Abstract
Purpose
Breathing and swallowing problems affect elderly people and may be related to age-associated tongue dysfunction. Hypoglossal motoneurons that innervate the tongue receive a robust, excitatory serotonergic (5HT) input and may be affected by aging. We used a rat model of aging and progressive resistance tongue exercise to determine if age-related alterations in 5HT inputs to the hypoglossal nucleus can be modifed. We hypothesized that tongue forces would increase with exercise, 5HT input to the tongue would decrease with age, and tongue exercise would augment 5HT input to the hypoglossal nucleus.
Method
Young (9-10 months), middle-aged (24-25 months), and old (32-33 months) male F344/BN rats received tongue exercise for 8 weeks. Immunoreactivity for 5HT was measured in digital images of sections through the hypoglossal nucleus using ImageJ software.
Results
Tongue exercise resulted in increased maximum tongue forces at all ages. There was a statistically significant increase in 5HT immunoreactivity in the hypoglossal nucleus in exercised, young rats, but only in the caudal third of the nucleus, and primarily in the ventral half.
Conclusions
Specificity found in serotonergic input following exercise may reflect the topographic organization of motoneurons in the hypoglossal nucleus and the tongue muscles engaged in the exercise paradigm.
Introduction
Hypoglossal motoneurons innervating tongue muscles contribute significantly to the maintenance of airway patency, especially during inspiration (Remmers, deGroot, Sauerland, & Anch, 1978). Reduced hypoglossal nerve activity during sleep or anesthesia can result in hypoxia due to upper airway obstruction. Hypoglossal motoneurons are activated primarily by glutamatergic drive from presynaptic neurons located in the pons and the medulla (Greer, Smith, & Feldman, 1991). Neuronal activity is modulated by a variety of neurotransmitters including serotonergic, noradrenergic, histaminergic and GABAergic inputs to hypoglossal motoneurons (Bastedo, Chan, Park, Liu, & Horner, 2009; V. B. Fenik, Davies, & Kubin, 2005; Horner, 2008; Rekling, Funk, Bayliss, Dong, & Feldman, 2000; Sood, Morrison, Liu, & Horner, 2005).
The hypoglossal nucleus receives serotonergic (5HT) input primarily from neurons in the medullary nucleus raphe pallidus and obscurus and the parapyramidal region (Barker, Thomas, & Behan, 2009; Manaker & Tischler, 1993). In vitro and in vivo studies have shown that 5HT activation of hypoglossal motoneurons is mediated by postsynaptic 5HT2A receptors (Brandes, et al., 2007; P. Fenik & Veasey, 2003; Schwarzacher, Pestean, Gunther, & Ballanyi, 2002). 5HT neurons are tonically active during wakefulness, and decrease their firing rate during sleep (Jacobs & Azmitia, 1992; Kubin, Tojima, Davies, & Pack, 1992). Withdrawal of 5HT excitatory drive to hypoglossal motoneurons during sleep is thought to reduce upper airway patency and contribute to sleep apnea (Kubin, Tojima, Reignier, Pack, & Davies, 1996; Remmers, et al., 1978).
Previous studies in our lab have shown that 5HT inputs to the hypoglossal nucleus undergo plasticity. 5HT inputs in the hypoglossal nucleus are influenced by age, with a reduction in the number of synaptic boutons and an increase in hypertrophied boutons seen in old rats (Behan & Brownfield, 1999). In female rats, hypoglossal 5HT levels vary with the 5-day estrous cycle (Behan, Zabka, Thomas, & Mitchell, 2003), possibly due to fluctuations in estrogen and progesterone levels (Behan & Wenninger, 2008). Taken together these data suggest that serotonergic axons and terminals in the hypoglossal nucleus have the capacity to remodel, not just with aging, but also dynamically. Remodeling of synaptic terminals has been described at the rat soleus neuromuscular junction following a 5-week regime of progressive resistance exercise in young rats (Deschenes, et al., 2000). The enhanced tongue protrusive force following exercise may be the result of an increase in 5HT modulation of hypoglossal motoneurons. We hypothesized that progressive resistance tongue exercise would result in increased voluntary tongue forces and remodeling of 5HT inputs to the hypoglossal nucleus, specifically by an increase in the number of synaptic boutons. Further, we hypothesized that exercise-induced augmentation in 5HT input would be age-dependent: greater in young rats compared with old rats based on the fact that there are age-associated changes in hypoglossal motoneurons (Schwarz, Thompson, Connor, & Behan, 2009) and the hypoglossal neuromuscular junction (Hodges, Anderson, & Connor, 2004). To address these hypotheses we quantified 5HT immunoreactivity in the hypoglossal nucleus in young, middle-aged and old, exercised and control male rats.
Methods
All procedures were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Wisconsin. At study completion, the animals were in one of three age groups: young (9-10 months), middle-aged (24-25 months), or old (32-33 months). A total of 48 (16 young, 16 middle-aged, 16 old) male Fischer 344/Brown Norway (F344/BN) rats were obtained from the National Institute of Aging colony. Within each age group 8 animals were divided into two different treatment groups: control and exercised. The median life expectancy of F344/BN rats is 36 months (Turturro, et al., 1999). Every effort was made to minimize the number of animals used and their suffering. Thus, tissues from these animals were assigned to more than one experiment.
Exercise
A detailed account of tongue exercise procedures can be found elsewhere (Connor, et al., 2009). Briefly, animals in the exercise group were obtained when they were either 6 months, 21 months or 29 months old to allow approximately 3 months of preparation, training, and tongue exercise prior to euthanizing them for study of the hypoglossal nuclei.
For the first 14 days after arrival in the animal care facility, exercise rats were gradually light-cycle reversed and water-restricted to exposure to water for only 3 hours per day. Following this period, an additional 10 days was spent training the animals to push the tongue against an instrumented disk to obtain a water reward. The instrumented disk was placed into an enclosure that was designed to accommodate a single rat with a depressed front panel and a small portal that allowed tongue access to the force operandum disk, with water presentation across the disk region. The apparatus functioned under computer control to measure parameters of the tongue press and to deliver a water reward if preset force challenges were met (Matrix Product Development, Cottage Grove, WI). A traditional learning paradigm was used to train the exercise group to push the tongue against the disk. A water reward was delivered according to a variable ratio (VR) 5 reinforcement schedule.
Tongue force maxima were individually estimated for each rat by averaging the 10 highest tongue forces exerted across three days of increment training. This estimated maximum press (EMP) value was then used to set thresholds for eight weeks of progressive resistance exercise and comprised the baseline maximum voluntary tongue force (MVTF) value. For the first two weeks, animals were trained to push the tongue against the disk at 50% of their EMP to obtain the water reward. For weeks 3 and 4, animals were required to press the tongue against the disk at 60% of the EMP. Similarly, weeks 5 and 6, and then 7 and 8 required 70% and 80% of the EMP to obtain the water reward, respectively. Increment training was repeated following the 8 weeks of tongue exercise to obtain post-exercise MVTF values.
Control animals did not receive tongue exercise or increment testing. As such, tongue force measures were available only for the animals within the exercise group. It was not possible to obtain voluntary tongue forces in the no-exercise group without training animals to perform the tongue force task, which would necessarily involve 6-10 days of tongue exercise at baseline and at 8 weeks following baseline. Accordingly, no-exercise control animals were used only for between-subjects analyses of the hypoglossal nuclei and not tongue force analyses.
Perfusion
Rats were anesthetized with isoflurane followed by sodium pentobarbital (120 mg/kg i.p.). Anesthetized rats were transcardially perfused with 200 ml of heparinized saline (10,000 units/liter) followed by 400 mL of 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1M sodium phosphate buffer (PB) (pH 7.4). Brains were removed and postfixed for 1 hour at 4°C, then cryoprotected for 24-36 hours at 4°C with 20% sucrose and 5% glycerol in 0.1M PB. Sections were cut coronally (50 μm) and stored in 0.1M phosphate buffer containing 0.02% sodium azide at 4°C. Each hypoglossal nucleus yielded approximately 45 sections. These sections were divided equally to represent rostral, middle, and caudal regions of the nucleus. Two old exercised rats died before the brains could be harvested for immunocytochemistry.
Immunocytochemistry
Two representative sections from rostral, middle and caudal regions of the hypoglossal nucleus were selected from each animal for 5HT immunoreactivity. Sections were washed in 0.1M phosphate buffered saline (PBS) for 3 × 5 minutes, and incubated in 3% hydrogen peroxide in distilled H2O (dH2O) for 20 minutes. Sections were washed again (3 × 5 minutes) and pre-treated with 1% sodium borohydride in dH2O for 30 minutes. Sections were washed in 0.1% Triton X-100 (Tx-100; Sigma, St. Louis, MO, USA) in 0.1M PBS (3 × 5 minutes), blocked with 5% normal goat serum (NGS) in 0.1% Tx-100 in 0.1M PBS for 60 minutes, and incubated with an antibody to 5HT made in rabbit (1:5000, (Brownfield, Yracheta, Chu, Lorenz, & Diaz, 1998) in 1% NGS with 0.75% Tx-100 for 18 hours at 4°C with gentle agitation. Sections were washed in 0.1M PBS with 0.1% Tx-100 (6 × 10 minutes). Sections were incubated with biotinylated goat anti-rabbit IgG (1:1000, Vector Laboratories, Burlingame, CA, USA) with 3% NGS solution in 0.1M PBS with 0.25% Tx -100 for 2 hours at room temperature. Sections were washed in 0.1M PBS with 0.1% Tx-100 (3 × 10 minutes) and then incubated with ABC Complex (0.6%; Vectastain Elite Kit, Vector Laboratories, Burlingame, CA, USA) in 0.1M PBS with 0.1% Tx-100 for 60 minutes at room temperature. Sections were washed in 0.1M PBS (2 × 10 minutes) and incubated with 0.08% DAB in 0.1M PBS with 0.02% H2O2 for 3 minutes. Sections were washed, mounted on slides, dried overnight, and coverslipped with Eukitt (Calibrated Instruments, Hawthorne, NY, USA).
Analysis
Digital images were taken using the SPOT (Advanced version) computer software and a SPOT RT Slider camera (Diagnostic Instruments, Sterling Heights, MI, USA) attached to a Nikon Eclipse E600 microscope. Microscope slides were coded such that the investigator was blinded to their identity throughout the analysis. The paired hypoglossal nuclei were divided into quadrants (Fig. 1). One picture was taken of each quadrant using the 60X objective, resulting in 8 images from each section. Images (area = 30,000 μm2; 8 pixels/μm2) were analyzed for 5HT staining density (ImageJ, version 1.42a; NIH). Images were adjusted to visually highlight 5HT staining. 5HT terminals range from 0.5–3.0 μm in diameter (Aldes, Chronister, Marco, Haycock, & Thibault, 1988). Thus, particles measuring 0.5–3.50 μm2 were counted. The area fraction (percentage of stained pixels) in each image was computed (Rasband, 1997-2001; Abramoff et al., 2004). To confirm that measurements were reliable, 10 ventrolateral or ventromedial caudal sections were randomly chosen, re-visualized and re-measured by the same microscopist (CFT). Area fractions were recalculated without reference to the original measures. Area fraction values from the original and remeasured slides were highly correlated (r=.82) with a mean difference of 0.1. Original values were used in statistical analyses.
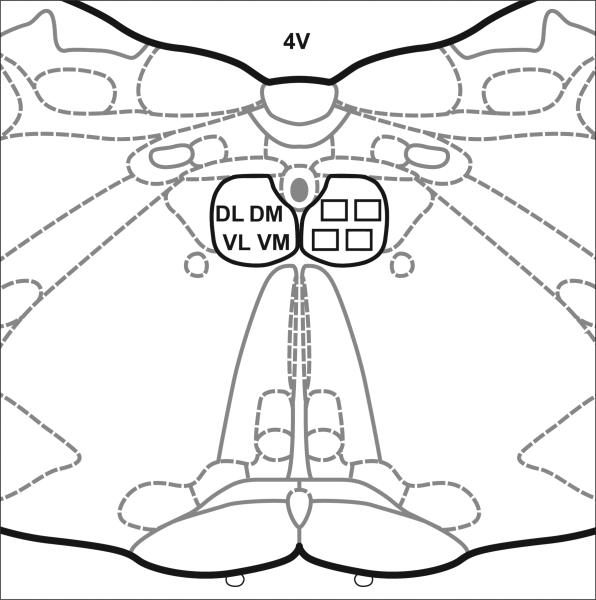
Figure 1.
The location of sample areas in the hypoglossal nucleus. The paired hypoglossal nuclei were divided into quadrants. One image was taken from each quadrant: dorsolateral (DL), dorsomedial (DM), ventrolateral (VL) and ventromedial (VM) on each side. 4V, fourth ventricle. Diagram modified from (Paxinos & Watson, 1998).
We used an analysis of covariance (ANCOVA) to compare tongue forces between age groups with the weight of the animal as the covariate. The pre-to-post exercise change in force was assessed within each age group using a paired t-test. Weights and changes in weight were compared between age and exercise groups using analysis of variance (ANOVA). The impact of age, exercise, weight, region, quadrant, and side on the percent of area in the hypoglossal nucleus that contained 5HT was examined using a repeated measures ANOVA. Post hoc tests were performed using Fisher's protected Lesat Significant Difference (LSD) tests. The Fisher LSD provides a t-statistic that is reported in the results along with the associated p-values.
All analyses were performed using SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC). All tests were two-tailed and p-values less than 0.05 were considered as significant. Experimentwise error was not controlled with an alpha correction because these conservative approaches may have led us to disregard biological meaningful contrasts, and as such increase the probability of Type II error.
Results
Weight
Prior to and following tongue exercise, animal body weight increased significantly with age (F5,39 = 18.8, p < 0.0001; F5,39 = 5.35, p < 0.0008). Post hoc tests revealed that the old and middle aged rats were significantly heavier than the young rats, with no difference between middle-aged and old (p > 0.05). However, there were no statistically significant differences in weight between control and exercised groups of rats at any age. Because difference in body weight among age groups may affect measurement of muscle strength (Jaric, 2002), force data were compared using analysis of covariance (ANCOVA) with weight as a covariate.
Force
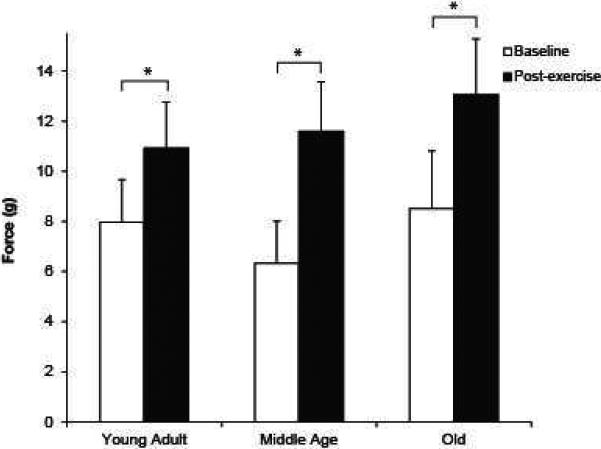
When adjusted for weight, tongue exercise resulted in increased maximum tongue force (g) at all ages compared with baseline values (Fig. 2; F2, 19 = 3.79, p = 0.04). Posthoc testing revealed that there was a significantly greater increase in tongue force with exercise in the middle aged rats compared with young adult rats (p < 0.01), and no difference between young adult and old, or middle aged and old rats. Although there were no significant differences in baseline force across the three age groups, there was a trend for middle-aged rats to exert less force than young and old rats (Fig. 2). The greatest change in force with exercise was exhibited by middle-aged and old rats (young: 46%; middle aged: 60.8%; old: 67.6%).
Figure 2.
Baseline and post-exercise tongue force in young, middle-aged and old rats. At all ages there was a statistically significant increase in force following 8 weeks of exercise training (p < 0.05).
5HT immunoreactivity in Exercised vs. Control Rats
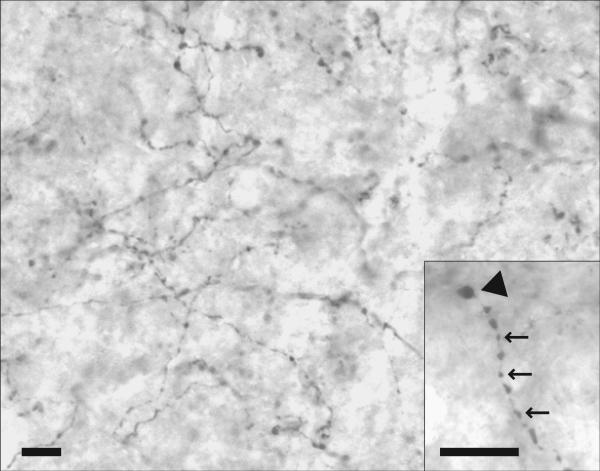
Dense 5HT immunoreactivity was present throughout the hypoglossal nucleus compared with the surrounding brain regions (Fig. 3). Synaptic boutons were present along axons (en passant) as well as at axon terminals. The majority of boutons measured 0.5–3.0 μm in diameter.
Figure 3.
Photomicrograph of 5HT immunoreactivity in the ventrolateral quadrant of the middle third of the hypoglossal nucleus in a middle-aged control rat. Synaptic boutons are present along axons (en passant) as well as at axon terminals. Boutons measure 0.5– 3.0 μm in diameter. Insert shows en passant (arrows) and terminal (arrowhead) boutons. Scale bars = 10 μm.
Rostrocaudal Extent of the Hypoglossal Nucleus
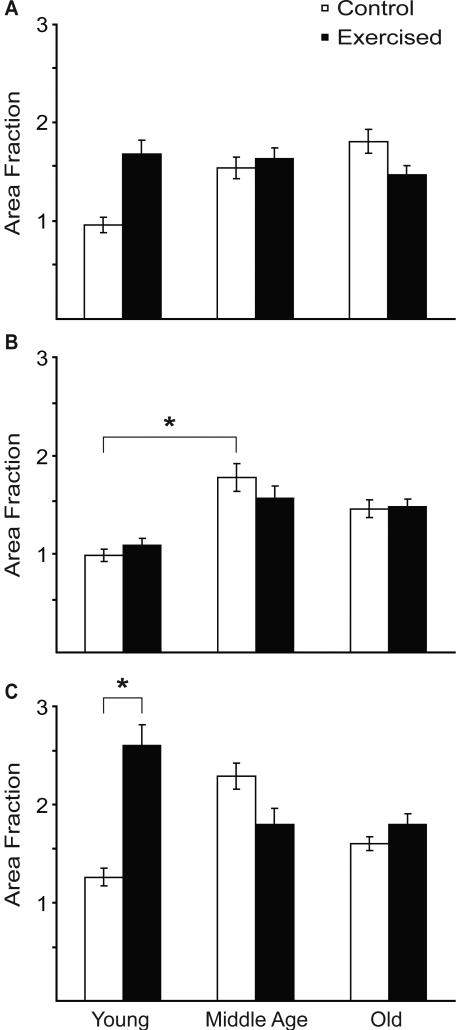
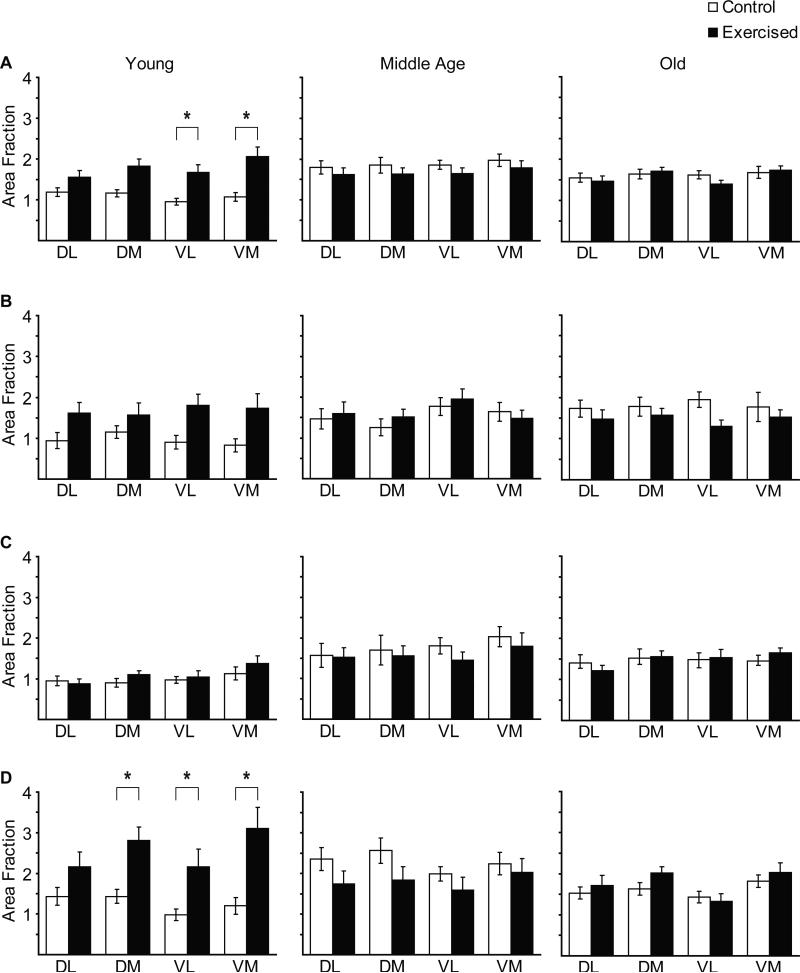
In the rostral and middle regions of the hypoglossal nucleus, there was no significant difference in 5HT immunoreactivity in young, middle aged or old rats as a function of exercise (p > 0.05) (Fig. 4A,B). However, in the caudal one-third of the nucleus, there was a statistically significant effect of exercise on 5HT immunoreactivity in young rats only (t37 = 2.58, p = 0.01) (Fig. 4C). When each of the four quadrants (dorsolateral, dorsomedial, ventrolateral, ventromedial) were analyzed individually throughout the rostro-caudal extent of the nucleus, there was a statistically significant increase in 5HT immunoreactivity as a function of exercise in the ventrolateral and ventromedial quadrants, but only in young exercised by comparison with young control rats (t37 = 2.53, p = 0.016 and t37 = 2.34, p = 0.02, respectively) (Fig. 5A).
Figure 4.
5HT immunoreactivity (area fraction) in rostral (A), middle (B) and caudal (C) regions of the hypoglossal nucleus in young, middle-aged and old, control and exercised rats. Asterisk denotes significance (p < 0.05).
Figure 5.
Quadrant-specific 5HT immunoreactivity (area fraction) in the entire hypoglossal nucleus (A), and in rostral (B), middle (C) and caudal (D) regions in young, middle-aged and old, control and exercised rats. Asterisk denotes significance (p < 0.05).
In the rostral and caudal regions of the hypoglossal nucleus, there was no statistically significant effect of age on 5HT immunoreactivity (Fig. 4A,C). In the middle region of the hypoglossal nucleus, there was an age-associated increase in 5HT immunoreactivity in middle-aged by comparison with young control rats (t37 = 2.35, p = 0.02) (Fig. 4B).
Quadrants of the Hypoglossal Nucleus
When each quadrant (dorsolateral, dorsomedial, ventrolateral, ventromedial) was analyzed in the rostral, the middle, or the caudal third of the nucleus (Fig. 5B,C,D), there was a statistically significant increase in 5HT immunoreactivity as a function of exercise only in the caudal third of the nucleus, and only in young animals. This increase was present in dorsomedial, ventrolateral and ventromedial quadrants (dorsomedial t37 = 2.71, p = 0.01; ventrolateral t37 = 2.37, p = 0.02; ventromedial t37 = 3.03, p = 0.004) (Fig. 5D).
When each of the four quadrants (dorsolateral, dorsomedial, ventrolateral, ventromedial) were analyzed individually throughout the rostro-caudal extent of the hypoglossal nucleus, there was not a statistically significant difference in 5HT immunoreactivity in exercised vs. control rats (Fig. 5A). Similarly, when each quadrant was analyzed in the rostral, the middle, or the caudal third of the hypoglossal nucleus, no statistically significant differences were found between exercised and control rats (Fig. 5B,C,D).
No statistically significant correlations were found between tongue force measurements and 5HT immunoreactivity in the caudal region of the hypoglossal nucleus in exercised rats at any age.
Discussion
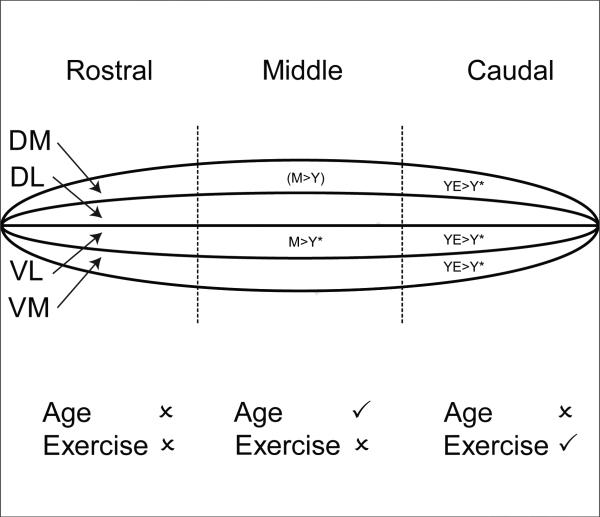
The hypotheses tested in this investigation were that progressive resistance tongue exercise would result in greater voluntary tongue forces relative to baseline force levels and age-dependent remodeling of 5HT inputs to the hypoglossal nucleus, with greater change manifested in young rats versus old rats. Our findings allowed us to accept these hypotheses in that: (1) tongue exercise resulted in significantly increased tongue forces at all ages; (2) tongue exercise resulted in a region-specific increase in 5HT immunoreactivity in the hypoglossal nucleus in young rats; (3) age-associated changes in 5HT immunoreactivity in the hypoglossal nucleus were region specific (Fig. 6). Regional changes in 5HT immunoreactivity may reflect the topographic organization of motoneurons in the hypoglossal nucleus and the specific tongue muscles engaged in the exercise paradigm.
Figure 6.
Summary diagram of region-specific exercise-induced and age-related changes in 5HT immunoreactivity in the hypoglossal nucleus. Top: The hypoglossal nucleus is represented as an elongated cylinder divided into three regions, rostral, middle and caudal. Within each region are four sample quadrants, dorsolateral (DL), dorsomedial (DM), ventrolateral (VL) and ventromedial (VM). Rat groups comprise young (Y), young-exercised (YE), middle-aged (M), middle-aged exercised (ME), old (O), old exercised (OE). Asterisks denote significance (p < 0.05). Brackets denote a strong trend. Bottom: Check marks denote a statistically significant effect of age or exercise in a region of the hypoglossal nucleus. X denotes no statistically significant effect.
Anatomy of the Hypoglossal Nucleus
A brief overview of hypoglossal nucleus anatomy is presented to provide potential explanations for our findings. In the hypoglossal nucleus of the rat, separate pools of motoneuronss innervate individual tongue muscles. Dividing the hypoglossal nucleus in half dorsoventrally, motoneuronss in the dorsal half innervate retrusor tongue muscles (styloglossus and hyoglossus) via the lateral branch of the hypoglossal nerve, and motoneurons in the ventral half innervate protrusor tongue muscles (genioglossus) via the medial branch of the hypoglossal nerve (Aldes, 1995; Altschuler, Bao, & Miselis, 1994; Dobbins & Feldman, 1995; McClung & Goldberg, 1999).
It is estimated that 20-30% of motoneurons in each subdivision innervate extrinsic muscles, with the remainder innervating a complex network of intrinsic tongue muscles oriented in longitudinal, vertical and transverse directions (McClung & Goldberg, 1999, 2002; Uemura-Sumi, Itoh, & Mizuno, 1988). Motoneurons innervating intrinsic muscles are present in both the dorsal (superior longitudinal, inferior longitudinal) and ventral (transverse, vertical) subdivisions of the hypoglossal nucleus. The rostrocaudal topography of the hypoglossal nucleus is such that genioglossal motoneurons innervating the main protrusor muscle of the tongue are found throughout the entire rostrocaudal extent of the ventral subdivision (Aldes, 1995; McClung & Goldberg, 2002). From the perspective of the tongue, muscles in the dorsal aspect (hyoglossus, superior longitudinal) are innervated by motoneurons in the caudal half of the hypoglossal nucleus whereas muscles in the ventral aspect (styloglossus, superior longitudinal) are innervated by motoneurons in the rostral half of the nucleus (McClung & Goldberg, 1999). Movements of the tongue tip are exclusively controlled by intrinsic muscles (Napadow, Chen, Wedeen, & Gilbert, 1999). Thus, increased 5HT immunoreactivity in ventrolateral regions of the hypoglossal nucleus with tongue exercise were expected and reasonable due to the nature of the tongue press task that likely engaged extrinsic tongue muscles, such as the genioglossus. However, involvement of intrinsic muscles of the tongue was also highly likely because the water reward provided to the rats necessitated shaping/contouring of the tongue to hold the water bolus and to allow delivery of the bolus into the oral cavity. Therefore, the interaction between the somatotoptic organization of the hypoglossal nucleus and the tongue press/water reward components of the task offer an explanation for primary regions of the hypoglossal nucleus in which significant differences were found. Because electromyography of specific tongue muscles was not performed during this task, we were unable to discern whether particular extrinsic or intrinsic tongue muscles were active during the tongue press task, or if particular muscles increased activity levels with exercise.
Exercise-induced remodeling
Potential sites of exercise-induced neuroplasticity include hypoglossal motoneurons, the hypoglossal neuromuscular junction, and the tongue musculature. Synaptic inputs to hypoglossal motoneurons (e.g., glutamateric, serotonergic) may undergo synaptogenesis following exercise, although the capacity for exercise-induced remodeling may diminish with age, as has been shown for glutamatergic inputs to the hippocampus of aged rats (McGahon et al., 1997; Davies et al., 2003). In addition to preand post-synaptic membrane-associated changes, second messenger signaling pathways that have been implicated in plasticity of respiratory motor output may also show age-related changes (Dale-Nagle et al., 2010; Gooney et al., 2004). Factors that promote synaptogenesis include the neurotrophins, a family of proteins that, when activated, initiate signaling cascades that promote the survival and function of neurons (Poo, 2001). Muscle activity has been shown to increase the expression of muscle-derived neurotrophins (Funakoshi et al., 1995), and increase nerve terminal branching at the neuromuscular junction (for review, see Marzetti and Leeuwenburgh, 2006). Changes have been described at the hypoglossal neuromuscular junction with increasing age including a reduction in cholinergic postsynaptic receptors (Hodges et al., 2004), which may be due to diminished neurotrophin release. Age-associated changes in muscle fiber type on the genioglossus muscle of the tongue may also alter the capacity for exercise-induced neuroplasticity in old animals (Schaser et al. in press). Thus, exercise-induced changes in tongue function may result from plasticity in both central and peripheral structures.
Tongue muscles engaged in pressing on a disk to get a water reward include protrusors (stick the tongue out, press down), intrinsic muscles (shaping the tongue, collecting a bolus of water) and retrusors (withdrawing of the tongue and transferring the bolus). Thus, regional changes in 5HT immunoreactivity may reflect 5HT modulation of neurons that activate muscles specifically engaged in the exercise paradigm: intrinsic muscles, the extrinsic styloglossus and genioglossus muscles, and neurons innervating muscles in the dorsal aspect of the tongue including the hyoglossus and superior longitudinal intrinsic muscles (Aldes, 1995; McClung & Goldberg, 1999, 2002). Consistent with this, increased 5HT immunoreactivity following exercise was measured in the ventral half of the nucleus (primarily associated with motoneurons innervating protrusor muscles), and the caudal third of the nucleus (primarily associated with motoneurons innervating intrinsic muscles) (Fig. 6).
5HT inputs terminate on the somata and dendrites of hypoglossal motoneurons, with up to 75% terminating on small to medium-sized dendrites (Aldes, et al., 1988). Dendrites of hypoglossal motoneurons are extensive (Altschuler, et al., 1994; Schwarz, et al., 2009) and would likely cross into adjacent quadrants to those in which measurements were made in the present study. Thus, the changes observed in this investigation of 5HT immunoreactivity are probably an underestimate, especially with the method of analysis we used (measuring particles < 3.5 μm in diameter). This may explain the absence of any detectable increase in 5HT immunoreactivity in middle-aged and old rats following exercise, despite a clear effect on tongue force (Fig. 2). Schwarz et al. (Schwarz, et al., 2009) recently reported a statistically significant decrease in the number of primary dendrites associated hypoglossal motoneurons in old F344/BN rats (36 months), which could have reduced the dendritic surface area available for synaptic contacts. However, as the number of primary dendrites was not significantly different in young and middle aged rats (Schwarz, et al., 2009), other mechanisms, such as changes at the muscle level (Connor, et al., 2009) may be contributing to the increase in tongue force seen in middle-aged and old rats, especially as middle-aged rats achieved the highest percent increase in tongue force after training (Fig. 2). Despite this, middle-aged rats demonstrated the lowest absolute baseline and post-exercise force levels, which may represent an age-related decrease in tongue strength not found in the oldest animals. An explanation for this finding may be a putative neuromuscular compensation that was suggested in a study of rat neuromuscular junction (NMJ) where maximal degeneration was found in 28-month-old animals compared with 31-month-old animals (Rosenheimer & Smith, 1985).
Age-associated changes in 5HT immunoreactivity
In the present study we found an age-associated increase in 5HT immunoreactivity by middle age, primarily in the middle region of the hypoglossal nucleus that did not decrease in old rats (Fig. 4). This finding contrasts with a previous study in Fisher 344 rats in which we found a decrease in 5HT immunoreactivity in the hypoglossal nucleus with age (3-6 months vs. 18-24 months; (Behan & Brownfield, 1999). However, the two studies differed methodologically: the earlier study used a non-quantitative analysis of 5HT immunoreactivity. In contrast, the particle analysis (measuring particles < 3.5 μm in diameter) used in the present study is designed to include 5HT synaptic boutons, but not axons, and it would also exclude the varicose terminals that were described in the earlier study in old rats. Rat strain differences (F344 vs. BN/F344), as well as the definition of young and old may also have contributed to the discrepancy between the two studies. The significance of an age-associated increase in 5HT terminals in the middle-third of the hypoglossal nucleus in BN/F344 rats is difficult to assess without additional information on 5HT metabolism, reuptake, and 5HT receptor expression.
Clinical implications
Oropharyngeal exercises, including tongue exercise, may be an effective therapy for patients with upper airway dysfunction including obstructive sleep apnea (Guimaraes, Drager, Genta, Marcondes, & Lorenzi-Filho, 2009; Puhan, et al., 2006) and swallowing disorders (Robbins, et al., 2005; Shaker, et al., 1997). Obstructive sleep apnea (OSA) affects 4% of adults, primarily older men and postmenopausal women (Bixler, et al., 2001; Young, Finn, Austin, & Peterson, 2003; Young, et al., 1993). During sleep airway resistance increases, and this can result in repetitive closure of the airway in OSA patients. OSA is associated with sleep fragmentation, daytime sleepiness and increased cardiovascular risk (Dempsey, Veasey, Morgan, & O'Donnell, 2010). The tongue has a major role in the maintenance of airway patency (Horner, 2007), and endogenous excitation of hypoglossal motoneurons by the neuromodulators 5HT and norepinephrnine help to maintain airway patency during wakefulness (Chan, Steenland, Liu, & Horner, 2006; Kubin & Davies, 2002). Two studies highlight the effectiveness of upper airway muscle exercise as a treatment for OSA. In a randomized controlled trial, playing the didgeridoo 5 days/week for 4 months reduced the severity and symptoms of patients with moderate OSA (Puhan, et al., 2006). In the second study a series of oropharyngeal exercises were shown to significantly reduce the severity of OSA (Guimaraes, et al., 2009). These exercises included a tongue press to palate task similar to exercises used in swallowing rehabilitation (see below).
With regard to swallowing, age-related decline in function has been reported in previous clinical studies that found the duration of the swallow to be longer, thus increasing the opportunity for airway penetration and aspiration of the bolus (Ekberg & Feinberg, 1991; Logemann, et al., 2000; Robbins, Hamilton, Lof, & Kempster, 1992). Because lingual weakness was implicated as a potentially causative factor for age-related dysphagia (Mortimore, Fiddes, Stephens, & Douglas, 1999; Nicosia, et al., 2000), the use of tongue exercise has been investigated with promising results (Kays & Robbins, 2006; Robbins, et al., 2008; Robbins, et al., 2005; Robbins, et al., 2007; Stathopoulos & Felson Duchan, 2006). Specifically, increased tongue pressures have been found during swallowing after 8 weeks of tongue exercise that appeared to translate to improved swallowing-related quality of life (Robbins, et al., 2007)
In conclusion, we have shown that repetitive tongue exercise elicits an increase in the number of 5HT-immunoreactive structures measuring 0.5 – 3.0 μm in diameter in the caudal hypoglossal nucleus in young rats. These varicosities most likely represent serotonergic synaptic boutons (Aldes, et al., 1988), which would suggest that there is sprouting of serotonergic inputs to the hypoglossal nucleus in the vicinity of motoneurons innervating the genioglossus muscle of the tongue. The density of 5HT terminals and receptors has also been reported to increase in the hypoglossal nucleus in response to chronic intermittent hypoxia which is a major component of OSA pathogenesis (Rukhadze, Fenik, Benincasa, Price, & Kubin, 2010). These authors suggest that brain-derived neurotrophic factor (BDNF) plays an important role in the sprouting of 5HT terminals in response to chronic intermittent hypoxia. The mechanisms by which tongue exercise generates increased tongue force may involve BDNF, as well as other trophic factors (Gomez-Pinilla, et al., 2007). Other neuromodulators of hypoglossal motoneurons such as neuropeptide Y, Substance P, CGRP, enkephalins and catecholamines (Horner, 2007) may also be upregulated by repetitive tongue exercise. Each of these possibilities should be explored further.
Acknowledgements
This study was supported by NIH R01AG18760 (MB) and R01DC008149 (NC). The authors acknowledge Dr. Keith Kluender for consulting on the development of the animal training paradigm.
REFERENCES
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Aldes LD. Subcompartmental organization of the ventral (protrusor) compartment in the hypoglossal nucleus of the rat. J Comp Neurol. 1995;353(1):89–108. doi: 10.1002/cne.903530109. [DOI] [PubMed] [Google Scholar]
- Aldes LD, Chronister RC, Marco LA, Haycock JW, Thibault J. Differential distribution of biogenic amines in the hypoglossal nucleus of the rat. Exp Brain Res. 1988;73(2):305–314. doi: 10.1007/BF00248222. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Miselis RR. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol. 1994;342(4):538–550. doi: 10.1002/cne.903420404. [DOI] [PubMed] [Google Scholar]
- Barker JR, Thomas CF, Behan M. Serotonergic projections from the caudal raphe nuclei to the hypoglossal nucleus in male and female rats. Respir Physiol Neurobiol. 2009;165(2-3):175–184. doi: 10.1016/j.resp.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastedo T, Chan E, Park E, Liu H, Horner RL. Modulation of genioglossus muscle activity across sleep-wake states by histamine at the hypoglossal motor pool. Sleep. 2009;32(10):1313–1324. doi: 10.1093/sleep/32.10.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Brownfield MS. Age-related changes in serotonin in the hypoglossal nucleus of rat: implications for sleep-disordered breathing. Neurosci Lett. 1999;267(2):133–136. doi: 10.1016/s0304-3940(99)00337-7. [DOI] [PubMed] [Google Scholar]
- Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol. 2008;164(1-2):213–221. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136(2-3):249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- Brandes IF, Zuperku EJ, Dean C, Hopp FA, Jakovcevic D, Stuth EA. Retrograde labeling reveals extensive distribution of genioglossal motoneurons possessing 5-HT2A receptors throughout the hypoglossal nucleus of adult dogs. Brain Res. 2007;1132(1):110–119. doi: 10.1016/j.brainres.2006.10.099. [DOI] [PubMed] [Google Scholar]
- Brownfield MS, Yracheta J, Chu F, Lorenz D, Diaz A. Functional chemical neuroanatomy of serotonergic neurons and their targets: antibody production and immunohistochemistry (IHC) for 5-HT, its precursor (5-HTP) and metabolite (5-HIAA), biosynthetic enzyme (TPH), transporter (SERT), and three receptors (5-HT2A, 5-ht5a, 5-HT7). Ann N Y Acad Sci. 1998;861:232–233. doi: 10.1111/j.1749-6632.1998.tb10195.x. [DOI] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174(11):1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52(3):732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman ES, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv. Exp. Med. Biol. 2010;699:225–230. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HA, Kelly A, Dhanrajan TM, Lynch MA, Rodriguez JJ, Stewart MG. Synaptophysin immunogold labelling of synapses decreases in dentate gyrus of the hippocampus of aged rats. Brain Res. 2003;986:191–195. doi: 10.1016/s0006-8993(03)03251-7. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR, Judelson DA, Kraemer WJ, Meskaitis VJ, Volek JS, Nindl BC, et al. Effects of resistance training on neuromuscular junction morphology. Muscle Nerve. 2000;23(10):1576–1581. doi: 10.1002/1097-4598(200010)23:10<1576::aid-mus15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357(3):376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: radiologic findings in 56 cases. AJR Am J Roentgenol. 1991;156(6):1181–1184. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167(4):563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172(10):1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, et al. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience. 2007;148(4):893–906. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooney M, Messaoudi E, Maher FO, Bramham CR, Lynch MA. BDNF-induced LTP in dentate gyrus is impaired with age: analysis of changes in cell signaling events. Neurobiology of Aging. 2004;25:1323–1331. doi: 10.1016/j.neurobiolaging.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179(10):962–966. doi: 10.1164/rccm.200806-981OC. [DOI] [PubMed] [Google Scholar]
- Hodges SH, Anderson AL, Connor NP. Remodeling of neuromuscular junctions in aged rat genioglossus muscle. Ann Otol Rhinol Laryngol. 2004;113(3 Pt 1):175–179. doi: 10.1177/000348940411300301. [DOI] [PubMed] [Google Scholar]
- Horner RL. Respiratory motor activity: influence of neuromodulators and implications for sleep disordered breathing. Can J Physiol Pharmacol. 2007;85(1):155–165. doi: 10.1139/y06-089. [DOI] [PubMed] [Google Scholar]
- Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol. 2008;164(1-2):179–196. doi: 10.1016/j.resp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jaric S. Muscle strength testing: use of normalisation for body size. Sports Med. 2002;32(10):615–631. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed] [Google Scholar]
- Kays S, Robbins J. Effects of sensorimotor exercise on swallowing outcomes relative to age and age-related disease. Semin Speech Lang. 2006;27(4):245–259. doi: 10.1055/s-2006-955115. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Pack AI, editor. Sleep apnea: pathogenesis, diagnosis and treatment. Dekker, NY: 2002. pp. 99–154. [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139(2):243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep. 1996;19(3):187–195. [PubMed] [Google Scholar]
- Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000;43(5):1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334(3):466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Experimental Gerontology. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Organization of motoneurons in the dorsal hypoglossal nucleus that innervate the retrusor muscles of the tongue in the rat. Anat Rec. 1999;254(2):222–230. doi: 10.1002/(SICI)1097-0185(19990201)254:2<222::AID-AR8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Organization of the hypoglossal motoneurons that innervate the horizontal and oblique components of the genioglossus muscle in the rat. Brain Res. 2002;950(1-2):321–324. doi: 10.1016/s0006-8993(02)03240-7. [DOI] [PubMed] [Google Scholar]
- McGahon B, Clements MP, Lynch MA. The ability of aged rats to sustain long-term potentiation is restored when the age-related decrease in membrane arachidonic acid concentration is reversed. Neuroscience. 1997;81:9–16. doi: 10.1016/s0306-4522(97)00116-4. [DOI] [PubMed] [Google Scholar]
- Mortimore IL, Fiddes P, Stephens S, Douglas NJ. Tongue protrusion force and fatiguability in male and female subjects. Eur Respir J. 1999;14(1):191–195. doi: 10.1034/j.1399-3003.1999.14a32.x. [DOI] [PubMed] [Google Scholar]
- Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Biomechanical basis for lingual muscular deformation during swallowing. Am J Physiol. 1999;277(3 Pt 1):G695–701. doi: 10.1152/ajpgi.1999.277.3.G695. [DOI] [PubMed] [Google Scholar]
- Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55(11):M634–640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Poo M. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Puhan MA, Suarez A, Lo Cascio C, Zahn A, Heitz M, Braendli O. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. BMJ. 2006;332(7536):266–270. doi: 10.1136/bmj.38705.470590.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997-2011. http://imagej.nih.gov/ij/ [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80(2):767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44(6):931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Robbins J, Butler SG, Daniels SK, Diez Gross R, Langmore S, Lazarus CL, et al. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear Res. 2008;51(1):S276–300. doi: 10.1044/1092-4388(2008/021). [DOI] [PubMed] [Google Scholar]
- Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53(9):1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103(3):823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Rosenheimer JL, Smith DO. Differential changes in the end-plate architecture of functionally diverse muscles during aging. J Neurophysiol. 1985;53(6):1567–1581. doi: 10.1152/jn.1985.53.6.1567. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Fenik VB, Benincasa KE, Price A, Kubin L. Chronic intermittent hypoxia alters density of aminergic terminals and receptors in the hypoglossal motor nucleus. Am J Respir Crit Care Med. 2010;182(10):1321–1329. doi: 10.1164/rccm.200912-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz EC, Thompson JM, Connor NP, Behan M. The effects of aging on hypoglossal motoneurons in rats. Dysphagia. 2009;24(1):40–48. doi: 10.1007/s00455-008-9169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Gunther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115(4):1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Shaker R, Kern M, Bardan E, Taylor A, Stewart ET, Hoffmann RG, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol. 1997;272(6 Pt 1):G1518–1522. doi: 10.1152/ajpgi.1997.272.6.G1518. [DOI] [PubMed] [Google Scholar]
- Schaser AJ, Volz LM, Wang H, Connor NP. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia. doi: 10.1007/s00455-010-9297-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172(10):1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- Stathopoulos E, Felson Duchan J. History and principles of exercise-based therapy: how they inform our current treatment. Semin Speech Lang. 2006;27(4):227–235. doi: 10.1055/s-2006-955113. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Uemura-Sumi M, Itoh M, Mizuno N. The distribution of hypoglossal motoneurons in the dog, rabbit and rat. Anat Embryol (Berl) 1988;177(5):389–394. doi: 10.1007/BF00304735. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]