 2 months (range 3–4.1 years). The fusion level was L3/4 in 3 patients, L4/5 in 50 patients. Thirty-eight patients underwent PLF and 15 underwent TLIF. The decision regarding the use of PLF or TLIF was based on patient presentation; patients presenting with greater than a 5° segmental kyphosis or foraminal stenosis at the slip segment underwent TLIF, and the remainder underwent PLF. Twenty-seven patients underwent SPS open PSF and 26 patients underwent conventional open PSF. No significant differences in age, gender, the level fused, or the type of fusion was observed between the two groups (Table 1).
Table 1.
Summary of clinical and demographic characteristics of the study population
| SPS open PSF group (N = 27) | Conventional open PSF group (N = 26) | P value | |
|---|---|---|---|
| Mean age (year) (range) | 64.5 (44–79) | 62.4 (45–76) | NS |
| Gender (female/male) | 16/11 | 11/15 | NS |
| Level fused | |||
| L3/4 | 2 | 1 | NS |
| L4/5 | 25 | 25 | |
| Type of fusion | |||
| PLF | 19 | 19 | NS |
| TLIF | 8 | 7 | |
Values represent number of patients unless otherwise indicated
PLF posterolateral lumbar fusion, TLIF transforaminal lumbar interbody fusion, NS not significant (p > 0.05)
Surgical procedures
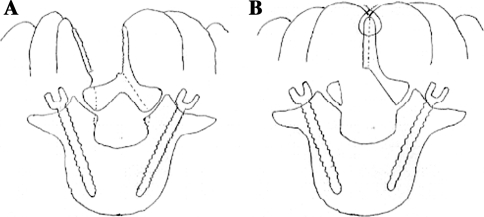
SPS open PSF. In the SPS open PSF procedure, a 6-cm midline skin incision was made, followed by bilateral paramedian longitudinal fascial incisions. The PSs were inserted using the free-hand technique, wherein the transverse process, which is visible because of blunt dissection between the MF and longissimus muscle, can be used as an orientation landmark. After the longitudinal splitting of the cranial and caudal spinous processes with a bone saw, the unilateral bases of the split spinous processes were broken and retracted to ensure minimal exposure of the lamina only on the side of the approach. The attachment of the MF muscle to the spinous process was left intact. Bilateral decompression was achieved through a unilateral laminotomy performed under a microscope, without the use of a tubular retractor. Facet joint fusion rather than PLF was predominantly performed as follows: the facet joint was exposed at the fusion level using the Wiltse approach, and the local bone was grafted into the gutters made by drilling the bilateral facet joint spaces. For a TLIF, an interbody cage filled with the local bone was inserted into the disc space after total facetectomy and subsequent complete discectomy. Split and floating spinous processes, with preserved attachment to the MF muscles, were placed in their original positions after the rod had been connected to the screw heads (Fig. 1).
Fig. 1.
a Paraspinal muscles are detached from the lamina while leaving the muscular attachment to the split spinous processes undisturbed, and then bilateral decompression is performed through a unilateral laminotomy. b After bilateral decompression, the split spinous processes are placed in their original positions
Conventional open PSF. In conventional open PSF, a midline incision approximately 12 cm long was made. The PSs were inserted after paraspinal muscle dissection, from the posterior aspect of the lumbar spine region. Decompression was performed by partial laminectomy. A local bone graft was used for the PLF and an interbody cage filled with local bone was used for the TLIF.
A rod was placed in situ in the PLF and was compressed between screw heads in the TLIF. Fluoroscopic imaging was not used in either group.
Assessment of outcomes
The Japanese Orthopedic Association (JOA) score, the Roland–Morris disability questionnaire (RDQ), and VAS (10 points) were used to assess outcomes. For the assessment of low back discomfort such as a heavy feeling or stiffness in the low back, VAS scores were used. Surgical time, intraoperative blood loss, and the ratio of the serum creatinine kinase (CK) level on the first postoperative day compared to preoperative level were measured. Approach-related paraspinal muscle damage was evaluated by assessing MF atrophy and changes in T2 signal in the MF using MRI. The cross-sectional area of the MF (MF-CSA) was measured bilaterally using a T2-axial image and Scion Image software (Scion Corp., Frederick, MD, USA). The total MF-CSA was calculated as the sum of the area on each side. The level of MF atrophy after the surgery was assessed by the ratio of postoperative MF-CSA to preoperative MF-CSA (atrophy ratio). Signal intensity of the MF muscle, corrected on the basis of the signal intensity of the psoas muscle in the same axial image, was measured quantitatively using T2-axial images and the Scion Image software. Lower scores indicated higher signal intensities. The signal change in the MF was quantified as the ratio of the postoperative T2-signal intensity of the MF to its preoperative signal intensity. MF-CSA and T2-signal intensity of the MF at the intervertebral level of the fused segment and adjacent segments (where the interference from the pedicle screw would be less), were determined three times, and an average was obtained. The MF atrophy ratio for a patient was determined as the mean value of the atrophy ratios from the fused and adjacent segments. A 5-mm-thick T2-weighted axial image was obtained using a 1.5 T MRI. Clinical and MRI evaluations were performed before the surgery and at 1 and 3 years after the surgery. Fusion was defined as less than 5° movement on lateral flexion and extension radiographs, the presence of a bone bridge between the transverse processes or continuous bone formation in the facet joint on computed tomography scans, and the presence of continuous trabecular bone formation through the cage.
Statistical analysis
Clinical parameters of the two groups were compared using the Fisher exact test and a Mann–Whitney U test. Correlation between the MF atrophy ratio of the patients and other clinical parameters was analyzed using Spearman’s rank correlation coefficient. A 2-factor factorial analysis of variance was used for the statistical analysis of the differences in MF atrophy ratio and the ratio of the signal intensity of the MF between the two groups. Tukey–Kramer’s test was used for post hoc pairwise comparisons. Statistical analysis was performed using the JMP program (version 8; SAS Institute Japan, Tokyo, Japan). P values <0.05 were considered statistically significant.
Results
Clinical results
The differences in the JOA scores, RDQ scores, or VAS scores for low back pain, and low back discomfort between the two groups before the surgery were insignificant (Table 2). The average surgical time in the SPS open PSF group was significantly longer than that in the conventional open PSF group (145.5 ± 31.7 vs. 117.7 ± 33.6 min, p < 0.01). The average intraoperative blood loss was comparable between the two groups (143.5 ± 53.3 vs. 141.5 ± 76.0 g). No complications such as wound infections and dural tears were observed, and no additional surgeries were needed in the two groups. The average length of hospital stay in the SPS open PSF group (23.0 days) was comparable to that in the conventional open PSF group (23.5 days). There was no difference in analgesics use immediately after the surgery and at follow-up between the two groups. Regarding the postoperative clinical results, no significant differences in the average JOA score, the improvement rate, or the RDQ score were observed between the two groups. However, 1 year after the surgery, the average VAS score for low back pain in the SPS open PSF group was significantly lower than that in the conventional open PSF group (1.5 ± 1.6 and 2.8 ± 2.3, respectively, p < 0.05) (Table 3). Moreover, a significant difference in the average VAS score for discomfort in the low back at 1 and 3 years after the surgery was observed between the two groups (1.4 ± 1.5 vs. 3.3 ± 2.1, p < 0.01, and 2.2 ± 2.0 vs. 3.7 ± 2.4, p < 0.05, respectively) (Table 3). Fusion rate was 85.2% in the SPS open PSF group and 88.5% in the conventional group.
Table 2.
Preoperative clinical parameters of the study population
| SPS open PSF group (N = 27) | Conventional open PSF group (N = 26) | P value | |
|---|---|---|---|
| Preoperative JOA score (points) | 13.5 ± 4.2 | 14.0 ± 4.9 | NS |
| Preoperative RDQ score (points) | 13.4 ± 4.8 | 12.8 ± 5.3 | NS |
| Preoperative VAS for low back pain (points) | 6.9 ± 2.1 | 5.9 ± 2.2 | NS |
| Preoperative VAS for discomfort in the low back (points) | 5.6 ± 2.1 | 5.2 ± 2.0 | NS |
Values are mean ± standard deviation
JOA Japanese Orthopedic Association, RDQ Roland–Morris disability questionnaire, VAS visual analog scale
NS not significant (p > 0.05)
Table 3.
Comparison of clinical result
| SPS open PSF group (N = 27) | Conventional open PSF group (N = 26) | P value | |
|---|---|---|---|
| Surgical time (min) | 145.5 ± 31.7 | 117.7 ± 33.6 | <0.01 |
| Intraoperative blood loss (g) | 143.5 ± 53.3 | 141.5 ± 76.0 | NS |
| Postoperative JOA score (points) | |||
| 1 year | 25.6 ± 3.2 | 25.7 ± 2.9 | NS |
| 3 year | 24.4 ± 4.1 | 25.2 ± 3.1 | NS |
| Postoperative improvement rate (%)a | |||
| 1 year | 78.1 ± 19.0 | 76.5 ± 23.6 | NS |
| 3 year | 73.2 ± 21.8 | 71.6 ± 25.0 | NS |
| Postoperative RDQ Score (points) | |||
| 1 year | 5.0 ± 5.3 | 6.0 ± 5.4 | NS |
| 3 year | 6.1 ± 5.9 | 7.2 ± 6.4 | NS |
| Postoperative VAS for low back pain (points) | |||
| 1 year | 1.5 ± 1.6 | 2.8 ± 2.3 | <0.05 |
| 3 year | 2.2 ± 2.0 | 2.7 ± 2.6 | NS |
| Postoperative VAS for discomfort in the low back (points) | |||
| 1 year | 1.4 ± 1.5 | 3.3 ± 2.1 | <0.01 |
| 3 year | 2.2 ± 2.0 | 3.7 ± 2.4 | <0.05 |
Values are mean ± standard deviation
JOA Japanese Orthopedic Association, RDQ Roland–Morris disability questionnaire, VAS visual analog scale
aImprovement rate (%) = (postoperative JOA score − preoperative JOA score) × 100/(29 − preoperative JOA score)
NS not significant (p > 0.05)
MRI evaluation (muscle invasiveness)
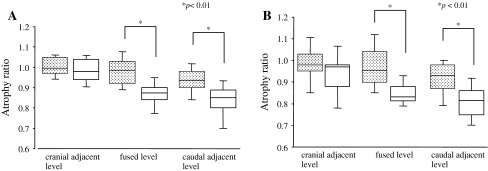
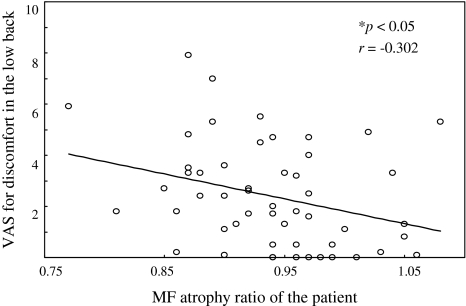
The average MF atrophy ratios at the fusion level in the SPS open PSF group were significantly greater than those in the conventional open PSF group at 1 and 3 years after the surgery (0.98 ± 0.08 vs. 0.87 ± 0.07 and 0.96 ± 0.11 vs. 0.84 ± 0.07, respectively, p < 0.01) (Figs. 2, 4). Moreover, significant differences were observed in the average MF atrophy ratio at the caudal adjacent level at both the 1- and 3-year postoperative follow-up between the two groups (0.93 ± 0.08 vs. 0.84 ± 0.09 and 0.92 ± 0.08 vs. 0.80 ± 0.09, respectively, p < 0.01) (Figs. 3, 4). However, at the cranial adjacent level, no significant differences were observed in the average MF atrophy ratio between the two groups, either at 1 year or 3 years after the surgery (Figs. 3, 4; Table 4). In all the patients, correlation between the MF atrophy ratio of the patient and VAS scores for low back pain and low back discomfort was analyzed. At the 1-year postoperative follow-up, the MF atrophy ratio of the patients significantly correlated with the VAS score for discomfort in the low back (p < 0.05) (Fig. 5). A lower level of MF atrophy indicated a lower score for low back discomfort. Regarding changes in the signal intensity in MF muscles, the average ratio in both the groups decreased 1 year after the surgery and increased 3 years after the surgery, compared to the preoperative level. However, the signal intensity was comparable between the two groups at the fusion level and at the caudal and cranial adjacent levels during the follow-up period (Table 5). Serum CK level is reported to reflect the level of muscle damage after lumbar surgery. We therefore examined the ratio between the serum CK level on the first postoperative day and the preoperative level. We did not find a significant difference between the two groups (6.6 ± 4.6 in the SPS open PSF group and 5.8 ± 5.6 in the conventional open group, p > 0.05).
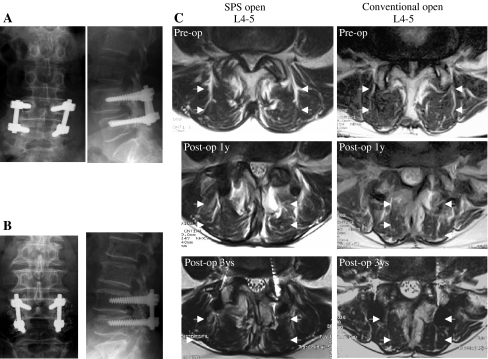
Fig. 2.
Representative plain radiographs of the lumbar spine after SPS open PSF with PLF (a) and conventional open PSF with PLF (b) for L4 degenerative spondylolisthesis. c T2-axial images at the L4-5 fused level in the same patients as in (a) and in (b) in the two groups. Note the significant multifidus muscle atrophy on the follow-up images of the patient in the conventional open PSF group
Fig. 4.
Postoperative MF atrophy. Box plots showing atrophy ratio of MF-CSA at the 1-year (a) and 3-year follow-up (b) at each level in the two groups. Dark box plots indicate the SPS open PSF group, and open box plots indicate the conventional open PSF group. The horizontal line in each box shows the median value. The box spans the 25th to the 75th percentiles and the error bars denote the 10th and 90th percentiles. Asterisks indicate statistical significance between groups (p < 0.01)
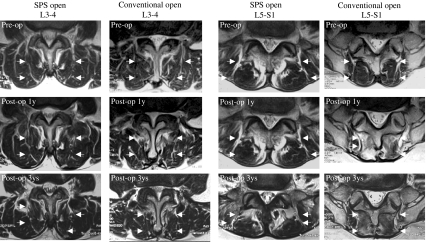
Fig. 3.
T2-axial images at the cranial adjacent site (L3–4) and the caudal adjacent site (L5-S1) of the L4–5 fused level in the same patients as presented in Fig. 2. Note the significant multifidus muscle atrophy at L5-S1 on the follow-up images of the patient in the conventional open PSF group
Table 4.
Comparison of the ratio of MF atrophy
| SPS open PSF group | Conventional open PSF group | P value | |
|---|---|---|---|
| (N = 27) | (N = 26) | ||
| Fused level | |||
| 1 year | 0.98 ± 0.08 | 0.87 ± 0.07 | <0.01 |
| 3 year | 0.96 ± 0.11 | 0.84 ± 0.07 | <0.01 |
| Cranial adjacent level | |||
| 1 year | 1.01 ± 0.06 | 0.99 ± 0.08 | NS |
| 3 year | 0.98 ± 0.09 | 0.94 ± 0.12 | NS |
| Caudal adjacent level | |||
| 1 year | 0.93 ± 0.08 | 0.84 ± 0.09 | <0.01 |
| 3 year | 0.92 ± 0.08 | 0.80 ± 0.09 | <0.01 |
Values are mean ± standard deviation, NS not significant (p > 0.05)
Fig. 5.
Scatterplots showing the association between VAS score for discomfort in the low back and MF atrophy ratio of the patient at the 1-year postoperative follow-up
Table 5.
Comparison of the ratio of T2-signal intensity
| SPS open PSF group (N = 27) | Conventional open PSF group (N = 26) | P value | |
|---|---|---|---|
| Fused level | |||
| 1 year | 0.94 ± 0.17 | 0.96 ± 0.08 | NS |
| 3 year | 1.27 ± 0.23 | 1.30 ± 0.21 | NS |
| Cranial adjacent level | |||
| 1 year | 0.97 ± 0.16 | 0.99 ± 0.12 | NS |
| 3 year | 1.25 ± 0.25 | 1.20 ± 0.15 | NS |
| Caudal adjacent level | |||
| 1 year | 0.88 ± 0.13 | 0.82 ± 0.13 | NS |
| 3 year | 1.17 ± 0.23 | 1.10 ± 0.29 | NS |
Values are mean ± standard deviation, NS not significant (p > 0.05)
Discussion
Several surgical techniques to reduce the detachment of paraspinal muscles from the posterior aspect of the lumbar spine are available [3, 12]. Avoidance of paraspinal muscular detachment from the posterior aspect of the lumbar spine could contribute to a reduction in paraspinal muscular atrophy. A clinical study conducted by Watanabe et al. [7] compared spinous process-longitudinal splitting laminectomy (wherein the muscular attachment was left intact) with conventional laminectomy. They concluded that the extent of paraspinal muscular atrophy was remarkably less in the former procedure. In addition, the interposition of the split spinous processes may act as a mechanical buffer for reducing paraspinal muscle retraction pressure. Microsurgical bilateral decompression via a unilateral laminotomy also does not require paraspinal muscle detachment from the posterior aspect of the contralateral side to the approach side [8]. Therefore, spinal canal decompression through unilateral laminotomy, after longitudinal splitting of the spinous processes while leaving the muscular attachments undisturbed, combined with the Wiltse et al. [9] approach for pedicle screw insertion, is expected to reduce the muscle damage, compared to the conventional open technique. By assessing the degree of MF atrophy, our current study showed that SPS open PSF was a less invasive technique to paraspinal musculature than the conventional open PSF.
An advantage of minimally invasive spine surgery (MIS) is that it reduces paraspinal muscular injury. Previous studies comparing MIS and conventional surgical techniques showed that MIS caused less damage to the paraspinal muscle, as determined by assessing the degree of atrophy and T2-signal intensity [6, 10, 12–14]. However, some controversy remains as to whether the advantages of MIS are reflected by improved clinical outcomes, as assessed using measures such as VAS scores for low back pain, the Oswestry disability index, or the JOA score [6, 10, 12–14].
Pathologies of the paraspinal musculature, such as atrophy or denervation, arising from iatrogenic injury are considered a major cause of “approach-related morbidity.” The problems caused include long-lasting low back pain, decreased trunk muscle strength after lumbar surgery and “failed back syndrome” or “fusion disease” [3, 11, 15, 16]. The MF muscle is an important deeply located back extensor muscle involved in the stabilization of the lumbar spine. Weakening of this muscle could lead to mechanical strain and thereby increase the risk of low back pain [17]. The current study showed that the degree of MF atrophy, in addition to the VAS scores for low back pain, was significantly greater in the conventional open PSF group than in the SPS open PSF group. This finding indicates that MF atrophy significantly influences postoperative low back pain.
In addition to a strong association between low back pain and paraspinal muscle atrophy, a significant correlation exists between low back pain and back muscle fatigue, as detected by electromyography performed in a study by Dedering et al. [18, 19] and Demoulin et al. [20]. These observations suggest a positive relationship between paraspinal muscle atrophy and muscular fatigue. They also suggest a close relationship between the subjective and objective assessment of muscle fatigue, and that muscle fatigue reflects the subjective “physical discomfort” [18, 19]. Thus, iatrogenic injury due to lumbar surgery results in paraspinal muscle atrophy, which leads to low back fatigue and discomfort. Hence, we examined whether prevention of paraspinal muscular atrophy indeed decreases low back discomfort such as a heavy feeling or stiffness as assessed using the VAS score for low back discomfort. The current study showed that VAS scores for low back discomfort, as well as the degree of MF atrophy, were significantly lower in the SPS open PSF group than in the conventional open PSF group. Moreover, the degree of MF atrophy was significantly correlated with the level of low back discomfort as observed 1 year after the surgery. These results suggest that a reduction in paraspinal muscle injury contributed to the decrease in low back discomfort. Interestingly, these differences in the degree of MF atrophy and VAS scores for low back discomfort were evident not only 1 year after the surgery but also 3 years after the surgery. Paraspinal muscle atrophy is reported to be counteracted by postoperative exercise of the back muscles. However, without special muscle training, recovery seems impossible [21, 22]. Thus, because the adverse effects caused by iatrogenic muscle damage may potentially persist for several years after the surgery, the use of techniques that cause less damage to the paraspinal muscles should be considered when performing lumbar surgery.
The current study did not find any differences in the JOA improvement rate or the RDQ score between the two groups. This may be attributed to the fact that the JOA scoring system is primarily concerned with the assessment of objective clinical outcomes such as motor score and gait ability and is therefore not sufficiently sensitive to detect symptoms associated with iatrogenic muscle atrophy. The RDQ, however, assesses the functional disability associated with low back pain. The RDQ scores improved significantly after the surgery in both the two groups. This scoring system is also not designed for the assessment of subjective low back discomfort.
High T2-signal intensity is reported to be associated with pathological changes such as edema, denervation, and fatty infiltration in paraspinal muscles [13, 23]. In this study, we observed high-signal intensity 1 year after the surgery. However, 3 years after the surgery, the high T2-signal intensity of the MF had almost returned to the preoperative level. Previous studies have shown that the reduction of edema up to approximately 1 year after the surgery and reinnervation of muscles that were denervated during the surgery is associated with recovery of signal intensity as shown by increased T2-signal intensity [13, 23]. Therefore, the detection of high T2-signal intensity in paraspinal muscles 1 year after the surgery is valuable as an indicator of paraspinal muscular damage. The evaluation of T2-signal intensity at 3 years after the surgery, however, may be less valuable. In several previous studies comparing postoperative MF T2-signal intensity between MIS and conventional approaches, MIS was fund to be less invasive to paraspinal musculature and associated with less T2-high-signal intensity [10, 14]. However, in the current study, changes in the T2-signal intensity in the MF did not differ between the two groups at either the fused or adjacent levels. The lack of difference between the two groups may be partially attributed to the significantly longer surgery time required for SPS open PSF than for conventional open PSF. This may obscure the reduced invasiveness into the paraspinal muscles afforded by SPS open PSF. In these procedures, the medial rami of the dorsal ramus innervating the MF muscle will be inevitably damaged, because this branch traverses the point of pedicle screw insertion. Therefore, high T2-signal intensity associated with the denervation of the MF muscle caused by injury to this branch is commonly observed in pedicle screw procedures.
No difference in the degree of MF atrophy on the cranial side of the surgical level was found between the two groups. This could be related to the anatomical characteristics of the MF muscle. Each MF muscle consists of several bundles that originate from the spinous processes, spread caudolaterally, and then insert into the facet joints at caudal segments 2–5 and the iliac crest; therefore, the MF muscle bundles are abundant at the caudal levels. The medial branch of the dorsal ramus, which innervates the MF muscle, enters the muscle from its cranial side [24]. Therefore, morphological changes in this muscle, which results from iatrogenic denervation and ischemic damage caused by dissection and retraction, at its caudal side and the surgical level and relatively less at the cranial side are observed on MR images.
Serum CK level also reflects muscle damage [25]. In many previous studies comparing postoperative serum CK levels between MIS and traditional approaches, increases in serum CK level immediately after the surgery were found to be significantly lesser in MIS than in traditional approaches. Hence, MIS is probably less damaging to paraspinal muscles [10, 12, 14]. In the current study, however, no significant difference in the ratio of serum CK level on the first postoperative day to the preoperative level was observed between the two groups. Gejo et al. [16] performed a clinical study and concluded that paraspinal muscle injury was directly related to the muscle retraction time during the surgery. Therefore, the lack of difference between the two groups is attributable to longer surgery time, and therefore longer retraction times, when using SPS open PSF than when using conventional open PSF. This could obscure the effect of reduced invasiveness of SPS open PSF. However, the ratio of serum CK level in the SPS open PSF group was comparable to that in a minimally invasive procedure in another study [10].
This study has some limitations. The sample size was small and the follow-up period was not of a sufficient duration to allow conclusions to be drawn about the long-term clinical outcomes. In addition, there were differences between the surgeries performed. For example, both the fusion method and fusion level differed between the patients. A prospective, randomized study with a larger sample size and a longer follow-up period would be required to compare the effect of the differences in the level of muscular damage on the clinical outcome of a minimally or less invasive procedure versus a conventional open procedure.
Conclusion
SPS combined with preserved muscle attachment, unilateral laminotomy for bilateral decompression, and a Wiltse approach for PS insertion is a surgical procedure that combines techniques that are expected to attenuate the damage to the paraspinal muscles by reducing paraspinal muscular detachment and retraction pressure. Our current study showed that compared to the conventional open PSF, this surgical procedure resulted in a significantly less degree of MF atrophy, as observed on MRI, and good results with low back pain and discomfort over 1 year after the surgery. SPS open PSF is a feasible approach for the alleviation of “fusion disease” after lumbar decompression and fusion surgery, because paraspinal muscle atrophy can contribute to muscular fatigue, low back pain, and lumbar discomfort.
Conflict of interest
No grant or any other funding has been received.
References
- 1.Sihvonen T, Herno A, Paljarvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575–581. doi: 10.1097/00007632-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Taylor H, McGregor AH, Medhi-Zadeh S, Richards S, Kahn N, Zadeh JA, et al. The impact of self-retaining retractors on the paraspinal muscles during posterior spinal surgery. Spine. 2002;27:2758–2762. doi: 10.1097/00007632-200212150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Khoo LT, Palmer S, Laich DT, Laich DT, Fessler RG. Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery. 2002;51(suppl 2):166–181. [PubMed] [Google Scholar]
- 4.Logroscino CA, Proietti L, Pola E, Scaramuzzo L, Tamburrelli FC. A Minimally invasive posterior lumbar interbody fusion for degenerative lumbar spine instabilities. Eur Spine J. 2011;20(suppl 1):S41–S45. doi: 10.1007/s00586-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantelhardt SR, Martinez R, Baerwinkel S, Burger R, Giese A, Rohde V. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicles screw placement. Eur Spine J. 2011;20:860–868. doi: 10.1007/s00586-011-1729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park T, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32:537–543. doi: 10.1097/01.brs.0000256473.49791.f4. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K, Hosoya T, Shiraishi T, Matsumoto M, Chiba K, Toyama Y. Lumbar spinous process-splitting laminectomy for lumbar canal stenosis. J Nuerosurg Spine. 2005;3:405–408. doi: 10.3171/spi.2005.3.5.0405. [DOI] [PubMed] [Google Scholar]
- 8.Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59:1264–1270. doi: 10.1227/01.NEU.0000245616.32226.58. [DOI] [PubMed] [Google Scholar]
- 9.Wiltse LL, Bateman JG, Hutchinson RH, Nelson WE. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Jt Surg Am. 1968;50:919–926. [PubMed] [Google Scholar]
- 10.Tsutsumimoto T, Shimogata M, Ohta H, Misawa H. Mini-open versus conventional open posterior lumbar interbody fusion for the treatment of lumbar degenerative spondylolisthesis. Spine. 2009;34:1923–1928. doi: 10.1097/BRS.0b013e3181a9d28e. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery: 2. Histologic and histochemical analyses in humans. Spine. 1994;19:2598–2602. doi: 10.1097/00007632-199411001-00018. [DOI] [PubMed] [Google Scholar]
- 12.Shunwu F, Xing Z, Fengdong Z, Xianggian F. Minimally invasive transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases. Spine. 2010;35:1615–1620. doi: 10.1097/BRS.0b013e3181c70fe3. [DOI] [PubMed] [Google Scholar]
- 13.Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine. 2005;30:123–129. doi: 10.1097/01.brs.0000157172.00635.3a. [DOI] [PubMed] [Google Scholar]
- 14.Shunwu F, Zhijun H, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19:316–324. doi: 10.1007/s00586-009-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratanen J, Hurme M, Falck B, Alaranta H, Nykvist F, Lehto M, et al. The lumbar multifidus muscle five years after surgery for a lumbar intervertebral disc herniation. Spine. 1993;18:568–574. doi: 10.1097/00007632-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Gejo R, Matsui H, Kawaguchi Y, Ishihara H, Tsuji H. Spinal changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999;24:1023–1028. doi: 10.1097/00007632-199905150-00017. [DOI] [PubMed] [Google Scholar]
- 17.Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dedering A, Nemeth G, Harms RK (1999) Correlation between electromyographic spectral changes and subjective assessment of lumbar muscle fatigue in subjects without pain from the lower back. Clin Biomech (Bristol, Avon) 14:103–111 [DOI] [PubMed]
- 19.Dedering A, Oddsson L, Harms-Ringdahi K, Nemeth G (2002) Electromyography and ratings of lumbar muscle fatigue using a four-level staircase protocol. Clin Biomech (Bristol, Avon) 17:171–176 [DOI] [PubMed]
- 20.Demoulin C, Crielaard JM, Vanderthommen M. Spinal muscle evaluation in healthy individuals and low-back-pain patients: a literature review. Jt Bone Spine. 2007;74:9–13. doi: 10.1016/j.jbspin.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Rissanen A, Kalimo H, Alaranta H. Effect of intensive training on the isokinetic strength and structure of lumbar muscles in patients with chronic low back pain. Spine. 1995;20:333–340. doi: 10.1097/00007632-199502000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Storheim K, Holm I, Gunderson R, Brox JI, Bø K. The effect of comprehensive group training on cross-sectional area, density, and strength of paraspinal muscles in patients sick-listed for subacute low back pain. J Spinal Disord Tech. 2003;16:271–279. doi: 10.1097/00024720-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi Y, Nakamura T, Takayama S, Horiuchi Y, Toyama Y. MR imaging in the diagnosis of denervated and reinnervated skeletalmuscles: experimental study in rats. Radiology. 2003;229:861–867. doi: 10.1148/radiol.2293020904. [DOI] [PubMed] [Google Scholar]
- 24.Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982;134:383–397. [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery: a histologic and enzymatic analyses. Spine. 1996;21:941–944. doi: 10.1097/00007632-199604150-00007. [DOI] [PubMed] [Google Scholar]