Abstract
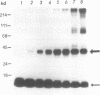
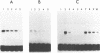
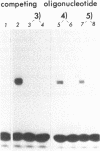
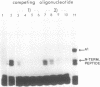
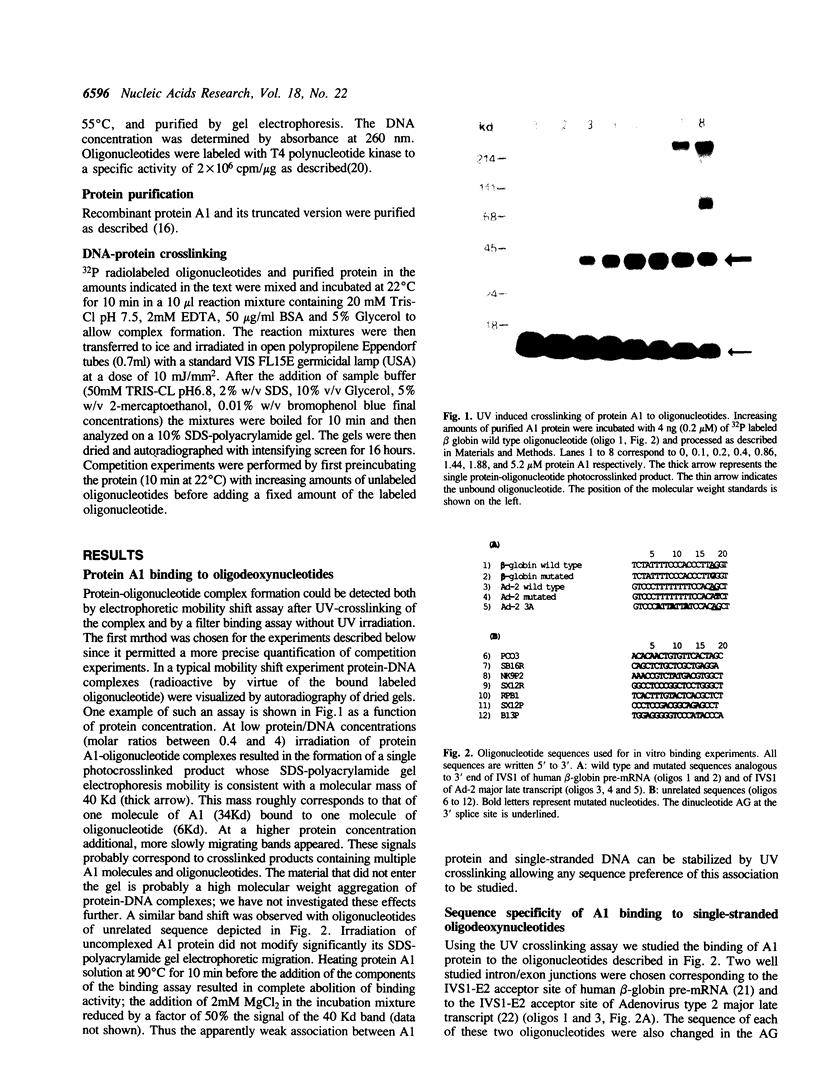
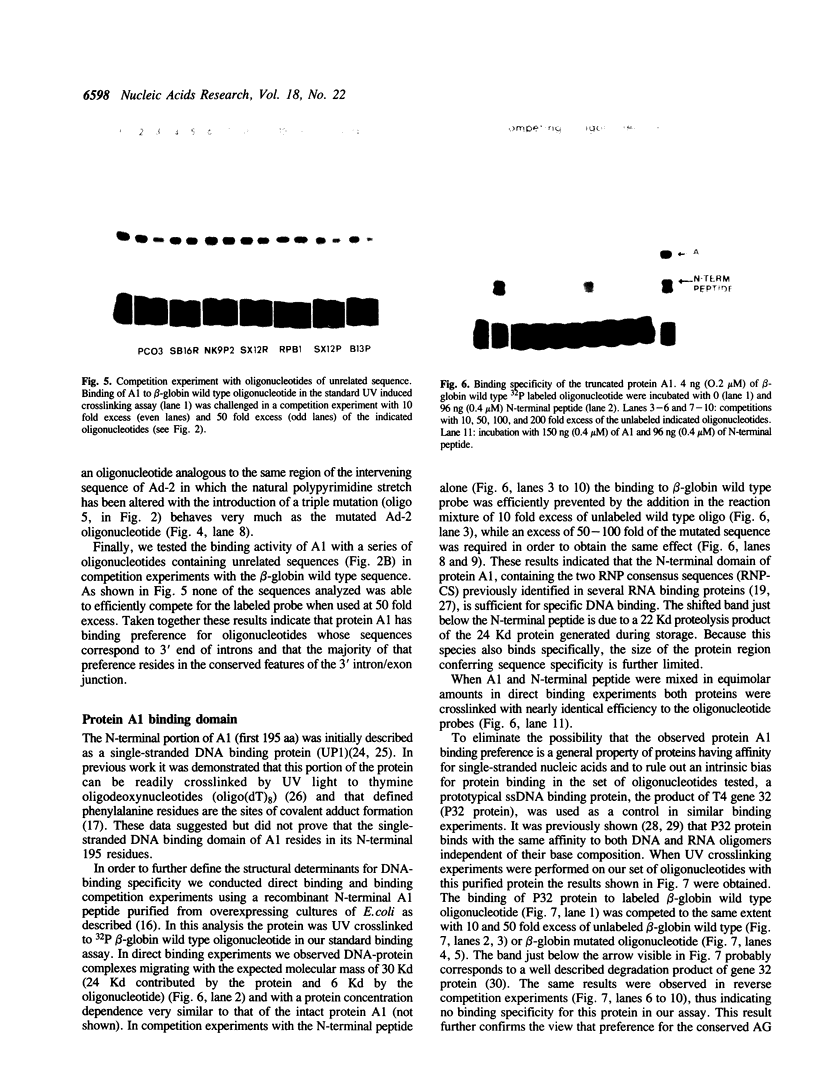
The reported binding preference of human hnRNP protein A1 for the 3'-splice site of some introns (Swanson and Dreyfuss (1988) EMBO J. 7, 3519-3529; Mayrand and Pederson (1990) Nucleic Acids Res. 18, 3307-3318) was tested by assaying in vitro the binding of purified recombinant A1 protein (expressed in bacteria) to synthetic oligodeoxynucleotides (21-mers) of suitable sequence. In such a minimal system we find preferential binding of protein A1 to oligodeoxynucleotide sequences corresponding to the 3'-splice site of IVS1 of human beta-globin pre-mRNA and of IVS1 of Adenovirus type 2 major late transcript. Mutation studies demonstrate that the binding specificity is dependent on the known critical domains of this intron region, the AG splice site dinucleotide and polypyrimidine tract, and resides entirely in the short oligonucleotide sequence. Moreover specific binding does not require the presence of other hnRNP proteins or of snRNP particles. Studies with a truncated recombinant protein demonstrated that the minimal protein sequence determinants for A1 recognition of 3'-splice acceptor site reside entirely in the N-terminal 195 aa of the unmodified protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Miller O. L., Jr, McKnight S. L. Ribonucleoprotein structure in nascent hnRNA is nonrandom and sequence-dependent. Cell. 1980 May;20(1):75–84. doi: 10.1016/0092-8674(80)90236-6. [DOI] [PubMed] [Google Scholar]
- Biamonti G., Buvoli M., Bassi M. T., Morandi C., Cobianchi F., Riva S. Isolation of an active gene encoding human hnRNP protein A1. Evidence for alternative splicing. J Mol Biol. 1989 Jun 5;207(3):491–503. doi: 10.1016/0022-2836(89)90459-2. [DOI] [PubMed] [Google Scholar]
- Buvoli M., Biamonti G., Tsoulfas P., Bassi M. T., Ghetti A., Riva S., Morandi C. cDNA cloning of human hnRNP protein A1 reveals the existence of multiple mRNA isoforms. Nucleic Acids Res. 1988 May 11;16(9):3751–3770. doi: 10.1093/nar/16.9.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvoli M., Cobianchi F., Bestagno M. G., Mangiarotti A., Bassi M. T., Biamonti G., Riva S. Alternative splicing in the human gene for the core protein A1 generates another hnRNP protein. EMBO J. 1990 Apr;9(4):1229–1235. doi: 10.1002/j.1460-2075.1990.tb08230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. D., Grabowski P. J., Sharp P. A., Dreyfuss G. Heterogeneous nuclear ribonucleoproteins: role in RNA splicing. Science. 1986 Mar 28;231(4745):1534–1539. doi: 10.1126/science.3952495. [DOI] [PubMed] [Google Scholar]
- Chung S. Y., Wooley J. Set of novel, conserved proteins fold pre-messenger RNA into ribonucleosomes. Proteins. 1986 Nov;1(3):195–210. doi: 10.1002/prot.340010302. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., Karpel R. L., Williams K. R., Notario V., Wilson S. H. Mammalian heterogeneous nuclear ribonucleoprotein complex protein A1. Large-scale overproduction in Escherichia coli and cooperative binding to single-stranded nucleic acids. J Biol Chem. 1988 Jan 15;263(2):1063–1071. [PubMed] [Google Scholar]
- Cobianchi F., SenGupta D. N., Zmudzka B. Z., Wilson S. H. Structure of rodent helix-destabilizing protein revealed by cDNA cloning. J Biol Chem. 1986 Mar 15;261(8):3536–3543. [PubMed] [Google Scholar]
- Conway G., Wooley J., Bibring T., LeStourgeon W. M. Ribonucleoproteins package 700 nucleotides of pre-mRNA into a repeating array of regular particles. Mol Cell Biol. 1988 Jul;8(7):2884–2895. doi: 10.1128/mcb.8.7.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G. Structure and function of nuclear and cytoplasmic ribonucleoprotein particles. Annu Rev Cell Biol. 1986;2:459–498. doi: 10.1146/annurev.cb.02.110186.002331. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Swanson M. S., Piñol-Roma S. Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem Sci. 1988 Mar;13(3):86–91. doi: 10.1016/0968-0004(88)90046-1. [DOI] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Structure of nuclear ribonucleoprotein: heterogeneous nuclear RNA is complexed with a major sextet of proteins in vivo. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1599–1602. doi: 10.1073/pnas.80.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Blanco M. A., Jamison S. F., Sharp P. A. Identification and purification of a 62,000-dalton protein that binds specifically to the polypyrimidine tract of introns. Genes Dev. 1989 Dec;3(12A):1874–1886. doi: 10.1101/gad.3.12a.1874. [DOI] [PubMed] [Google Scholar]
- Gerke V., Steitz J. A. A protein associated with small nuclear ribonucleoprotein particles recognizes the 3' splice site of premessenger RNA. Cell. 1986 Dec 26;47(6):973–984. doi: 10.1016/0092-8674(86)90812-3. [DOI] [PubMed] [Google Scholar]
- Herrick G., Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2124–2132. [PubMed] [Google Scholar]
- Hockensmith J. W., Kubasek W. L., Vorachek W. R., von Hippel P. H. Laser cross-linking of nucleic acids to proteins. Methodology and first applications to the phage T4 DNA replication system. J Biol Chem. 1986 Mar 15;261(8):3512–3518. [PubMed] [Google Scholar]
- Kowalczykowski S. C., Lonberg N., Newport J. W., von Hippel P. H. Interactions of bacteriophage T4-coded gene 32 protein with nucleic acids. I. Characterization of the binding interactions. J Mol Biol. 1981 Jan 5;145(1):75–104. doi: 10.1016/0022-2836(81)90335-1. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Mayrand S. H., Pederson T. Crosslinking of hnRNP proteins to pre-mRNA requires U1 and U2 snRNPs. Nucleic Acids Res. 1990 Jun 11;18(11):3307–3318. doi: 10.1093/nar/18.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill B. M., LoPresti M. B., Stone K. L., Williams K. R. High pressure liquid chromatography purification of UP1 and UP2, two related single-stranded nucleic acid-binding proteins from calf thymus. J Biol Chem. 1986 Jan 15;261(2):878–883. [PubMed] [Google Scholar]
- Merrill B. M., Stone K. L., Cobianchi F., Wilson S. H., Williams K. R. Phenylalanines that are conserved among several RNA-binding proteins form part of a nucleic acid-binding pocket in the A1 heterogeneous nuclear ribonucleoprotein. J Biol Chem. 1988 Mar 5;263(7):3307–3313. [PubMed] [Google Scholar]
- Merrill B. M., Williams K. R., Chase J. W., Konigsberg W. H. Photochemical cross-linking of the Escherichia coli single-stranded DNA-binding protein to oligodeoxynucleotides. Identification of phenylalanine 60 as the site of cross-linking. J Biol Chem. 1984 Sep 10;259(17):10850–10856. [PubMed] [Google Scholar]
- Michel B., Zinder N. D. In vitro binding of the bacteriophage f1 gene V protein to the gene II RNA-operator and its DNA analog. Nucleic Acids Res. 1989 Sep 25;17(18):7333–7344. doi: 10.1093/nar/17.18.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. K., Green M. R. Mammalian U2 snRNP has a sequence-specific RNA-binding activity. Genes Dev. 1989 Oct;3(10):1562–1571. doi: 10.1101/gad.3.10.1562. [DOI] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr, Beyer A. L. RNP particles at splice junction sequences on Drosophila chorion transcripts. Cell. 1985 Nov;43(1):143–151. doi: 10.1016/0092-8674(85)90019-4. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Konarska M. M., Grabowski P. J., Hardy S. F., Sharp P. A. Lariat RNA's as intermediates and products in the splicing of messenger RNA precursors. Science. 1984 Aug 31;225(4665):898–903. doi: 10.1126/science.6206566. [DOI] [PubMed] [Google Scholar]
- Pullman J. M., Martin T. E. Reconstitution of nucleoprotein complexes with mammalian heterogeneous nuclear ribonucleoprotein (hnRNP) core proteins. J Cell Biol. 1983 Jul;97(1):99–111. doi: 10.1083/jcb.97.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Riva S., Morandi C., Tsoulfas P., Pandolfo M., Biamonti G., Merrill B., Williams K. R., Multhaup G., Beyreuther K., Werr H. Mammalian single-stranded DNA binding protein UP I is derived from the hnRNP core protein A1. EMBO J. 1986 Sep;5(9):2267–2273. doi: 10.1002/j.1460-2075.1986.tb04494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Boelens W., Dathan N. A., van Venrooij W. J., Mattaj I. W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B'' and their cognate RNAs. Nature. 1990 Jun 7;345(6275):502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- Schleif R. DNA binding by proteins. Science. 1988 Sep 2;241(4870):1182–1187. doi: 10.1126/science.2842864. [DOI] [PubMed] [Google Scholar]
- Sierakowska H., Szer W., Furdon P. J., Kole R. Antibodies to hnRNP core proteins inhibit in vitro splicing of human beta-globin pre-mRNA. Nucleic Acids Res. 1986 Jul 11;14(13):5241–5254. doi: 10.1093/nar/14.13.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. RNA binding specificity of hnRNP proteins: a subset bind to the 3' end of introns. EMBO J. 1988 Nov;7(11):3519–3529. doi: 10.1002/j.1460-2075.1988.tb03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi J., Alibert C., Temsamani J., Reveillaud I., Cathala G., Brunel C., Jeanteur P. A protein that specifically recognizes the 3' splice site of mammalian pre-mRNA introns is associated with a small nuclear ribonucleoprotein. Cell. 1986 Dec 5;47(5):755–766. doi: 10.1016/0092-8674(86)90518-0. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Glowacka S. K., Szer W. Structure of complexes between a major protein of heterogeneous nuclear ribonucleoprotein particles and polyribonucleotides. J Mol Biol. 1983 Dec 25;171(4):439–455. doi: 10.1016/0022-2836(83)90039-6. [DOI] [PubMed] [Google Scholar]
- Wilk H. E., Angeli G., Schäfer K. P. In vitro reconstitution of 35S ribonucleoprotein complexes. Biochemistry. 1983 Sep 13;22(19):4592–4600. doi: 10.1021/bi00288a038. [DOI] [PubMed] [Google Scholar]