Abstract
The development of therapeutic proteins requires the understanding of the relationship between the dose, exposure, efficacy, and toxicity of these molecules. Several intrinsic and extrinsic factors contribute to the challenges for measuring therapeutic proteins in a precise and accurate manner. In addition, induction of an immune response to therapeutic protein results in additional complexities in the analysis of the pharmacokinetic profile, toxicity, safety, and efficacy of this class of molecules. Assessment of immunogenicity of therapeutic proteins is a required aspect of regulatory filings for a licensing application and for the safe and efficacious use of these compounds. A systematic strategy and well-defined criteria for measuring anti-drug antibodies (ADA) have been established, to a large extent, through coordinated efforts. These recommendations are based on risk assessment and include the determination of ADA content (concentration/titer), affinity, immunoglobulin isotype/subtype, and neutralization capacity. This manuscript reviews the requirements necessary for understanding the nature of an ADA response in order to discern the impact of immunogenicity on pharmacokinetics/pharmacodynamics and efficacy.
KEY WORDS: antibody, immunogenicity, pharmacodynamics, pharmacokinetics, protein therapeutics
INTRODUCTION AND BACKGROUND
The clinical development of protein therapeutics has made significant advances as evidenced by the increasing number of approvals. Protein therapeutics have complex structures which result in unique pharmacokinetic and pharmacodynamic (PK/PD) properties, including an oftentimes high degree of nonlinearity in the dose–exposure–response relationship due to high-affinity interactions with pharmacologic target structures and other endogenous proteins (1,2). Accurate measurement and modeling of PK/PD characteristics of therapeutic proteins are required to make decisions on adequate exposure in preclinical toxicology studies, select first-in-human starting doses for single and multiple dosing regimens, determine the efficacious dose for phase III trials, and assess drug–drug interactions (3). Administration of protein therapeutics can induce anti-drug antibodies (ADA), which can have an impact on their PK/PD characteristics. When produced in “high1” amounts, these “high”-affinity mature ADA have an increased likelihood of modulating and even neutralizing the drug's therapeutic effects. The characterization of these ADA responses presents bioanalytical challenges. Recent publications and regulatory guidance documents have provided significant insights and direction to meet the regulatory requirements of characterizing and quantifying immune responses to protein therapeutics (4–6).
The ADA formation may impact either the pharmacokinetics of the protein therapeutic, i.e., the relationship between dose and the obtained concentrations in plasma or other organs and tissues, or the pharmacodynamics, which describes the relationship between systemic concentrations (exposure) and therapeutic effects, or both. Thus, ADA may ultimately affect the efficacy and/or toxicity profile of therapeutic proteins (7,8). In order to delineate the impact of the immunogenicity on modulating PK and PD, drug development scientists are challenged with simultaneously considering the interplay of multiple influential factors that are major determinants in the occurrence, frequency, severity, quantifiability, and clinical impact of immune reactions to a protein therapeutic.
Several factors influence the immunogenicity of therapeutic proteins. The European Medicine Agency guidance document and other reviews (4,6,9) have classified them into disease-, patient-, or product-related factors. Briefly, disease- and patient-related factors have the potential to predispose an individual to an immune response. Examples for disease-related factors include dysregulation of immune responses in autoimmune conditions, inflammatory responses due to an infectious agent, or an existing immune response in a patient due to a disease condition (4). A patient-related factor is for example the major histocompatibility complex background of subjects that has been demonstrated to influence the immunogenicity of protein therapeutics (10). Product-related factors that can influence immune response to a therapeutic protein have also been reported (11,12), such as the induction of an unanticipated inflammatory response in healthy subjects after administration of an immunomodulatory biologic (e.g., anti-CD28 mAb) (13). In summary, there is a multitude of interacting factors responsible for ADA formation against protein therapeutics that comprises product-specific critical quality attributes (14) including (1) amino acid sequence differences between therapeutic protein and endogenous proteins; (2) post-translational modification-associated changes; (3) structural alterations such as aggregation, oxidation, deamidation, degradation, and conformational changes; (4) changes due to storage conditions; (5) production/purification-associated modifications; and (6) formulation-associated changes, as well as other factors such as (7) route, frequency, and dose of administration; (8) immune status of the clinical subject/animal; and (9) genetic background of the subject.
This review highlights some of the challenges in understanding the impact of immunogenicity on PK/PD through a series of questions and partial answers with examples. Listings of the current gaps and suggestions for further analyses are provided in Tables I and II.
Table I.
Considerations for Analyzing the Impact of Immunogenicity on PK/PD
| Considerations: | |
|---|---|
| 1. | The assessment of the impact of immunogenicity on PK/PD should consider different dosing schedules and well-defined sampling schedules, which could include peak and trough concentrations and late time points after the circulating drug has been cleared. |
| 2. | The bioanalytical methods should detect ADA in the presence of circulating drug, and conversely, therapeutic proteins should be measured in the presence of ADA. These data will allow for advances in modeling the total kinetics of ADA responses in subjects. |
| 3. | Differentiation of ADA responses by concentrations should be performed prior to an accurate assessment of impact on PK profiles of therapeutic proteins. |
| 4. | In certain cases, pharmacokinetic profiles of therapeutic proteins could be assessed through cross-sectional analysis of PK bioanalytical data, using population PK data analysis methods. |
| 5. | ADA with higher concentrations have a greater likelihood of having an impact on PK profiles of therapeutic proteins. |
Table II.
Gaps in Our Current Knowledge on the Impact of Immunogenicity on PK/PD Ordered by Priority for the Need to Be Addressed
| GAPS: | |
|---|---|
| 1. | There is a lack of consistency in reporting types of ADA responses; thus, characteristics of the ADA that have impact on PK/PD are reported in diverse ways (e.g., titers, concentrations, isotypes, etc.) and are difficult to compare among compounds. |
| 2. | High concentrations of circulating protein therapeutics and ADA interfere with accuracy of bioanalytical measurements and make simultaneous quantification challenging. |
| 3. | The inability to accurately measure the concentrations of antibodies, especially during the course of the study, hampers the ability to model the time course of an immune response to therapeutic proteins. |
| 4. | The mechanism of action of ADA-induced impact on PK of therapeutic proteins has not been extensively investigated and remains unclear. |
| 5. | There is limited knowledge of the effect of endogenous modification or partial degradation on the immunogenicity of protein therapeutics. |
| 6. | There is a lack of a database of product- and patient-related factors that influence the impact of immunogenicity on PK/PD and efficacy. |
WHAT ARE THE CHALLENGES IN THE METHODS FOR BIOANALYTICAL QUANTIFICATION OF ADA AND THERAPEUTIC PROTEINS?
The primary objective of accurate bioanalytical measurements of therapeutic proteins is to ensure the presence of drug concentrations in the target organ or tissue that are sufficient for inhibition or activation of its molecular target. Several factors affect the accurate measurement of circulating protein therapeutics; these include interference of soluble target, bound ADA, and serum components (15,16). Advances in bioanalytical assays may allow detection and measurement of free protein therapeutics and various bound forms (17), e.g., therapeutic bound to ADA, therapeutic bound to target, modified or truncated therapeutics, and metabolites of the therapeutic (18). The bound form of a therapeutic protein can become biologically inactive as it can be neutralized by the target (e.g., through binding to shed target receptors) or ADA. An assay for free therapeutic protein allows for the detection of a bioactive drug that is not complexed to a soluble target or ADA. There are instances where the terminal half-life (t½) is much shorter for the therapeutic measured in the “free” assay than in the “total” assay, as the ADA–therapeutic protein complex is oftentimes cleared slower than the free therapeutic protein. In addition, compensatory upregulation of shed target may result in concentration increases in “total,” while “free” concentrations are actually decreasing (16). In addition, the accuracy of quantification of protein therapeutics can be confounded by the presence of ADA. Thus, the challenges in measuring concentrations of therapeutic proteins include understanding the characteristics of free and bound forms and the impact of circulating ADA on assay accuracy.
Significant advances have been made in the bioanalytical methods to measure ADA which involve three stages, namely screening, confirmation, and characterization (5,6). The screening and confirmatory assays utilize binding immunoassays to detect the presence of ADA. The ADA response is likely to be a polyclonal response with (1) diverse ADA concentrations and affinity, and (2) different isotype and subtype responses and (3) directed to different epitopes. The samples that are positive in the confirmatory assay are subsequently characterized in a functional assay to determine the neutralization potential. The lower limit of detection of ADA is highly dependent on the affinity of the antibodies used in the immunoassays and has been reported as approximately ~20 and 1,000 ng/mL for capturing antibodies with an approximate KD of 10−9 and 10−8 M, respectively (19,20). There are instances where the described quantification methods for therapeutic proteins fail, for example, if the drug is dosed in low amounts and is immediately consumed by the target, resulting in no quantifiable concentrations. In these instances, PD measurements confirming target binding to the drug can be used as a surrogate marker for drug exposure. For some modalities like fusion proteins or conjugated proteins, where enhanced degradation through biotransformation might occur, a PD effect could also be a more reliable surrogate for understanding exposure as PK assays can detect only the intact molecule while metabolites that are potentially still bioactive can usually not be detected by these assays.
WHAT TYPES OF ADA RESPONSES TO THERAPEUTIC PROTEINS HAVE BEEN OBSERVED IN CLINICAL TRIALS?
The concentrations and types of ADA are variable between individuals and can therefore have a different impact on PK/PD and the efficacy of therapeutic proteins in different individuals. In addition, a similar concentration of ADA in two different individuals could also have a variable impact due to the polyclonal nature of the ADA response. Hence, characterizing the nature of the antibody response could provide a mechanism to differentiate the range of ADA by strength and type, thereby facilitating the development of robust statistical methods to evaluate their impact on PK, PD, efficacy, and safety. ADA isotype responses from IgM to the various IgG subtypes have been reported. These responses have been shown to be directed to epitopes which target both the active site (ligand/receptor binding epitopes, which result in neutralizing activity) and non-neutralizing sites on therapeutic proteins. Table III shows the immunogenicity rates and characteristics of a short list of therapeutic proteins, which represent the major classes of drug products, i.e., monoclonal antibodies, recombinant cytokines, enzymes, and other proteins. A more detailed list can be found at www.fda.gov and in other recent reviews (21–23).
Table III.
Immunogenicity Rates and Characteristics for a Selection of Representative Therapeutic Proteins
| Product | Mechanism of action | Type | Immunogenicity ratea | Estimated ADA concentration (type) |
|---|---|---|---|---|
| Adalimumab | Anti-TNF-α Ab | Human IgG1 κ | 1–12 % | NR |
| Infliximab | Anti-TNF-α Ab | Chimeric IgG1 κ | 10 % | NR |
| Alemtuzumab | Anti-CD52 Ab | Humanized IgG1 κ | 8.3 % | NR |
| Muromonab-CD3 | Anti-CD3 Ab | Murine IgG1 κ | 86 % | (IgM, G, E) |
| Cetuximab | Anti-EGFR Ab | Chimeric IgG1 κ | 5 % | NR (IgE) |
| Natalizumab | Anti-α4β1 Ab | IgG1 κ | 9 % | NR |
| Rituximab | Anti-CD20 | Chimeric IgG1 κ | 1 % | NR |
| Darbepoietin α | Analog of erythropoietin | Protein | 3–4 % | 0.2–1 μg/mL (IgG1-4) |
| Interferons | Interferons | Protein | 6–24 % | NR |
| Anakinra | IL1R antagonist | Protein | 28 % | NR |
| Recombinant FVIII, IX | Factor VIII, IX replacement | Proteins | 3.6–20 % | ~1.2–3 BU |
| Alglucosidase α | Alglucosidase α replacement | Protein | 89 % | >12,800 titer (IgG) |
As noted in the previous section, the bioanalytical methods to measure free circulating ADA are influenced by high concentrations of circulating drug, especially during the dosing phase of clinical studies. Thus, assessment of the ADA of the subjects is preferably performed at the end of the study in the absence of circulating drug, but this cannot be done in all clinical instances, for example if drug therapy is continued after study completion for ethical reasons.
The inability to accurately measure the concentrations of antibodies, especially during the course of the study, hampers the ability to characterize and model the time course of an immune response to therapeutic proteins. In order to be able to determine the impact of antibodies on PK completely and accurately, both samples for PK and ADA analyses need to be collected at the same appropriate time points. The time period of the first occurrence of therapeutic protein induced ADA ranges from ~2 to 4 weeks post-administration. Although much is known about the kinetics of the immune response to vaccine antigens and autoantigens in disease states (24), the precise kinetics and the nature of the immune response induced by ~10–300 mg doses of human and humanized therapeutic proteins in humans are largely unknown. Notwithstanding, studies on immunogenicity to natalizumab indicate that ADA were detected by 12 weeks post-treatment and that persistent ADA (rather than transient ADA) had a strong correlation with a decrease in drug serum concentrations and resultant reduced efficacy (25). Two anti-TNF-α antibody therapeutics, infliximab and adalimumab, have also shown more rapid clearance resulting in loss of efficacy when ADA were formed (8,26). ADA that have an impact on clearance have been reported in a range of 8–16 weeks after initiation of therapy (25,27). Since measurements of ADA and drug concentrations at the same time points are significantly impacted by assay interferences, improved bioanalytical methods to define the characteristics of ADA are needed to allow for advances in characterizing the kinetics of ADA responses.
WHAT ARE THE CHARACTERISTICS OF ADA LIKELY HAVING AN IMPACT ON PK/PD OR EFFICACY OF A THERAPEUTIC PROTEIN?
ADA can impact the PK of therapeutic proteins in diverse ways. On the one hand, ADA can enhance clearance, and on the other sustain the circulation of therapeutic proteins. The mechanism of action of an ADA-induced impact on the PK of therapeutic proteins has not been extensively investigated. Both binding and neutralizing ADA have likely the potential to impact the clearance of therapeutic proteins (28). Generally, ADA that are present at high concentrations and have high affinity for the therapeutic protein are considered to have the highest potential of impact. The isotype of the ADA may also affect clearance, based on interactions of the various isotypes (e.g., IgG1, IgG2, IgG3, etc.) with Fc receptor (FcR) types (29,30). The increased likelihood of ADA formation at >4 weeks after initiation of therapy can be explained by the kinetics of the somatic hypermutation process of immunoglobulins (31).
Treatment of Pompe disease patients with alglucosidase-α (Myozyme) represents an example for the effect of ADA on PK/PD and efficacy. Alglucosidase-α is administered to subjects with mutations in the glucosidase gene and induces ADA in the majority of the subjects. It was estimated that subjects with an ADA titer of >1:12,800 had an increase in clearance by 50 % (range, 5–90 %) from weeks 1 to 12 (32). Furthermore, patients with high sustained ADA titer have an attenuated therapeutic response to this enzyme replacement therapy (33).
We have recently analyzed the impact of ADA concentrations on the PK of a fully human monoclonal antibody therapeutic. Several individuals developed ADA ranging in concentration from 250 to >2,000 ng/mL. (Note: the concentrations of ADA are relative, since these have been extrapolated from a rabbit polyclonal standard). Using a statistical approach, we have observed that higher ADA concentrations were associated with lower therapeutic trough concentrations and that the association was strongest at later time points (week 12 of weekly dosing) (Starcevic M. et al., manuscript submitted).
WHAT ARE THE MECHANISMS OF ADA-INDUCED CLEARANCE AND SUSTENANCE OF THERAPEUTIC PROTEINS?
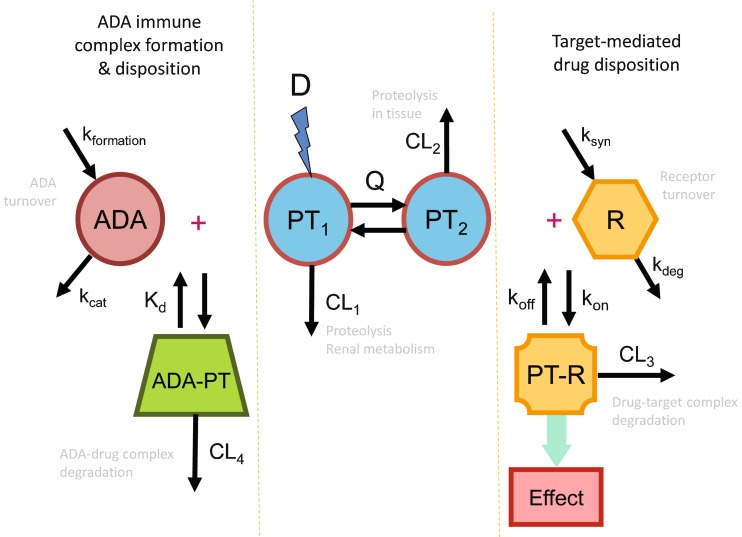
The binding of protein therapeutics to ADA directed against them results in the formation of immune complexes. Similar to immune complexes formed from endogenous IgG molecules and their natural targets, ADA–protein therapeutic immune complexes circulating in the bloodstream trigger regular endogenous elimination processes. This elimination is mediated through the reticuloendothelial system, predominantly phagocytic cells in the liver and spleen such as monocytes and macrophages as well as endothelial cells. The immune complexes are internalized and undergo subsequent lysosomal degradation. Since this process results in the degradation of the protein therapeutic and the ADA, the ADA-induced clearance constitutes an additional elimination pathway for the protein therapeutic (Fig. 1). While there is still limited mechanistic knowledge on the uptake of the immune complex, Fcγ receptors have been suggested as a major route for immune complex internalization (34).
Fig. 1.
Example of multiple clearance pathways affecting the pharmacokinetics of a typical protein therapeutic. Depicted is a two-compartment pharmacokinetic model with intravenous administration of a dose (D), concentrations of the protein therapeutic in the central (PT 1) and peripheral (PT 2) compartment, and interdepartmental clearance Q. The pharmacokinetic model includes two clearance pathways, one from the central compartment (CL 1) representative of for example renal metabolism or proteolytic degradation through the reticuloendothelial system, and a second unspecific proteolytic degradation pathway from the peripheral compartment (CL 2) Added to these two clearance pathways is, on the right side, a target-mediated disposition pathway that constitutes interaction of the protein therapeutic with its pharmacologic target receptor, which is in a homeostatic equilibrium of synthesis and degradation (synthesis rate k syn and degradation rate constant k deg). The dynamic equilibrium for the formation of the resulting protein therapeutic–receptor complex (PT–R) is determined through the association rate constant k on and the dissociation rate constant k off. The formation of PT–R does not only elicit the pharmacologic effect, but also triggers degradation of the complex. Thus, target binding and subsequent PT–R degradation constitute an additional clearance pathway for the protein therapeutic (CL 3). The left side of the graphic depicts the effect of an immune response to the protein therapeutic resulting in ADA formation. Again, the circulating concentration of the ADA is determined by a homeostatic equilibrium between its formation rate (k formation) and a catabolic turnover process (rate constant k cat). The ADA response results in the formation of immune complexes with the drug (ADA–PT). Dependent on the size and structure of the immune complexes, endogenous elimination pathways through the reticuloendothelial system may be triggered, most likely via Fcγ-mediated endocytosis. Thus, immune complex formation and subsequent degradation may constitute an additional clearance pathway (CL 4) for protein therapeutics
Binding of ADA to therapeutic proteins occurs either through the active site of the protein which can neutralize its activity or through other portions of the protein. ADA can bind to the therapeutic proteins and clear the drug through Fc receptor pathways. The characteristics of an ADA response that can clear versus sustain therapeutic proteins are not clearly understood. One hypothesis for the mechanism of the clearing versus sustaining ADA involves the size of the ADA–protein therapeutic immune complex. Herein, larger immune complexes can be cleared by endogenous mechanisms. Thus, clearing ADA increases the clearance of the affected protein therapeutic as their immune complex formation triggers elimination through the reticuloendothelial system. This additional elimination process results in a decrease in the systemic exposure and shortening of the elimination half-life of the affected protein drug. In contrast, sustaining antibodies also form ADA–protein drug immune complexes, but the size and structure of the formed complex are insufficient to trigger the elimination process through the reticuloendothelial system. These complexes serve as a storage depot for the protein therapeutic. They can thereby reduce the clearance of protein therapeutics and increase their systemic exposure and elimination half-life. Recycling of the immune complex through interaction of the ADA component of the complex with the neonatal Fc receptor may be an additional mechanism for the observed prolongation in half-life (35). As other hypotheses about the mechanism of the ADA effect on PK have been proposed, further studies on the characteristics of ADA responses are needed to provide insight into those ADA that result in clearing versus sustaining circulating concentrations of therapeutic proteins (36).
WHAT EXPERIMENTS AND DATA ANALYSIS ARE UTILIZED FOR EVALUATING THE IMPACT OF IMMUNOGENICITY ON PK/PD?
In order to evaluate the impact of immunogenicity on PK/PD, the following factors should be considered: different dosing schedules and appropriate timing of sample collection which should include samples at the peak and trough concentration, at late time points after dosing, and samples after the circulating protein drug has been cleared, if possible. At every sampling time point for ADA assessment, a PK sample for corresponding concentration measurements of the therapeutic protein should also be collected. Thus, the ADA sampling strategy involves collection as early as 2 h post dosing to as late as several weeks after dosing. The interference of soluble targets, extracellular domain of the target receptors, or ADA on the PK assay need to be considered in the design of the sampling strategy by looking for free or total drug. The ADA sampling for shorter studies frequently includes a pre-dose sample as reference, a sample approximately 2 weeks after dosing to capture early low-affinity response, and late-stage sampling after approximately a month to capture mature IgG-mediated response. For long-term studies, quarterly sampling ensures monitoring for a transient vs. a persistent response and maturation to a neutralizing response. In instances where a high magnitude of immune response is expected, drug–ADA immune complex sampling can also occur. Such a sampling would require capture of time points following dosing to ensure capture of the immune complexes before they are cleared by the reticuloendothelial system.
In certain cases, pharmacokinetic profiles of therapeutic proteins can be assessed through cross-study analysis of concentration measurements using population PK analysis methods (37,38). The analysis of effects of immunogenicity on PK has been described for several molecules (22,24,25). However, the use of pharmacostatistical techniques in a population PK analysis across multiple clinical studies can provide a more robust dataset for these analyses. In this approach, box plot analysis between steady-state area-under-the-concentration-time curve of the therapeutic proteins can assess the variability of systemic exposure in the subjects with ADA. Through analysis of individual subjects, it can be assessed whether subjects with different characteristics of ADA response clear the circulating drug faster or slower.
The interpretation of these results, although statistically correct, can be hampered by the bioanalytical methods used to measure neutralizing antibodies which usually have a very low tolerance to the presence of circulating drug. In this respect, it is possible that some subjects, who have high circulating concentrations of therapeutic protein, could score negative for the presence of neutralizing antibodies in the bioassay, but the ADA could influence the circulating drug concentrations. In order to be able to address this issue, relative quantitative analysis of antibody concentrations can be used to further stratify the analysis. Using this approach, a more in-depth analysis of the impact of immunogenicity can be performed.
WHAT ARE THE CONSEQUENCES OF ADA USUALLY BEING A POLYCLONAL RESPONSE FOR ADA ASSESSMENTS?
Due to induction of a polyclonal ADA response following administration of protein therapeutics, multiple ADA species circulating in the serum exist, each of which has its own specificity and binding affinity. As a result, these different ADA responses may have divergent effects on the PK/PD of the protein therapeutic: (1) neutralizing effect on activity by interfering with the protein therapeutic's ability to bind to its pharmacologic target, (2) non-neutralizing effect on activity and a sustaining effect on the PK, and (3) non-neutralizing effect on activity, but an enhanced elimination of the protein therapeutic. The net clinical effect will be determined by the ADA responses that are expressed in the largest amounts and/or have the most prominent effects with regard to the PK/PD. Therefore, quantifying the concentrations of ADA responses along with circulating drug concentrations may provide a pathway for a better understanding of the impact of immunogenicity (37). Nevertheless, there are still major gaps in the understanding of many aspects of the polyclonal ADA response.
CAN AN ADA RESPONSE BE OVERCOME CLINICALLY?
The field of immunogenicity to therapeutic proteins has evolved from methods of detecting ADA into the development of treatment regimens that manipulate the immune system and abrogate immunogenicity. In this respect, methotrexate, rituximab, and intravenous immunoglobulins have been utilized to inhibit ADA responses to therapeutic proteins (39–41). These immunomodulatory regimens are particularly relevant to therapeutic proteins with a high risk to breaking the B cell tolerance towards their endogenous analogs, such as hemophilic factors IX and VIII, or replacement enzymes such as alglucosidase alfa (Myozyme). In these cases, mitigating antibody responses by induction and maintenance of immune tolerance can be key to the success of a therapy in patients.
The primary solution to the presence of ADA has been to “dose through” with higher dose regimens, i.e., stoichiometrically overcoming the neutralizing effect of an ADA response by supplying a large number of molecules of the protein therapeutic that outnumber the available ADA molecules. The doses selected for this approach have been established empirically. This could for example be shown for the treatment of multiple sclerosis patients with interferon-β. A loss of bioactivity due to the formation of neutralizing ADA could be overcome by intravenous application of high-dose interferon-β (42).
WHAT ARE THE PITFALLS OF COMPARING ADA INCIDENCE RATES AMONG DIFFERENT PROTEIN THERAPEUTICS?
Although significant advances have been made to standardize methodologies to measure ADA through collaborative efforts of industry and regulatory agencies, several challenges continue to manifest in measuring immunogenicity to therapeutic proteins. Some of these include the inability to measure total and free ADA during the dosing phase, use of different technology platforms in measuring diverse antibody polyclonal responses (such as low affinity, high concentrations, specific isotype), or the limited assessment of sensitivity and specificity of assays due to use of polyclonal rabbit/monkey positive controls (43,44). For these reasons, it remains difficult to compare the immunogenicity of different therapeutic proteins. It is possible that a concerted effort to develop international standards for classes of therapeutic proteins can provide some value in comparative immunogenicity. Such efforts have been made in vaccine development (45), and their establishment for protein therapeutics, although challenging, will be valuable to compare immunogenicity rates of molecules, especially with the advent of expanding development activities for biosimilars.
SUMMARY
Many of the questions raised in this review regarding ADA formation and its impact on the PK/PD and efficacy of protein therapeutics could only be partially answered based on the current knowledge. Table I summarizes major considerations that should be taken into account in the assessment of ADA responses, while Table II highlights some of the major gaps in our current understanding of immunogenicity. Further work on immunogenicity and clinical immunology will have to address many of these current gaps in our knowledgebase on the immune response to protein therapeutics in order to improve the safety and ensure the efficacy of the clinically used protein drugs today and in the future.
Footnotes
The methods to define “high” amount/affinity of ADA that can have an impact of PK–PD are one of the major challenges in the field.
This manuscript was developed based on discussions at the symposium “Impact of Immunogenicity on PK and PD of Protein Therapeutics” at the AAPS National Biotechnology Conference in San Francisco, CA, May 19, 2010.
REFERENCES
- 1.Meibohm B, Braeckman RA. Pharmacokinetics and pharmacodynamics of peptides and proteins. In: Crommelin DJA, Sindelar RD, Meibohm B, editors. Pharmaceutical biotechnology: concepts and applications. 3. New York: Informa Healthcare; 2007. pp. 95–123. [Google Scholar]
- 2.Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93:2184–2204. doi: 10.1002/jps.20125. [DOI] [PubMed] [Google Scholar]
- 3.Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, et al. Quantitative prediction of human pharmacokinetics for monoclonal antibodies: retrospective analysis of monkey as a single species for first-in-human prediction. Clin Pharmacokinet. 2011;50:131–142. doi: 10.2165/11537430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.CHMP. Guideline of immunogenicity assessment of biotechnology-derived therapeutic proteins. London: EMEA Guidance Document; 2008.
- 5.Gupta S, Devanarayan V, Finco D, Gunn GR, 3rd, Kirshner S, Richards S, et al. Recommendations for the validation of cell-based assays used for the detection of neutralizing antibody immune responses elicited against biological therapeutics. J Pharm Biomed Anal. 2011;55:878–888. doi: 10.1016/j.jpba.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48:1267–1281. doi: 10.1016/j.jpba.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Jamnitski A, Bartelds GM, Nurmohamed MT, van Schouwenburg PA, van Schaardenburg D, Stapel SO, et al. The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann Rheum Dis. 2011;70:284–288. doi: 10.1136/ard.2010.135111. [DOI] [PubMed] [Google Scholar]
- 8.Lecluse LL, Driessen RJ, Spuls PI, de Jong EM, Stapel SO, van Doorn MB, et al. Extent and clinical consequences of antibody formation against adalimumab in patients with plaque psoriasis. Arch Dermatol. 2010;146:127–132. doi: 10.1001/archdermatol.2009.347. [DOI] [PubMed] [Google Scholar]
- 9.Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289:1–16. doi: 10.1016/j.jim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa MD, Vielmetter J, Chu S, Smith DD, Jacinto J. Clinical link between MHC class II haplotype and interferon-beta (IFN-beta) immunogenicity. Clin Immunol. 2006;118:42–50. doi: 10.1016/j.clim.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter J, et al. Meeting report on protein particles and immunogenicity of therapeutic proteins: filling the gaps in risk evaluation and mitigation. Biologicals. 2011;38:602–611. doi: 10.1016/j.biologicals.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1:457–462. doi: 10.1038/nrd818. [DOI] [PubMed] [Google Scholar]
- 13.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 14.Rathore AS, Winkle H. Quality by design for biopharmaceuticals. Nat Biotechnol. 2009;27:26–34. doi: 10.1038/nbt0109-26. [DOI] [PubMed] [Google Scholar]
- 15.Bautista AC, Wullner D, Moxness M, Swanson SJ, Chirmule N, Jawa V. Impact of matrix-associated soluble factors on the specificity of the immunogenicity assessment. Bioanalysis. 2010;2:721–731. doi: 10.4155/bio.10.24. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Kelley M, King LE, Yang J, Salimi-Moosavi H, Tang MT, et al. Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J. 2011;13:99–110. doi: 10.1208/s12248-011-9251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salimi-Moosavi H, Lee J, Desilva B, Doellgast G. Novel approaches using alkaline or acid/guanidine treatment to eliminate therapeutic antibody interference in the measurement of total target ligand. J Pharm Biomed Anal. 2009;51:1128–1133. doi: 10.1016/j.jpba.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Hall MP, Gegg C, Walker K, Spahr C, Ortiz R, Patel V, et al. Ligand-binding mass spectrometry to study biotransformation of fusion protein drugs and guide immunoassay development: strategic approach and application to peptibodies targeting the thrombopoietin receptor. AAPS J. 2010;12:576–585. doi: 10.1208/s12248-010-9218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang M, Klakamp SL, Funelas C, Lu H, Lam B, Herl C, et al. Detection of high- and low-affinity antibodies against a human monoclonal antibody using various technology platforms. Assay Drug Dev Technol. 2007;5:655–662. doi: 10.1089/adt.2007.089. [DOI] [PubMed] [Google Scholar]
- 20.Lofgren JA, Dhandapani S, Pennucci JJ, Abbott CM, Mytych DT, Kaliyaperumal A, et al. Comparing ELISA and surface plasmon resonance for assessing clinical immunogenicity of panitumumab. J Immunol. 2007;178:7467–7472. doi: 10.4049/jimmunol.178.11.7467. [DOI] [PubMed] [Google Scholar]
- 21.Hay C, Recht M, Carcao M, Reipert B. Current and future approaches to inhibitor management and aversion. Semin Thromb Hemost. 2006;32(Suppl 2):15–21. doi: 10.1055/s-2006-946910. [DOI] [PubMed] [Google Scholar]
- 22.Swann PG, Tolnay M, Muthukkumar S, Shapiro MA, Rellahan BL, Clouse KA. Considerations for the development of therapeutic monoclonal antibodies. Curr Opin Immunol. 2008;20:493–499. doi: 10.1016/j.coi.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Thorpe R, Swanson SJ. Assays for detecting and diagnosing antibody-mediated pure red cell aplasia (PRCA): an assessment of available procedures. Nephrol Dial Transplant. 2005;20(Suppl 4):iv16–iv22. doi: 10.1093/ndt/gfh1086. [DOI] [PubMed] [Google Scholar]
- 24.Plotkin S, Orenstein W. Vaccines. Philadelphia: Saunders; 1999. [Google Scholar]
- 25.Buttel IC, Chamberlain P, Chowers Y, Ehmann F, Greinacher A, Jefferis R, et al. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals. 2011;39:100–109. doi: 10.1016/j.biologicals.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 26.de Vries MK, Wolbink GJ, Stapel SO, de Groot ER, Dijkmans BA, Aarden LA, et al. Inefficacy of infliximab in ankylosing spondylitis is correlated with antibody formation. Ann Rheum Dis. 2007;66:133–134. doi: 10.1136/ard.2006.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305:1460–1468. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- 28.Jawa V, Hokom M, Hu Z, El-Abaadi N, Zhuang Y, Berger D, et al. Assessment of immunogenicity of romiplostim in clinical studies with ITP subjects. Ann Hematol. 2010;89:S75–S85. doi: 10.1007/s00277-010-0908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–659. doi: 10.2165/11535960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Lanning DK, Knight KL. Somatic hypermutation: mutations 3′ of rabbit VDJ H-chain genes. J Immunol. 1997;159:4403–4407. [PubMed] [Google Scholar]
- 32.Genzyme. Myozyme prescribing information. Cambridge: Genzyme; 2009.
- 33.Banugaria SG, Prater SN, Ng YK, Kobori JA, Finkel RS, Ladda RL, et al. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med. 2011;13:729–736. doi: 10.1097/GIM.0b013e3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mousavi SA, Sporstol M, Fladeby C, Kjeken R, Barois N, Berg T. Receptor-mediated endocytosis of immune complexes in rat liver sinusoidal endothelial cells is mediated by FcgammaRIIb2. Hepatology. 2007;46:871–884. doi: 10.1002/hep.21748. [DOI] [PubMed] [Google Scholar]
- 35.Montero-Julian FA, Klein B, Gautherot E, Brailly H. Pharmacokinetic study of anti-interleukin-6 (IL-6) therapy with monoclonal antibodies: enhancement of IL-6 clearance by cocktails of anti-IL-6 antibodies. Blood. 1995;85:917–924. [PubMed] [Google Scholar]
- 36.Rehlaender BN, Cho MJ. Antibodies as carrier proteins. Pharm Res. 1998;15:1652–1656. doi: 10.1023/A:1011936007457. [DOI] [PubMed] [Google Scholar]
- 37.Kakkar T, Sung C, Gibiansky L, Vu T, Narayanan A, Lin SL, et al. Population PK and IgE pharmacodynamic analysis of a fully human monoclonal antibody against IL4 receptor. Pharm Res. 2011;28:2530–2542. doi: 10.1007/s11095-011-0481-y. [DOI] [PubMed] [Google Scholar]
- 38.Tabrizi MA, Roskos LK. Preclinical and clinical safety of monoclonal antibodies. Drug Discov Today. 2007;12:540–547. doi: 10.1016/j.drudis.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Baert F, Noman M, Vermeire S, Van Assche G, D'Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 40.Collins PW, Mathias M, Hanley J, Keeling D, Keenan R, Laffan M, et al. Rituximab and immune tolerance in severe hemophilia A: a consecutive national cohort. J Thromb Haemost. 2009;7:787–794. doi: 10.1111/j.1538-7836.2009.03332.x. [DOI] [PubMed] [Google Scholar]
- 41.Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millonig A, Rudzki D, Holzl M, Ehling R, Gneiss C, Kunz B, et al. High-dose intravenous interferon beta in patients with neutralizing antibodies (HINABS): a pilot study. Mult Scler. 2009;15:977–983. doi: 10.1177/1352458509105384. [DOI] [PubMed] [Google Scholar]
- 43.Patton A, Mullenix MC, Swanson SJ, Koren E. An acid dissociation bridging ELISA for detection of antibodies directed against therapeutic proteins in the presence of antigen. J Immunol Methods. 2005;304:189–195. doi: 10.1016/j.jim.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Peng K, Siradze K, Quarmby V, Fischer SK. Clinical immunogenicity specificity assessments: a platform evaluation. J Pharm Biomed Anal. 2011;54:629–635. doi: 10.1016/j.jpba.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Quataert SA, Kirch CS, Wiedl LJ, Phipps DC, Strohmeyer S, Cimino CO, et al. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]