Abstract
While each of the two key parameters of oral drug absorption, the solubility and the permeability, has been comprehensively studied separately, the relationship and interplay between the two have been largely ignored. For instance, when formulating a low-solubility drug using various solubilization techniques: what are we doing to the apparent permeability when we increase the solubility? Permeability is equal to the drug’s diffusion coefficient through the membrane times the membrane/aqueous partition coefficient divided by the membrane thickness. The direct correlation between the intestinal permeability and the membrane/aqueous partitioning, which in turn is dependent on the drug’s apparent solubility in the GI milieu, suggests that the solubility and the permeability are closely associated, exhibiting a certain interplay between them, and the current view of treating the one irrespectively of the other may not be sufficient. In this paper, we describe the research that has been done thus far, and present new data, to shed light on this solubility–permeability interplay. It has been shown that decreased apparent permeability accompanies the solubility increase when using different solubilization methods. Overall, the weight of the evidence indicates that the solubility–permeability interplay cannot be ignored when using solubility-enabling formulations; looking solely at the solubility enhancement that the formulation enables may be misleading with regards to predicting the resulting absorption, and hence, the solubility–permeability interplay must be taken into account to strike the optimal solubility–permeability balance, in order to maximize the overall absorption.
KEY WORDS: BCS class II compounds, drug solubility, intestinal permeability, oral absorption, poor aqueous solubility, solubility-enabling formulations, solubility–permeability tradeoff
INTRODUCTION
The rate and extent of drug absorption from the gastrointestinal (GI) tract are very complex and affected by many factors. These include physicochemical factors, physiological factors, and factors related to the dosage form (1–3). Despite this complexity, the Biopharmaceutics Classification System (BCS) developed by Amidon et al. (4) revealed that the fundamental key parameters controlling oral drug absorption are the permeability of the drug through the GI membrane and the solubility/dissolution of the drug dose in the GI milieu. These key parameters are characterized in the BCS, one of the most substantial tools in modern pharmaceutics and biopharmaceutics of oral drug products (5–10). While each of these two key parameters, the solubility and the permeability, has been comprehensively studied separately, and the validity and broad applicability of the BCS have been the subject of extensive research and discussion, the relationship and interplay between the two have been largely disregarded, in spite of the crucial significance and applicability this interplay may have, as will be presented in this paper.
The number of low-solubility drug candidates is rising constantly in today’s drug discovery, and by some estimates, more than 40% of new drug candidates are lipophilic and have poor aqueous solubility (11–15). Dissolution of the drug in the aqueous milieu of the GI is almost always a precondition for oral absorption, and hence, inadequate aqueous solubility often causes limited oral bioavailability. Low aqueous solubility hence is a common problem plaguing the drug candidates in the pipeline of most major pharmaceutical companies. It should be noted that the degree of solubility needs to be evaluated with relation to the intended dose, i.e., dose number >1, according to the BCS principles.
A wide variety of solubility-enabling formulation approaches have been developed and are routinely used to tackle the problem of inadequate aqueous solubility, e.g., the use of surface active agents, lipid-based formulations, self-emulsifying drug delivery systems, cyclodextrins, cosolvents, amorphous solid dispersions, and other techniques. While significant increase of the apparent solubility may certainly be achieved by these solubility-enabling formulations, the effect on the overall fraction dose absorbed is rather erratic; it is quite often that these formulations fail to deliver the desired result, and outcomes of increased, unchanged, or decreased absorption following the use of solubility-enabling formulations have been reported in the literature.
Although the intestinal permeability is, alongside the aqueous solubility, a key parameter that governs oral absorption, the impact of solubility-enabling formulations on the intestinal membrane permeability of a lipophilic drug is often overlooked and poorly understood. It is not the effects of the formulation’s excipients on the permeability mechanisms (e.g., transporters, gap junctions, etc.) that we raise here, rather we focus on the direct interplay between the apparent solubility and permeability, or asked differently: what are we doing to the apparent intestinal permeability of the drug when we use solubility-enabling formulations to increase the apparent aqueous solubility?
Intestinal permeability refers to the flow of a substance across the organ, how deep can a substance penetrate into the intestinal wall per time unit. Mathematically, permeability is equal to the diffusion coefficient of the drug through the membrane times the membrane/aqueous partition coefficient of the drug divided by the membrane thickness. The direct correlation between the intestinal permeability and the drug’s GI membrane/GI milieu partitioning, which in turn is dependent on the drug’s apparent solubility in the GI milieu, suggests that the two key parameters dictating oral drug absorption, the solubility and the permeability, may be closely associated, exhibiting a certain interplay between them, and the current view of treating the one irrespectively of the other may not be sufficient.
In this paper, we will present the research that has been done thus far to shed light on the solubility–permeability (S-P) interplay. This interplay may be system dependent; the effect of increasing the apparent solubility by, e.g., surfactants on the apparent permeability of the drug may be different than the effect when utilizing other solubility enhancement techniques. Hence, we will present the data according to the different solubilizing methods that have been investigated. Overall, this review highlights that the weight of the evidence indicates that the solubility–permeability interplay cannot be ignored when using solubility-enabling formulations; looking solely at the solubility enhancement that the formulation enables may be misleading with regards to predicting the resulting absorption, and hence, the S-P interplay must be taken into account in order to strike the optimal solubility–permeability balance and maximize the overall oral absorption.
S-P INTERPLAY FROM CYCLODEXTRIN-BASED SYSTEMS
Over the last 20 years, cyclodextrins have become a very popular and useful drug delivery option for increasing the aqueous solubility and oral absorption of hydrophobic drugs (16–19). Cyclodextrins are crystalline, cyclic oligosaccharides consisting of a hydrophilic outer surface that enables high aqueous solubility and a hydrophobic central cavity that can host hydrophobic solutes. In this way, cyclodextrins are structurally designed to increase the apparent water solubility of lipophilic drugs through the formation of more water-soluble inclusion complexes (18,19).
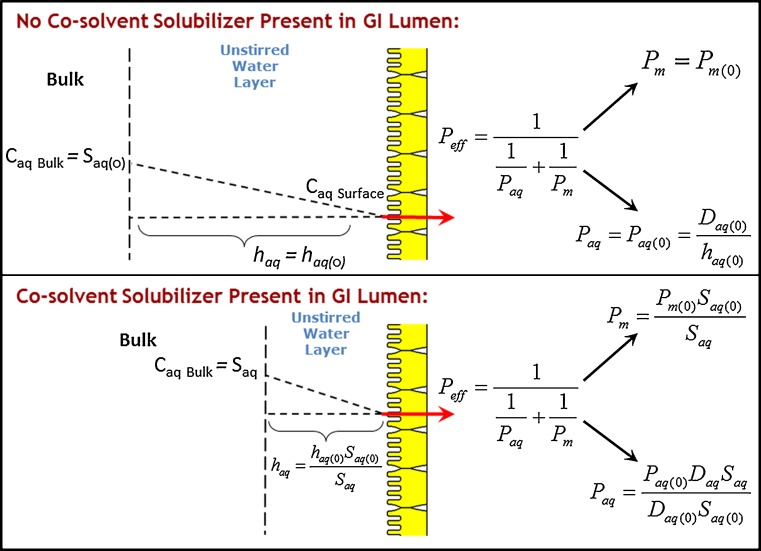
Although the extraordinary solubility advantage afforded by the use of cyclodextrins has led to their widespread use, several reports have emerged that cyclodextrins may also reduce the apparent permeability of the coadministered drug (20–22). Intuitively, this effect may be qualitatively explained by the decrease in the drug’s free fraction available for membrane permeability; it is easy to grasp that when the drug is bound to the cyclodextrin complex, it cannot be absorbed. This tradeoff between solubility increase and permeability decrease can lead to paradoxical effects on the overall absorption. For example, a critical review of the literature reveals that the use of cyclodextrins may lead to improved, unchanged, or even reduced fraction of drug absorbed (23,24). Further reports have emerged in the literature which has made apparent these opposing effects of cyclodextrins on the solubility and the permeability, and qualitative guidelines have been suggested for the proper use of cyclodextrins (20,24,25). More recently, quantitative analyses have emerged that enable simulation of the solubility–permeability interplay and the overall effect of cyclodextrins on intestinal membrane permeability (26–29). For instance, we have recently developed a mathematical mass transport model to elucidate the impact of molecular complexation with cyclodextrins on the intestinal permeability, and to mechanistically elucidate the solubility–permeability interplay when using cyclodextrin-based formulations (26). A schematic of the model is shown in Fig. 1. The model takes into consideration the effects of cyclodextrins on the intestinal membrane permeability (Pm) as well as the unstirred water layer (UWL) permeability (Paq), to allow prediction of the overall effective permeability (Peff) dependence on the concentration of cyclodextrin (CCD). The analysis revealed that: (1) the UWL permeability increases with increasing CCD due to quick decrease in the effective thickness of the UWL with increasing CCD; (2) the permeability through the intestinal membrane decreases with increasing CCD, attributed to the decrease in the drug’s free fraction; and (3) since Paq increases and Pm decreases with increasing CCD, the unstirred water layer is effectively abolished and the overall Peff tends toward membrane controlled, i.e., Peff ≈ Pm above a certain CCD. This mass transport model enabled excellent quantitative prediction of progesterone Peff as a function of HPβCD concentrations in several permeability models, including the PAMPA assay, Caco-2 studies, and the in situ rat jejunal perfusion model. It was demonstrated, hence, that when using cyclodextrins in solubility-enabling formulations, the overall fraction of drug absorbed is governed by a tradeoff that exists between the solubility increase and permeability decrease. Given these opposing effects, one must take into account both solubility and permeability considerations, in order to strike the appropriate balance between the two that will allow to maximize the overall absorption from a cyclodextrin-based formulation.
Fig. 1.
Schematic illustration of the quasi-equilibrium transport model describing the effect of cyclodextrins or surfactants on the drug transport through the unstirred water layer and the intestinal membrane, developed by Dahan et al. (26) and Miller et al. (35)
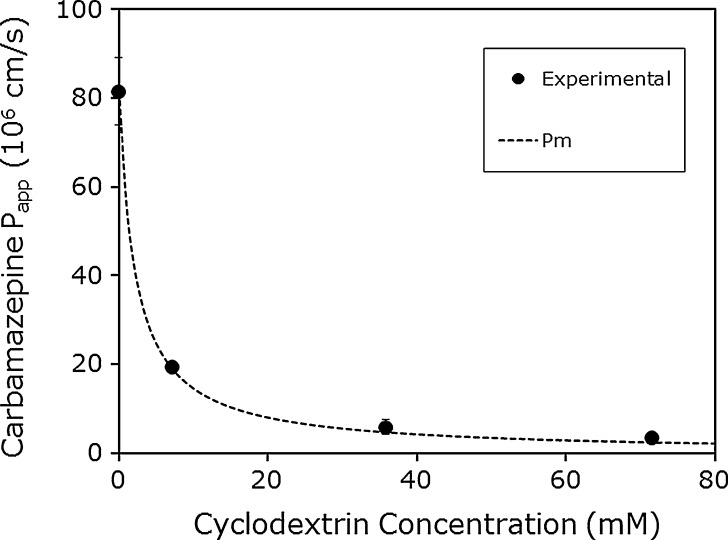
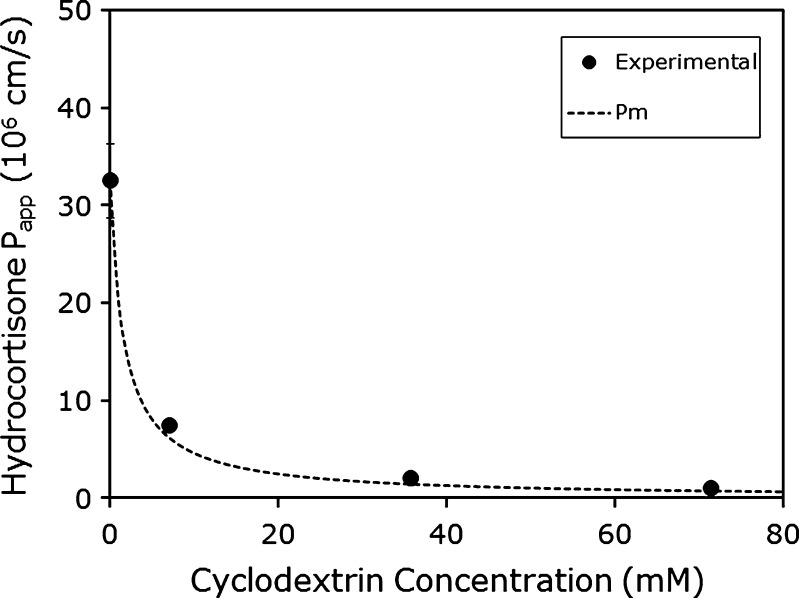
To further evaluate our quasi-equilibrium membrane transport model sketched in Fig. 1 (26), we applied the model to some experimental permeability as a function of cyclodextrin concentration data from the literature. Brewster et al. (30) determined the Peff of carbamazepine and hydrocortisone as a function of HPβCD concentration using the PAMPA assay, with a 2% (w/v) dioleylphosphadityl choline in dodecane solution as the artificial membrane. The authors determined the Peff of the compounds at varying stir speeds in the absence of HPβCD to create approximate UWL lengths of 25, 40, and >100 μm. The Peff was essentially the same at UWL lengths of 25 and 40 μm, indicating that the overall transport was under membrane control at these UWL lengths. Therefore, the Peff at UWL length of 25 μm was assumed to be equal to Pm for both compounds (Pm = 81.5 × 10−6 cm/s for carbamazepine and Pm = 32.5 × 10−6 cm/s for hydrocortisone). Likewise, the Peff at UWL length of >100 μm was assumed to be equal to Paq for both compounds (Paq = 23.7 × 10−6 cm/s for carbamazepine and Paq = 14.6 × 10−6 cm/s for hydrocortisone). Similar permeabilities were obtained at UWL lengths of 40 and >100 μm for both compounds at all HPβCD concentrations studied (7.1 to 71.4 mM). This indicates that the UWL is non-rate limiting and the overall Peff is under membrane control. Moreover, Peff < Paq over the entire range of HPβCD concentrations that was studied (7.1 to 71.4 mM). Our quasi-equilibrium membrane transport model (Fig. 1) may be applied. Figures 2 and 3 compare the theoretical Peff as a function of HPβCD predicted by the theoretical model to the experimentally observed values reported by Brewster et al. (30) for carbamazepine and hydrocortisone, respectively. It can be seen that under all of the concentrations of HPβCD tested, and for both compounds, an excellent agreement was achieved between the predicted lines and the experimental Peff values.
Fig. 2.
The apparent permeability (P app; in centimeters per second) of carbamazepine as a function of HPβCD concentration in the PAMPA model. The theoretical line was calculated via the transport model shown in Fig. 1 (26,35), and the experimental data points were derived from Brewster et al. (30)
Fig. 3.
The apparent permeability (P app; in centimeters per second) of hydrocortisone as a function of HPβCD concentration in the PAMPA model. The theoretical line was calculated via the transport model shown in Fig. 1 (26,35), and the experimental data points were derived from Brewster et al. (30)
S-P INTERPLAY FROM SURFACTANT-BASED SYSTEMS
The use of surfactants has long been a primary drug delivery strategy to increase the apparent aqueous solubility of lipophilic drugs. Indeed, surfactants often play a crucial role in providing the solubilization power to enable the oral absorption of poorly soluble drugs that otherwise could not be delivered. Along the GI tract, naturally occurring surfactants such as sodium taurocholate (STC) are present and play important roles in solubilization of lipophilic drugs. Likewise, artificial surfactants such as sodium lauryl sulfate (SLS) are routinely added to the dosage form to increase the apparent aqueous solubility of poorly soluble drugs.
While it is well recognized that surfactants can enable extraordinary increases in the aqueous solubility of hydrophobic drugs, the impact of micellar solubilization on the intestinal membrane permeability is often overlooked. Previous studies have shown that the use of surfactants may lead to increased, decreased, or unchanged membrane permeability (31–42). It is important to note that surfactants may increase intestinal membrane permeability for drugs with inherently low-permeability and high aqueous solubility (i.e., BCS class III compounds), e.g., through the disruption of membrane integrity to increase paracellular transport (e.g., tight junction opening, which may induce potentially toxic effects) and/or the inhibition of efflux transporters (36,39,42–45). However, for lipophilic drugs with inherently high transcellular membrane permeability (e.g., BCS class II), surfactants can decrease the free fraction of drug which results in decreased intestinal membrane permeability (31,35,39–42).
In using surfactants for the solubilization of lipophilic drugs, it is well recognized that above the critical micelle concentration (CMC), drugs may be incorporated into surfactant micelles. And it is this micellization process which drives the extraordinary increases in apparent aqueous solubility often afforded by surfactants. However, the micellar solubilization which gives rise to this important apparent solubility enhancement also results in decreased free fraction of drug. Similar to the case of cyclodextrins, this incorporation of drug in micelles significantly decreases the amount of free drug available for permeation through the intestinal membrane (31,32,34,35,40–42).
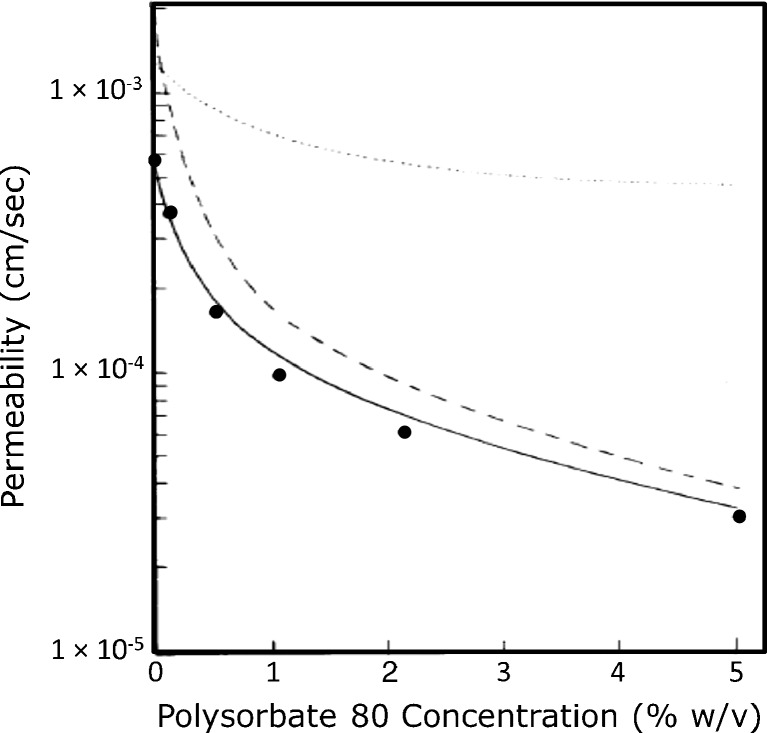
Amidon et al. (31) first established a theoretical model of the impact of micellar solubilization on the apparent membrane permeability of a poorly soluble drug. In this seminal work, they showed that the apparent permeability of progesterone across an artificial dimethicone membrane was decreased with increasing concentration of Tween 80 above the CMC (Fig. 4). We have since extended this work and the mechanistic model by looking at the impact of micellar solubilization by SLS and STC on the apparent permeability of progesterone in the rat intestinal perfusion model (35). The model for the solubility–permeability interplay when using surfactants is closely related to the cyclodextrins model, and hence, the schematic in Fig. 1 may represent the surfactant case as well. Similar to the case of cyclodextrins, this incorporation of drug in micelles significantly decreases the amount of free drug available for intestinal membrane permeation, such that Pm decreases with increasing surfactant concentration above CMC. At the same time, the micellar solubilization causes the effective UWL thickness to quickly decrease, such that Paq markedly increases with increasing surfactant concentration above the CMC. Since Paq increases and Pm decreases with increasing surfactant concentration above CMC, the unstirred water layer is effectively eliminated and the overall Peff tends toward membrane controlled as surfactant concentration is increasing. Our work demonstrates that when surfactants are used to enhance the apparent solubility of lipophilic drugs, there exists an interplay between solubility increase and permeability decrease. Most importantly, the opposing effects on the solubility and the permeability must be accounted for, in order to fully understand the impact on the overall absorption when surfactants are employed to enhance the oral exposure of a low-solubility drug. Our work offers a quantitative model that may be used to understand this solubility–permeability interplay, and predict a priori the tradeoff that occurs between apparent solubility increase and intestinal permeability decrease when surfactants are used as pharmaceutical solubilizers.
Fig. 4.
Theoretical predictions of P eff (solid line) and P m (dashed line) as a function of polysorbate 80 concentration. Black circle, experimentally determined P eff. Data derived from Amidon et al. (31)
It should be noted that the effective aqueous boundary layer thickness refers to the drug concentration gradient from the membrane surface to the bulk; it is important to distinguish this parameter from the hydrodynamic boundary layer thickness, which is the distance from the membrane surface to the point at which the fluid hydrodynamics (i.e., mixing) becomes constant and equal to bulk. The hydrodynamic boundary layer thickness is fixed under set hydrodynamic conditions, while the effective aqueous boundary layer thickness may change depending on the length of the concentration gradient. This analysis also explains the reason that the unstirred water boundary layer serves as a permeability barrier for low-solubility compounds, while high-solubility drugs are not affected by this boundary layer.
S-P INTERPLAY FROM NONBINDING SYSTEMS
In all of the cases presented thus far, the solubility increase occurred due to micellization/complexation, which reduces the free fraction of the drug. Decreased free fraction is directly translated to lower concentration gradient and hence thermodynamic driving force for membrane permeation. Therefore, in all of these cases, the decreased permeability with increased apparent solubility could be attributed to free fraction considerations, and not necessarily to direct interrelationship between the solubility and the permeability. While the tradeoff between the apparent solubility and intestinal permeability in binding systems (e.g., cyclodextrins, surfactants) is now well established and understood, the question at this point was whether this solubility–permeability tradeoff is unique to binding solubilization methods, or is it a general phenomenon attributable to the nature of increased apparent solubility per se, regardless of the solubilization method being used.
An elegant approach to isolate the increased apparent solubility from the free fraction may be the use of cosolvents. Solubilization by cosolvent does not involve complexation with the drug, and therefore, the issue of free fraction is not relevant for these systems. Hence, such a system may allow to investigate the direct solubility–permeability interplay. Riad and Sawchuk (46) evaluated the effect of different levels of polyethylene glycol 400 (PEG-400) on the intestinal permeability of the antiepileptic lipophilic drug carbamazepine using rabbit intestinal perfusion. The intestinal permeability varied inversely with the percentage of PEG-400 (Fig. 5). The authors explained the decreased permeability by a reduction in the thermodynamic activity of carbamazepine with increased concentrations of PEG-400, as well as by solvent drag considerations due to osmolarity of the perfusing solutions (46).
Fig. 5.
The effect of PEG-400 concentration on carbamazepine intestinal permeability in rabbit colon perfusion. Data derived from Riad and Sawchuk (46)
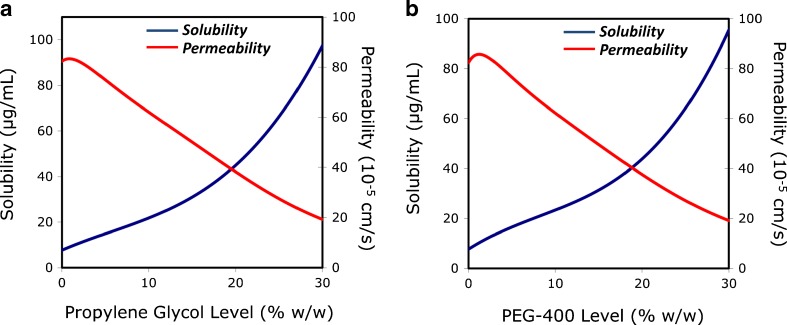
We have recently studied the apparent aqueous solubility (Saq) and rat intestinal permeability (Peff) of the lipophilic drug progesterone in systems containing various levels of the cosolvents propylene glycol and PEG-400, to reveal the solubility–permeability interplay from nonbinding systems (47). To eliminate possible influences on the permeability results, thermodynamic activity was maintained equivalent in all permeability studies (75% equilibrium solubility). Both cosolvents increased progesterone Saq in nonlinear fashion. Decreased Peff with increased Saq was observed, despite the constant thermodynamic activity, and the nonrelevance of free fraction. A mass transport analysis was developed to describe this interplay. A schematic of the model is shown in Fig. 6. The model considers the effects of solubilization on the permeability through the intestinal membrane (Pm) and the permeability through the UWL (Paq), to predict the overall Peff dependence on Saq. The analysis revealed that: (1) the effective UWL thickness quickly decreases with ↑Saq, such that Paq markedly increases with ↑Saq; (2) the apparent membrane/aqueous partitioning decreases with ↑Saq, thereby reducing the thermodynamic driving force for permeability such that ↓Pm with ↑Saq; and (3) since ↑Paq and ↓Pm with ↑Saq, the UWL is eliminated and Peff becomes membrane controlled with ↑Saq. The model allowed excellent quantitative prediction of Peff as a function of Saq. The overall apparent solubility vs. permeability predicted by the model is presented in Fig. 7. This work demonstrated that a direct tradeoff exists between the apparent solubility and permeability irrespectively of the free fraction, which must be taken into account when developing solubility-enabling formulations to strike the optimal solubility–permeability balance, in order to maximize the overall oral absorption.
Fig. 6.
Schematic illustration of the quasi-equilibrium transport model describing the effect of cosolvent on the drug transport through the unstirred water layer and the intestinal membrane. Complete derivation of the equations can be found in Miller et al. (47)
Fig. 7.
The effects of increasing propylene glycol (a, left panel) and PEG-400 (b, right panel) concentration on progesterone apparent aqueous solubility and intestinal permeability based on the theoretical quasi-equilibrium transport model shown in Fig. 6 (47)
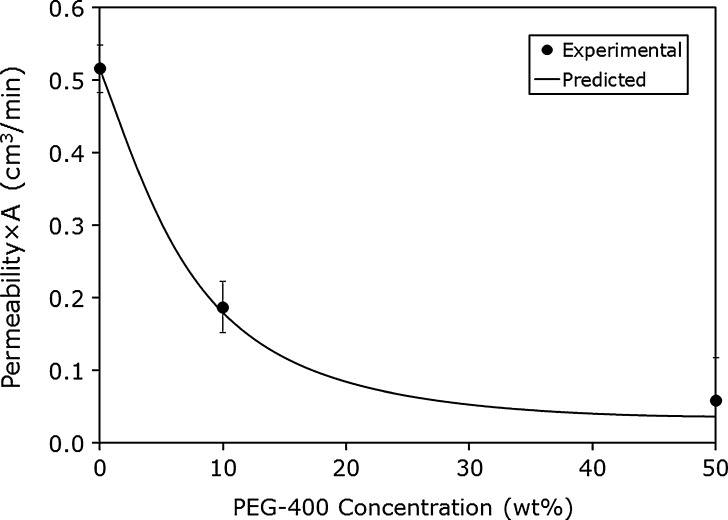
To further evaluate our mass transport model sketched in Fig. 6, we have applied the model to the literature experimental carbamazepine permeability data from Riad and Sawchuk (46) using our experimental carbamazepine solubility data (48). It can be seen in Fig. 8 that the model enabled excellent prediction of the experimental results. More recently, we have revealed that the intestinal absorption of carbamazepine is membrane controlled and that the unstirred water layer is not effective as an absorption barrier (48). Still, the apparent permeability of carbamazepine decreased significantly with increased solubility in the presence of PEG-400; the increased solubility in the aqueous GI milieu reduced the apparent membrane/aqueous partitioning, thereby reducing the driving force for membrane permeability. Hence, we have shown that the solubility–permeability interplay exists whether the aqueous boundary layer is an effective barrier to the absorption, as in the previous examples with progesterone, or not, as in the case of carbamazepine.
Fig. 8.
The effective permeability (P eff; in centimeters per second) of carbamazepine as a function of PEG-400 concentration in the rabbit intestinal perfusion model. The theoretical line was calculated via the transport model shown in Fig. 6 (47), and the experimental data points were derived from Riad and Sawchuk (46)
CONCLUSIONS
In conclusion, the weight of the evidence indicates that the solubility–permeability interplay/tradeoff cannot be ignored when using solubility-enabling formulations; the formulator must assure that the solubility gain triumphs the permeability loss, and then the result will be higher absorption. It is important to know and realize the solubility–permeability tradeoff, to avoid the opposite scenario in which the permeability loss triumphs the solubility gain. Overall, looking solely at the solubility enhancement that the formulation enables may be misleading with regards to predicting the resulted absorption, and hence, the solubility–permeability interplay must be accounted for to strike the optimal solubility–permeability balance and to maximize the overall absorption.
References
- 1.Lennernas H. Human intestinal permeability. J Pharm Sci. 1998;87(4):403–410. doi: 10.1021/js970332a. [DOI] [PubMed] [Google Scholar]
- 2.Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv Drug Deliv Rev. 1996;19(3):359–376. doi: 10.1016/0169-409X(96)00009-9. [DOI] [PubMed] [Google Scholar]
- 3.Sun D, Yu LX, Hussain MA, Wall DA, Smith RL, Amidon GL. In vitro testing of drug absorption for drug ‘developability’ assessment: forming an interface between in vitro preclinical data and clinical outcome. Curr Opin Drug Discov Devel. 2004;7(1):75–85. [PubMed] [Google Scholar]
- 4.Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 5.Dahan A, Amidon GL. Segmental dependent transport of low permeability compounds along the small intestine due to P-glycoprotein: the role of efflux transport in the oral absorption of BCS class III drugs. Mol Pharmaceutics. 2009;6(1):19–28. doi: 10.1021/mp800088f. [DOI] [PubMed] [Google Scholar]
- 6.Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. AAPS J. 2009;11(4):740–746. doi: 10.1208/s12248-009-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahan A, Miller JM, Hilfinger JM, Yamashita S, Yu LX, Lennernas H, et al. High-permeability criterion for BCS classification: segmental/pH dependent permeability considerations. Mol Pharmaceutics. 2010;7(5):1827–1834. doi: 10.1021/mp100175a. [DOI] [PubMed] [Google Scholar]
- 8.Lennernas H. Human jejunal effective permeability and its correlation with preclinical drug absorption models. J Pharm Pharmacol. 1997;49(7):627–638. doi: 10.1111/j.2042-7158.1997.tb06084.x. [DOI] [PubMed] [Google Scholar]
- 9.Lobenberg R, Amidon GL. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50(1):3–12. doi: 10.1016/S0939-6411(00)00091-6. [DOI] [PubMed] [Google Scholar]
- 10.Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42(6):620–643. doi: 10.1177/00970002042006005. [DOI] [PubMed] [Google Scholar]
- 11.Dahan A, Hoffman A. Use of a dynamic in vitro lipolysis model to rationalize oral formulation development for poor water soluble drugs: correlation with in vivo data and the relationship to intra-enterocyte processes in rats. Pharm Res. 2006;23(9):2165–2174. doi: 10.1007/s11095-006-9054-x. [DOI] [PubMed] [Google Scholar]
- 12.Dahan A, Hoffman A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release. 2008;129(1):1–10. doi: 10.1016/j.jconrel.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Carr RA, Spence JK, Wang WW, Turner TM, Lipari JM, et al. A pH-dilution method for estimation of biorelevant drug solubility along the gastrointestinal tract: application to physiologically based pharmacokinetic modeling. Mol Pharmaceutics. 2010;7(5):1516–1526. doi: 10.1021/mp100157s. [DOI] [PubMed] [Google Scholar]
- 14.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 15.Stenberg P, Bergström CAS, Luthman K, Artursson P. Theoretical predictions of drug absorption in drug discovery and development. Clin Pharmacokinet. 2002;41(11):877–899. doi: 10.2165/00003088-200241110-00005. [DOI] [PubMed] [Google Scholar]
- 16.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59(7):645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3(12):1023. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 18.Loftsson T, Brewster .. ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 19.Rajewski RA, Stella VJ. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J Pharm Sci. 1996;85(11):1142–1169. doi: 10.1021/js960075u. [DOI] [PubMed] [Google Scholar]
- 20.Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123(2):78. doi: 10.1016/j.jconrel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Loftsson T, Vogensen SB, Brewster ME, Konráðsdóttir F. Effects of cyclodextrins on drug delivery through biological membranes. J Pharm Sci. 2007;96(10):2532–2546. doi: 10.1002/jps.20992. [DOI] [PubMed] [Google Scholar]
- 22.Loftsson T, Jarho P, Másson M, Järvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005;2(2):335–351. doi: 10.1517/17425247.2.1.335. [DOI] [PubMed] [Google Scholar]
- 23.Loftsson T, Brewster ME, Masson M. Role of cyclodextrins in improving oral drug delivery. Am J Drug Deliv. 2004;2(4):261. doi: 10.2165/00137696-200402040-00006. [DOI] [Google Scholar]
- 24.Rao VM, Stella VJ. When can cyclodextrins be considered for solubilization purposes? J Pharm Sci. 2003;92(5):927–932. doi: 10.1002/jps.10341. [DOI] [PubMed] [Google Scholar]
- 25.Miller LA, Carrier RL, Ahmed I. Practical considerations in development of solid dosage forms that contain cyclodextrin. J Pharm Sci. 2007;96(7):1691–1707. doi: 10.1002/jps.20831. [DOI] [PubMed] [Google Scholar]
- 26.Dahan A, Miller JM, Hoffman A, Amidon GE, Amidon GL. The solubility–permeability interplay in using cyclodextrins as pharmaceutical solubilizers: mechanistic modeling and application to progesterone. J Pharm Sci. 2010;99(6):2739–2749. doi: 10.1002/jps.22033. [DOI] [PubMed] [Google Scholar]
- 27.Gamsiz E, Miller L, Thombre A, Ahmed I, Carrier RL. Modeling the influence of cyclodextrins on oral absorption of low-solubility drugs: I. Model development. Biotech Bioeng. 2010;105(2):409–420. doi: 10.1002/bit.22523. [DOI] [PubMed] [Google Scholar]
- 28.Gamsiz E, Thombre A, Ahmed I, Carrier RL. Drug salts and solubilization: modeling the influence of cyclodextrins on oral absorption. Ann Biomed Eng. 2011;39(1):455–468. doi: 10.1007/s10439-010-0169-1. [DOI] [PubMed] [Google Scholar]
- 29.Gamsiz ED, Miller L, Thombre AG, Ahmed I, Carrier RL. Modeling the influence of cyclodextrins on oral absorption of low solubility drugs: II. Experimental validation. Biotechnol Bioeng. 2010;105(2):421–430. doi: 10.1002/bit.22524. [DOI] [PubMed] [Google Scholar]
- 30.Brewster ME, Noppe M, Peeters J, Loftsson T. Effect of the unstirred water layer on permeability enhancement by hydrophilic cyclodextrins. Int J Pharm. 2007;342(1–2):250–253. doi: 10.1016/j.ijpharm.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Amidon GE, Higuchi WI, Ho NFH. Theoretical and experimental studies of transport of micelle-solubilized solutes. J Pharm Sci. 1982;71(1):77–84. doi: 10.1002/jps.2600710120. [DOI] [PubMed] [Google Scholar]
- 32.Katneni K, Charman SA, Porter CJH. Permeability assessment of poorly water-soluble compounds under solubilizing conditions: the reciprocal permeability approach. J Pharm Sci. 2006;95(10):2170–2185. doi: 10.1002/jps.20687. [DOI] [PubMed] [Google Scholar]
- 33.Katneni K, Charman SA, Porter CJH. Impact of cremophor-EL and polysorbate-80 on digoxin permeability across rat jejunum: delineation of thermodynamic and transporter related events using the reciprocal permeability approach. J Pharm Sci. 2007;96(2):280–293. doi: 10.1002/jps.20779. [DOI] [PubMed] [Google Scholar]
- 34.Katneni K, Charman SA, Porter CJH. Use of plasma proteins as solubilizing agents in in vitro permeability experiments: correction for unbound drug concentration using the reciprocal permeability approach. J Pharm Sci. 2008;97(1):209–224. doi: 10.1002/jps.20877. [DOI] [PubMed] [Google Scholar]
- 35.Miller JM, Beig A, Krieg BJ, Carr RA, Borchardt TB, Amidon GE, et al. The solubility–permeability interplay: mechanistic modeling and predictive application of the impact of micellar solubilization on intestinal permeation. Mol Pharmaceutics. 2011;8(5):1848–1856. doi: 10.1021/mp200181v. [DOI] [PubMed] [Google Scholar]
- 36.Mudra DR, Borchardt RT. Absorption barriers in the rat intestinal mucosa. 3: effects of polyethoxylated solubilizing agents on drug permeation and metabolism. J Pharm Sci. 2010;99(2):1016–1027. doi: 10.1002/jps.21836. [DOI] [PubMed] [Google Scholar]
- 37.Mudra DR, Borchardt RT. Absorption barriers in the rat intestinal mucosa: 1. Application of an in situ perfusion model to simultaneously assess drug permeation and metabolism. J Pharm Sci. 2010;99(2):982–998. doi: 10.1002/jps.21912. [DOI] [PubMed] [Google Scholar]
- 38.Mudra DR, Jin JY, Borchardt RT. Absorption barriers in the rat intestinal mucosa: 2. Application of physiologically based mathematical models to quantify mechanisms of drug permeation and metabolism. J Pharm Sci. 2010;99(2):999–1015. doi: 10.1002/jps.21965. [DOI] [PubMed] [Google Scholar]
- 39.Nerurkar MM, Ho NFH, Burton PS, Vidmar TJ, Borchardt RT. Mechanistic roles of neutral surfactants on concurrent polarized and passive membrane transport of a model peptide in Caco-2 cells. J Pharm Sci. 1997;86(7):813–821. doi: 10.1021/js960483y. [DOI] [PubMed] [Google Scholar]
- 40.Poelma FGJ, Breäs R, Tukker JJ. Intestinal absorption of drugs. III. The influence of taurocholate on the disappearance kinetics of hydrophilic and lipophilic drugs from the small intestine of the rat. Pharm Res. 1990;7(4):392–397. doi: 10.1023/A:1015827624296. [DOI] [PubMed] [Google Scholar]
- 41.Poelma FGJ, Breäs R, Tukker JJ, Crommelin DJA. Intestinal absorption of drugs. The influence of mixed micelles on the disappearance kinetics of drugs from the small intestine of the rat. J Pharm Pharmacol. 1991;43(5):317–324. doi: 10.1111/j.2042-7158.1991.tb06697.x. [DOI] [PubMed] [Google Scholar]
- 42.Yano K, Masaoka Y, Kataoka M, Sakuma S, Yamashita S. Mechanisms of membrane transport of poorly soluble drugs: role of micelles in oral absorption processes. J Pharm Sci. 2010;99(3):1336–1345. doi: 10.1002/jps.21919. [DOI] [PubMed] [Google Scholar]
- 43.Buyukozturk F, Benneyan JC, Carrier RL. Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J Control Release. 2010;142(1):22–30. doi: 10.1016/j.jconrel.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Dahan A, Hoffman A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: the ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur J Pharm Biopharm. 2007;67(1):96–105. doi: 10.1016/j.ejpb.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 45.Giacomini K, Huang S, Tweedie D, Benet L, Brouwer K, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riad LE, Sawchuk RJ. Effect of polyethylene glycol 400 on the intestinal permeability of carbamazepine in the rabbit. Pharm Res. 1991;8(4):491–497. doi: 10.1023/A:1015803312233. [DOI] [PubMed] [Google Scholar]
- 47.Miller JM, Beig A, Carr RA, Webster GK, Dahan A. The solubility–permeability interplay when using cosolvents for solubilization: revising the way we use solubility-enabling formulations. Mol Pharmaceutics. 2012. doi:10.1021/mp200460u. [DOI] [PubMed]
- 48.Beig A, Miller JM, Dahan A. Accounting for the solubility–permeability interplay in oral formulation development for poor water solubility drugs: the effect of PEG-400 on carbamazepine absorption. Eur J Pharm Biopharm. 2012. doi:10.1016/j.ejpb.2012.02.012. [DOI] [PubMed]