Abstract
This study compared outcomes in methamphetamine use and sexual risk behaviors from a modified gay-specific, cognitive behavioral therapy (GCBT) combined with a low-cost contingency management (CM; [GCBT+CM]) intervention to prior findings from clinical trials of the original GCBT. Effect sizes for primary outcomes were compared using meta analysis. Comparisons of effect sizes at end of treatment showed the modified GCBT+CM produced significantly fewer consecutive weeks of methamphetamine abstinence (−0.44, CI: −0.79, −0.09) and fewer male sexual partners (−0.36, CI: −0.71, −0.02) than the first trial of GCBT, and more days of methamphetamine use (0.35, CI: 0.02, 0.68) than the second trial of GCBT. At 26-week follow-up, the modified GCBT+CM produced greater effects in reducing the number of male sexual partners (−0.54, CI: −0.89, −0.19; −0.51, CI: −0.84, −0.18). The original GCBT produced more and mostly short-term beneficial drug use outcomes, though sexual behavior changes consistently favored the modified GCBT+CM. On balance, most benefits are retained with the modified GCBT+CM intervention.
Keywords: methamphetamine abuse treatment, gay and bisexual men, sexual risk behavior, cognitive behavioral therapy, contingency management
1. Introduction
In the United States, methamphetamine use is endemic among gay and bisexual males (GBM), particularly in major urban centers such as New York City, Los Angeles and San Francisco where the drug is easily accessible and integrated into the social and sexual contexts of GBM (Halkitis, Parsons, & Stirratt, 2001; Mansergh et al., 2001; Mattison, Ross, Wolfson, & Franklin, 2001; Reback, 1997; Woody et al., 2001). Methamphetamine abuse is a major public health concern for communities of GBM, with high prevalence (>10%) of use of the drug reported in New York (Grov, Bimbi, Nanin, & Parsons, 2006), Los Angeles, San Francisco (Stall et al., 2001) and among GBM who frequent the Internet (Hirshfield, Remien, Humberstone, Walavalkar, & Chiasson, 2004) and sex venues (Halkitis, Fischgrund, & Parsons, 2005) to find sexual partners.
Among GBM who use methamphetamine, the drug is frequently integrated into sexual behaviors that confer risk, providing opportunities for transmission of sexually transmitted diseases, including HIV (Buchacz et al., 2005; Chesney, Barrett, & Stall, 1998; Plankey et al., 2007). In the process of using the drug, methamphetamine often becomes incorporated with many of the identities held by the user, including the identity as a gay or bisexual man, as a methamphetamine user, and as a person living with (or without) HIV (Reback, 1997). Given the association between methamphetamine use, high-risk sexual behaviors and HIV seropositivity (Shoptaw & Reback, 2006), the development of a low-cost, efficacious and evidenced-based intervention for use in community settings would serve as a tremendous public health benefit.
Our initial efforts to intervene on the interwoven problems of methamphetamine misuse and high-risk sexual behaviors yielded a tailored, gay-specific, cognitive behavioral therapy (GCBT) intervention that integrated core elements from a standard cognitive behavioral therapy (CBT) intervention (Rawson et al., 1995) with elements that addressed cultural and social aspects of methamphetamine use by GBM. This tailored intervention equally addressed methamphetamine use and HIV-related sexual risk reductions among GBM. In the initial randomized controlled trial, the GCBT intervention was developed and evaluated against three evidence-based conditions: contingency management (CM), standard CBT, and a combination of standard CBT+CM. The original GCBT intervention consisted of 48 sessions delivered in group format over 16 weeks and was shown to significantly reduce sexual risk behaviors over standard CBT during treatment, with comparable reductions of methamphetamine use at follow-up visits to one-year (Reback, Larkins, & Shoptaw, 2004; Shoptaw et al., 2005).
Developmental work continued on the intervention in a replication study that evidenced the specificity of GCBT to statistically reduce methamphetamine use compared to a control condition (Gay Social Support Therapy [GSST]). In broader groups of substance-using GBM, i.e., abuse of all stimulants and alcohol, both GCBT and the comparator GSST performed equally in reducing substance use during treatment and to one year, with GCBT outperforming GSST in reducing methamphetamine use among methamphetamine abusing GBM (Shoptaw et al., 2008).
In this study, we evaluate whether the size of outcomes in retention, methamphetamine use, and sexual risk behaviors between a final modification to the GCBT intervention for methamphetamine-abusing GBM differed from those measured using the original and replication versions of the GCBT intervention. In this final stage of therapy development, GCBT was coupled with CM in order to combine optimally effective interventions for reducing HIV-related sexual behaviors (GCBT) and methamphetamine use (CM).
The modified GCBT+CM intervention was specifically designed to be cost and time effective for community application, thus moving research on efficacious treatments into practice. This open label study continued development of the GCBT intervention by adopting, tailoring and transferring the original intervention for use in a community-based HIV prevention setting. Shortened, modified versions of the original and replicated GCBT interventions would increase feasibility of implementation in community-based organizations. Modifications consisted of reducing the intervention from 16 weeks and 48 sessions to 8 weeks and 24 sessions while maintaining the core elements. Given that the modified intervention has fewer sessions and is easier to implement, outcome findings that demonstrated only minor reductions in outcomes from the more expansive model would be important to advise adaptation of evidence-based interventions into community settings that intervene with this high-risk population.
Given the reductions in intensity and coverage of drug treatment, this open label trial predicted that effect sizes for outcomes when using the modified GCBT+CM intervention would be significantly lower than those for the original GCBT intervention in reducing methamphetamine use and HIV-sexual risk behaviors.
2. Materials and methods
2.1 Participants
Study participants for the modified GCBT+CM intervention were self-identified GBM, between the ages of 18 and 65, who were seeking treatment for methamphetamine abuse. Participants were excluded from the study if they presented with psychiatric or medical conditions that precluded safe study participation (e.g., suicidal ideation), were unable to comply with study requirements, or required more intense treatment than outpatient care. The study was approved by the Institutional Review Board of Friends Research Institute, Inc., and provided oversight for all study activities.
2.2 Interventions
The designs of the original and replicated interventions are thoroughly described elsewhere (Shoptaw et al., 2008; Shoptaw et al., 2005).
2.2.1 The original intervention (Study #1)
Participants in this study were randomized into one of four treatment conditions: CM, standard CBT, a combination of standard CBT+CM, or the GCBT intervention. Participants attended clinic in the early evening on each Monday, Wednesday and Friday to receive treatment, submit an observed urine sample and complete research assessments. Briefly, the CM intervention used in this study was a voucher-based reinforcement therapy. The maximum in possible vouchers earned, if all urine samples were negative for methamphetamine metabolites, was $1,277.50. Vouchers could be redeemed at any study visit for pro-social and healthy purchases such as groceries, gift certificates, or to pay bills. The standard CBT intervention was implemented in a group format and taught cognitive skills to initiate and maintain methamphetamine abstinence. The CM+CBT intervention integrated the complete CM and CBT interventions into a combined condition. The GCBT built upon the standard CBT intervention with modified culturally relevant reference to gay culture. Also delivered in a group format, this tailored intervention equally targeted methamphetamine use and high-risk HIV-related sexual behaviors.
2.2.2 The replicated intervention (Study #2)
Participants in this study were randomized into one of two treatment conditions: the GCBT intervention or a gay-specific social support therapy (GSST) intervention. Clinic attendance for the GCBT intervention was the same as in the original intervention. The GCBT intervention used in this study replicated the original intervention however the substances of abuse were expanded to target all stimulants and alcohol. The GSST comparison condition was based on a social model of recovery but tailored to include gay-specific references. Participants in the GSST intervention attended one weekly HIV health education/risk reduction group, one weekly open discussion social support group, and one weekly individual counseling session with a peer professional who served as a quasi “sponsor.”
2.2.3. The modified intervention (Study #3)
All participants enrolled in this open label study received the modified GCBT intervention (reduced from 16 weeks and 48 sessions to 8 weeks and 24 sessions) and a low-cost CM intervention (reduced from a maximum payout of $1,277.50 over 16 weeks to $178 over 8 weeks). Clinic attendance for the modified intervention was the same as in the original intervention, i.e., in the early evening on each Monday, Wednesday and Friday to receive treatment, submit a urine sample and complete research assessments.
2.3 Procedure
As study procedures for the original and replicated GCBT intervention are described elsewhere (Shoptaw et al., 2008; Shoptaw et al., 2005), procedures presented here focus on the modified GCBT+CM study. Potential participants were recruited through referrals from local community-based organizations serving the population and advertisements designed to target GBM who were seeking treatment for methamphetamine abuse or dependence. At intake participants provided informed consent, a baseline urine screen, and completed baseline assessments. Participants started treatment at the first Monday, Wednesday or Friday after completing baseline assessments. GCBT+CM visits continued for each Monday, Wednesday, and Friday for a total of 8 weeks, with weekly aftercare sessions for weeks 9–16. Follow-up assessments were conducted at 8, 16, and 26 weeks post baseline.
2.4 Assessments
Study measures were administered to participants by trained research assistants at baseline, during treatment and at follow-up visits. The Addiction Severity Index (McLellan et al., 1992) and a modified Behavioral Questionnaire – Amphetamine (Chesney, Chambers, & Kahn, 1997) was administered at baseline and at 8-, 16- and 26-week follow-up evaluations. Urine drug screening for metabolites of methamphetamine used radioimmuneassay strips (Phamatech, San Diego, CA) and was conducted each Monday, Wednesday, Friday for the first 8 weeks and weekly thereafter. The treatment retention was calculated as the number of days from the start of treatment to the last date of clinic attendance.
2.5 Statistical analysis
To determine whether the original and replicated interventions differed from the modified intervention in the size of outcomes measured, we utilized meta analytic software, Review Manager (RevMan) Version 5.0 (The Cochrane Collaboration, 2008), to calculate effect sizes for relevant outcomes reported for end of treatment and 26-week follow-up evaluations for each of the three trials evaluating GCBT. Substance use treatment outcomes included average retention in weeks in the intervention, longest consecutive abstinence period verified by urine drug screen results (in weeks), treatment effectiveness score (TES; Ling et al., 1997), percent of urine samples negative for methamphetamine metabolite, and number of days of reported methamphetamine use in the previous 30 days. HIV-sexual risk behaviors included self-reported behaviors in the previous 30 days including: number of male sexual partners, number of times unprotected receptive anal intercourse (URAI), and number of times unprotected insertive anal intercourse (UIAI). Most outcomes were continuous. As such, the effect size (ES) calculated was the standardized mean difference d (Cohen, 1988). For each intervention measure, d was calculated as the difference between group scores for two treatment approaches (i.e., original GCBT vs. modified GCBT+CM intervention and replicated GCBT vs. modified GCBT+CM intervention) and divided by the pooled standard deviation for each comparison group (Kazis, Anderson, & Meenan, 1989; Lipsey & Wilson, 2001). Significant differences were assessed for each ES using a Z test statistic with alpha set at 0.05. For each ES, the sign was set so that it was positive when the mean outcome for any measure was higher for the modified GCBT+CM intervention. Additionally, the risk ratio was calculated at a 95% confidence interval.
3. Results
3.1 Study progress
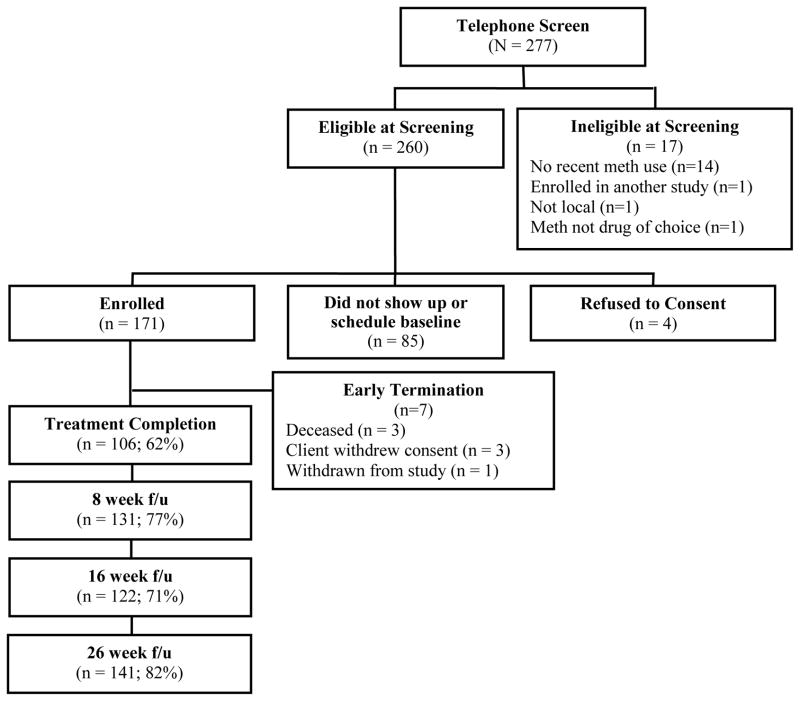
Figure 1 shows the progress of participants from initial telephone screening to last follow-up evaluation for the modified GCBT+CM intervention trial. From those initially screened by telephone, 61.7% (N = 171) were enrolled in the intervention. Participant follow-up rates were 76.6%, 71.3%, and 82.4% for follow-up visits at week 8, 16, and 26, respectively.
Figure 1.
Study Progress and Retention
3.2 Baseline characteristics
A descriptive summary of baseline demographic, methamphetamine use, and sexual risk behaviors for each of the studies is provided in Table 1. Participants for all three studies included GBM residing in Los Angeles, CA. The samples in the studies were primarily Caucasian/white (65%), with the original study having the largest proportion on Caucasian participants. The average age of participants was 38.5 years. The HIV-seropositivity was relatively consistent in each study, ranging from 55.0% (the original intervention, study #1) to 63.2% (the modified intervention, study #3). Across the three studies, current and reported methamphetamine use variables did not differ statistically. Similarly, reported HIV-related sexual risk behaviors showed no statistically significant differences across the studies.
Table 1.
Baseline demographic, drug use, and sexual risk characteristics of participants for each study
| Variable | Studies
|
||
|---|---|---|---|
| Original Intervention study #1 (n=40) M (S.D.) or % |
Replicated Intervention study #2 (n=46) M (S.D.) or % |
Modified Intervention study #3 (n=171) M (S.D.) or % |
|
| Age (years) | 38.5 (5.4) | 37.0 (6.4) | 39.9 (8.2) |
| Education (years) | 14.8 (2.1) | 15.5 (2.1) | 14.9 (2.6) |
| Ethnicity (%) | |||
| Caucasian | 85.0 | 72.0 | 59.0 |
| Hispanic | 5.0 | 22.0 | 21.0 |
| African American | 5.0 | 0.0 | 8.0 |
| Asian American | 0.0 | 4.0 | 10.0 |
| Native American | 5.0 | 0.0 | 0.0 |
| Multi/Other | 0.0 | 2.0 | 15.0 |
| HIV Seropositivity (%) | 55.0 | 63.0 | 63.2 |
| Reported drug use | |||
| Lifetime methamphetamine use (in years) | 5.6 (5.0) | 6.7 (4.5) | 4.0 (4.8) |
| Methamphetamine use (days in previous 30) | 10.4 (7.8) | 6.3 (7.7) | 11.2 (8.3) |
| Days of using >1 drug (previous 30 days) | 4.0 (5.5) | 3.0 (4.6) | 6.59 (7.1) |
| Any injection methamphetamine use (%) | 40.0 | 36.0 | 48.0 |
| Sexual risk behaviorsa | |||
| Sexual partners (# in previous 30 days) | 10.1 (13.3) | 9.4 (15.6) | 9.8 (11.9) |
| Sexual partners (# in previous 6 months) | 39.9 (45.5) | 48.0 (42.0) | 48.1 (68.8) |
| URAI with other than primary partner (times in previous 30 days) | 3.1 (6.8) | 3.2 (5.0) | 5.4 (12.5) |
| UIAI with other than primary partner (times in previous 30 days) | 7.1 (12.8) | 2.5 (4.9) | 5.7 (15.9) |
Note. URAI = unprotected receptive anal intercourse with other than primary partner; UIAI = unprotected insertive anal intercourse with other than primary partner.
Self-reported sexual risk behaviors in the previous 30 days at baseline and all follow-up visits.
3.3 Difference in effect sizes for retention, drug use, and sexual risk behaviors
3.3.1 Original GCBT intervention vs. modified GCBT+CM intervention
Results showed statistically significant differences between effect sizes along outcomes measured for the original GCBT and modified GCBT+CM interventions at the end of treatment for longest consecutive negative urine samples. No other differences were observed between the treatments along any drug use variables. We did find a statistically significant difference favoring the modified GCBT+CM intervention for reducing the number of male partners in the previous 30 days at the end of treatment. At 26-week follow-up visits, the difference in size of effect in reducing number of male sexual partners favoring the modified GCBT+CM condition remained statistically significant.
3.3.2 Replicated GCBT intervention vs. modified GCBT+CM intervention
Results showed a statistically significant difference in effect sizes favoring the replicated GCBT intervention over the modified GCBT+CM intervention in producing lower reported days of methamphetamine use in the previous 30 days at the end of treatment (Table 2). No parallel measure of consecutive weeks of negative urine samples was available for the modified GCBT+CM intervention, but outcomes along other markers of treatment outcome, including retention and treatment effectiveness score, appeared to be of similar magnitude for the two conditions at the end of treatment. At 26-week follow-up visits, the modified GCBT+CM condition produced better outcomes in reducing number of male sexual partners compared to the original GCBT condition.
Table 2.
Standardized mean difference effect sizes for the original intervention (study #1) and the modified intervention (study #3) and the replicated intervention (study #2) and the modified intervention (study #3) for measures at end of treatment and at 26-week follow-up
| Studies
|
Comparisons
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Original Intervention (Study #1) (n = 40) | Replicated Intervention (Study #2) (n = 46) | Modified Intervention (Study #3) (n=171) | Original Intervention (Study #1) vs. Modified Intervention (Study #3) | Replicated Intervention (Study #2) vs. Modified Intervention (Study #3) | ||||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Std Mean Difference | Std Mean Difference | |||
| (SMD)a | (95% C. I.) | (SMD)a | (95% C. I.) | |||||||
| End of Treatment Measures | ||||||||||
| Retention (weeks) Standardizedb | 11.3 | 6.3 | 12.78 | 4.8 | 11.02 | 6.24 | −0.04 | −0.39, 0.30 | −0.29 | −0.62, 0.03 |
| Longest consecutive negative urine samples (weeks) | 3.5 | 3.4 | NA | NA | 2.35 | 2.39 | −0.44 ** | −0.79, −0.09 | NA | NA |
| Treatment Effectiveness Score (Standardized)b | 23.4 | 16.7 | 22.2 | 12.3 | 22 | 17.12 | −0.09 | −0.43, 0.26 | −0.01 | −0.34, 0.31 |
| Reported days methamphetamine use in previous 30 days | 2.9 | 5.7 | 2.1 | 4.1 | 4.32 | 6.78 | 0.21 | −0.13, 0.56 | 0.35 * | 0.02, 0.68 |
| Percent of negative urine samples (%) and (Risk Ratio) | (80.0%) | (81.0%) | (82.8%) | (0.97) | (0.78, 1.20) | (0.98) | (0.78, 1.23) | |||
| Sexual Behaviorc | ||||||||||
| Number male partners | 5.2 | 8.4 | 3.3 | 4.4 | 3.21 | 4.52 | −0.36* | −0.71, −0.02 | −0.02 | −0.35, 0.31 |
| URAI | 0.2 | 0.6 | 1.1 | 3.1 | 1.05 | 2.70 | 0.35 | 0.00, 0.69 | −0.02 | −0.34, 0.31 |
| UIAI | 0.4 | 1.2 | 0.9 | 2.2 | 1.38 | 4.05 | 0.26 | −0.08, 0.61 | 0.13 | −0.20, 0.45 |
| Week 26 | ||||||||||
| Reported days methamphetamine use in previous 30 days | 3.6 | 7.0 | 3.6 | 7.0 | 3.89 | 6.72 | 0.04 | −0.30, 0.39 | 0.04 | −0.28, 0.37 |
| Percent of negative urine samples (%) and (Risk Ratio) | (69.7%) | (82.1%) | (85.1%) | (0.81) | (0.63, 1.03) | (0.96) | (0.82, 1.13) | |||
| Sexual Behaviorc | ||||||||||
| Number male partners | 5.4 | 9.4 | 5.2 | 8.8 | 2.51 | 3.86 | −0.54** | −0.89, −0.19 | −0.51** | −0.84, −0.18 |
| URAI | 0.9 | 1.8 | 2.1 | 4.5 | 1.17 | 3.04 | 0.09 | −0.25, 0.44 | −0.27 | −0.60, 0.05 |
| UIAI | 0.6 | 1.2 | 1.1 | 2.4 | .83 | 1.78 | 0.14 | −0.21, 0.48 | −0.14 | −0.47, 0.19 |
Standardized mean difference effect sizes are based on Cohen’s d utilizing a pooled standard deviation. An effect size 0.2 to 0.3 would be a small effect, around 0.4 to 0.7 a medium effect and 0.8 or greater a large effect (Cohen, 1988)
Original values adjusted for comparison
Self-reported sexual risk behaviors in the previous 30 days
p < .05,
p < .01
4. Discussion
Findings from comparing the effect sizes observed when implementing original and modified versions of a GCBT intervention for methamphetamine-abusing GBM showed a pattern of generally similar outcomes along drug use and sexual risk behaviors at the end of treatment and to 26-week follow-up visit with minor exceptions. The original GCBT intervention demonstrated superior efficacy along one marker of methamphetamine use at the end of treatment (consecutive weeks of abstinence). The replicated GCBT intervention also demonstrated superior efficacy along a different marker of methamphetamine use at the end of treatment (self-reported days of use in the previous 30). Yet, no other differences in the size of outcomes for methamphetamine use or retention in treatment were observed. Along sexual risk behaviors, the modified GCBT+CM intervention produced greater effects in reducing the number of male sexual partners over the original GCBT intervention at end of treatment and over both original and replicated GCBT interventions at 26-week follow-up visits. These findings indicate that while the modified GCBT+CM intervention has slightly less potency at reducing use of methamphetamine than its progenitor versions, it has consistently stronger effects in reducing the number of male sexual partners. These analyses suggest that most of the benefits to the evidence-based interventions for reducing methamphetamine use and concomitant HIV-related sexual risk behaviors are retained when utilizing the condensed, modified GCBT+CM intervention in a community setting.
There are several possible explanations for observed differences in the size of treatment effects along methamphetamine use outcomes. The simplest explanation for these differences may be the length of time individuals received exposure to the elements in the GCBT interventions (16 weeks for the original and replicated interventions vs. 8 weeks for the modified intervention). It is important to note that by the 26-week follow-up evaluation this difference in size of treatment effects had dissipated, with measures of methamphetamine use at follow-up showing similar effect sizes across the three GCBT conditions. What is of some interest is that inclusion of the low-value CM schedule with the modified GCBT intervention did not boost measures of methamphetamine abstinence during treatment toward approximating those observed for the original GCBT interventions that did not contain CM. The low value for the CM schedule may have severely limited its potential for improving markers of drug abstinence (Petry et al., 2004; Sindelar, Elbel, & Petry, 2007).
The modified intervention, compared to the original and replicated interventions, had a greater impact on reducing the number of male sex partners in the previous 30 days, both at end of treatment and at 26-week follow-up evaluations. All GCBT conditions reduced unprotected sexual behaviors at similar levels, which is encouraging in considering use of this intervention for addressing risk behaviors for sexual disease transmission. The differential efficacy of the modified GCBT+CM condition in reducing the number of sexual partners may point to a mechanism by which overall disease transmission risk is reduced.
The aim of the open label trial of the modified intervention was to expand the preliminary work by integrating GCBT – the intervention optimal for reducing sexual risks – with CM – the intervention optimal for reducing stimulant use – into one behavioral intervention and to adopt, tailor and transfer the integrated intervention into a community HIV prevention setting. This goal was successfully achieved. Additionally, to accommodate application in a community setting, the CM intervention was reduced from a maximum payout of $1,277.50 over 16 weeks to $178 over 8 weeks, demonstrating the feasibility of this approach. What is not answered by this study is whether the observed pattern of differences in the size of methamphetamine use and sexual behavior outcomes would have been similar had the modified GCBT been delivered in the absence of the low-value CM schedule. Specifically, it is not known whether the low-value CM schedule adds significant impact to findings over the condensed GCBT intervention alone.
There are limitations to this report. As the trial of the modified GCBT+CM intervention did not contain a comparison condition, there is potential that observed outcomes were attributable to unmeasured third variables. While it is possible that the reduced CM schedule for the modified intervention was too low to produce equivalent reductions to methamphetamine use evidenced in the original study, it is encouraging that outcomes using the modified GCBT+CM intervention in this open label trial were well within ranges expected from our prior work and were comparable to outcomes from more time and resource intensive therapies.
This body of work in intervention studies clearly demonstrates that methamphetamine abuse treatment can function as a part of comprehensive HIV prevention efforts for GBM with a concomitant focus on sexual and drug behaviors. Findings show the feasibility of using interventions that reduce methamphetamine use to mediate sexual risks in venues outside of traditional drug abuse treatment settings such as community HIV prevention settings. Given the growing scarcity of public funding, implementing low-cost, efficacious and evidenced-based interventions in community settings may provide an important lever for addressing public health impact.
Research Highlights.
aim of the trial was to expand earlier work and adapt into a community setting
this study compared outcomes in meth use and sex risk behaviors to prior findings
effect sizes for primary outcomes were compared using meta analysis
meth abuse treatment can function as a part of comprehensive HIV prevention efforts
findings show feasibility of using intervention in community HIV prevention setting
Acknowledgments
Role of Funding Source
Funding for this study was provided by the California HIV/AIDS Research Program (CHRP), grant #MU04-FRII-704 to Dr. Reback and NIDA grant 1R01DA11031 and SAMHSA grant 1KD112043 to Drs. Shoptaw and Reback; the CHRP, NIDA and SAMHSA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors would like to acknowledge James A. Peck, Psy.D., Xiaowei Yang, Ph.D., Erin Rotheram-Fuller, Ph.D., Sherry Larkins, Ph.D. Rosemary C. Veniegas, Ph.D., Thomas E. Freese, Ph.D., Christopher Hucks-Ortiz, M.P.H., Pin-Chieh Wang, Ph.D., and Jeff Dang, Ph.D. for their work on either the original or replicated GCBT intervention; and to Peter Theodore, Ph.D., Joshua Riley, M.S., and Kevin Shone for their contributions during the implementation of the modified GCBT+CM intervention. Finally, the authors would like to thank Ronald Brooks, Ph.D. and Uyen Kao, MPH for their assistance during the manuscript development.
Footnotes
Contributors
C.J. Reback wrote the protocol and had primary responsibility for implementing the research study. S. Shoptaw oversaw the statistical analysis. C.J. Reback and S. Shoptaw contributed equally to the writing of the manuscript and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, Klausner JD. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19:1423–1424. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Barrett DC, Stall R. Histories of substance use and risk behavior: precursors to HIV seroconversion in homosexual men. American Journal of Public Health. 1998;88:113–116. doi: 10.2105/ajph.88.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney MA, Chambers DB, Kahn JO. Risk behavior for HIV infection in participants in preventive HIV vaccine trials: a cautionary note. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1997;16:266–271. doi: 10.1097/00042560-199712010-00007. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: LEA; 1988. [Google Scholar]
- Grov C, Bimbi DS, Nanin JE, Parsons JT. Exploring racial and ethnic differences in recreational drug use among gay and bisexual men in New York city and Los Angeles. Journal of Drug Education. 2006;36:105–123. doi: 10.2190/1G84-ENA1-UAD5-U8VJ. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Fischgrund BN, Parsons JT. Explanations for methamphetamine use among gay and bisexual men in New York City. Substance Use and Misuse. 2005;40:1331–1345. doi: 10.1081/JA-200066900. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Parsons JT, Stirratt MJ. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. Journal of Homosexuality. 2001;41:17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- Hirshfield S, Remien RH, Humberstone M, Walavalkar I, Chiasson MA. Substance use and high-risk sex among men who have sex with men: a national online study in the USA. AIDS Care. 2004;16:1036–1047. doi: 10.1080/09540120412331292525. [DOI] [PubMed] [Google Scholar]
- Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Medical Care. 1989;27:S178–189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ. Treatment effectiveness score as an outcome measure in clinical trials. NIDA Research Monograph. 1997;175:208–220. [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Mansergh G, Colfax GN, Marks G, Rader M, Guzman R, Buchbinder S. The Circuit Party Men’s Health Survey: findings and implications for gay and bisexual men. American Journal of Public Health. 2001;91:953–958. doi: 10.2105/ajph.91.6.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison AM, Ross MW, Wolfson T, Franklin D. Circuit party attendance, club drug use, and unsafe sex in gay men. Journal of Substance Abuse. 2001;13:119–126. doi: 10.1016/s0899-3289(01)00060-8. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: how low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plankey MW, Ostrow DG, Stall R, Cox C, Li X, Peck JA, Jacobson LP. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. Journal of Acquired Immune Deficiency Syndrome. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Shoptaw SJ, Obert JL, McCann MJ, Hasson AL, Marinelli-Casey PJ, Brethen PR, Ling W. An intensive outpatient approach for cocaine abuse treatment. The Matrix model. Journal of Substance Abuse Treatment. 1995;12:117–127. doi: 10.1016/0740-5472(94)00080-b. [DOI] [PubMed] [Google Scholar]
- Reback CJ. The social construction of a gay drug: methamphetamine use among gay and bisexual males in Los Angeles. City of Los Angeles: AIDS Coordinator’s Office; 1997. [Google Scholar]
- Reback CJ, Larkins S, Shoptaw S. Changes in the meaning of sexual risk behaviors among gay and bisexual male methamphetamine abusers before and after drug treatment. AIDS and Behavior. 2004;8:87–98. doi: 10.1023/b:aibe.0000017528.39338.75. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ. Associations between methamphetamine use and HIV among men who have sex with men: a model for guiding public policy. Journal of Urban Health. 2006;83:1151–1157. doi: 10.1007/s11524-006-9119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Larkins S, Wang PC, Rotheram-Fuller E, Dang J, Yang X. Outcomes using two tailored behavioral treatments for substance abuse in urban gay and bisexual men. Journal of Substance Abuse Treatment. 2008;35:285–293. doi: 10.1016/j.jsat.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug and Alcohol Dependence. 2005;78:125–134. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Sindelar J, Elbel B, Petry NM. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction. 2007;102:309–316. doi: 10.1111/j.1360-0443.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Stall R, Paul JP, Greenwood G, Pollack LM, Bein E, Crosby GM, Mills TC, Binson D, Coates TJ, Catania JA. Alcohol use, drug use and alcohol-related problems among men who have sex with men: the Urban Men’s Health Study. Addiction. 2001;96:1589–1601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration. Review Manager. 5.0. Copenhagen: The Nordic Cochrane Center; 2008. [Google Scholar]
- Woody GE, VanEtten-Lee ML, McKirnan D, Donnell D, Metzger D, Seage G, 3rd, Gross M. Substance use among men who have sex with men: Comparison with a national household survey. Journal of Acquired Immune Deficiency Syndrome. 2001;27:86–90. doi: 10.1097/00126334-200105010-00015. [DOI] [PubMed] [Google Scholar]