Abstract
Lipid rafts are microdomains present within membranes of most cell types. These membrane microdomains, which are enriched in cholesterol and glycosphingolipids, have been implicated in the regulation of certain signal transduction and membrane traffic pathways. To investigate the possibility that lipid rafts organize exocytotic pathways in neuroendocrine cells, we examined the association of proteins of the exocytotic machinery with rafts purified from PC12 cells. The target soluble N-ethylmaleimide-sensitive factor attachment protein receptor (tSNARE) proteins syntaxin 1A and synaptosomal-associated protein of 25 kDa (SNAP-25) were both found to be highly enriched in lipid rafts (≈25-fold). The vesicle SNARE vesicle-associated membrane protein (VAMP)2 was also present in raft fractions, but the extent of this recovery was variable. However, further analysis revealed that the majority of VAMP2 was associated with a distinct class of raft with different detergent solubility characteristics to the rafts containing syntaxin 1A and SNAP-25. Interestingly, no other studied secretory proteins were significantly associated with lipid rafts, including SNARE effector proteins such as nSec1. Chemical crosslinking experiments showed that syntaxin1A/SNAP-25 heterodimers were equally present in raft and nonraft fractions, whereas syntaxin1A/nSec1 complexes were detected only in nonraft fractions. SDS-resistance assays revealed that raft-associated syntaxin1A/SNAP-25 heterodimers were able to interact with VAMP2. Finally, reduction of cellular cholesterol levels decreased the extent of regulated exocytosis of dopamine from PC12 cells. The results described suggest that the interaction of SNARE proteins with lipid rafts is important for exocytosis and may allow structural and spatial organization of the secretory machinery.
Membrane fusion is a highly orchestrated process requiring the participation of a large number of proteins. Many of the protein components of the fusion machinery are highly conserved, functioning in a wide range of different fusion events in all eukaryotic cells. Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins are localized to both donor and acceptor membranes and are essential for membrane fusion. Indeed, these proteins form a high-affinity complex that is sufficient to promote membrane fusion in vitro (1). However, the rate and fidelity of membrane fusion in vivo require a large number of other regulatory proteins (2). For example, α soluble N-ethylmaleimide sensitive factor attachment protein (αSNAP) and N-ethylmaleimide-sensitive factor (NSF) act at an early stage of secretion, probably by disassembling vesicular cis-SNARE complexes, allowing the subsequent assembly of trans-SNARE pairs between the vesicle and plasma membrane (3–5). Another key component of the exocytotic machinery is nSec1. The interaction of nSec1 with monomeric syntaxin 1A is mutually exclusive of SNARE complex assembly (6, 7), and nSec1 has been shown to function in a late step of exocytosis, controlling the rate of fusion pore expansion (8).
A plethora of other proteins have been implicated in regulated exocytosis, and gene disruption and protein-binding assays have suggested functions for these proteins. However, a key question that remains unanswered is how these proteins are organized in vivo. For example, the target (t)SNARE syntaxin 1A interacts with a large number of protein components of the secretory machinery, including synaptosomal-associated protein of 25 kDa (SNAP-25), vesicle-associated membrane protein (VAMP)2, αSNAP, nSec1, synaptotagmin, Munc13, Csp, tomosyn, and calcium channels (6, 9–13). How are these proteins organized to allow the appropriate interaction to proceed, while preventing interference from other binding partners of syntaxin 1A? Clearly, some of these interactions are mutually exclusive, but intricate regulatory mechanisms must exist to ensure that the appropriate interactions take place when required. The need for structural organization of the exocytotic machinery is underlined by the demonstration that SNAP-25 and syntaxin 1A are found not only in nerve terminals (where synaptic vesicle exocytosis occurs) but also along the axonal plasma membrane (14). Also, yeast homologues of syntaxin and SNAP-25 are localized around the entire periphery of the cell in addition to the bud, the active site of exocytosis (15). Thus, despite target (t)SNAREs being widely distributed on plasma membranes, exocytosis occurs only at defined sites of the membrane, implying that only certain tSNAREs are functionally active.
There has been much interest recently in the role played by the lipid components of membranes in various cell processes. For example, membrane microdomains referred to as “lipid rafts” (16) have received much attention as potential regulators and organizing centers for signal transduction and membrane traffic pathways (17–19). Lipid rafts are noncaveolar microdomains that are enriched in cholesterol and glycosphingolipids and are characterized biochemically by their relative insolubility in cold Triton X-100. In addition, a distinct type of lipid raft has recently been discovered that is soluble in Triton X-100 but insoluble in Lubrol WX (20). The ability of lipid rafts to selectively recruit specific proteins while excluding others makes them ideally suited to organize cell processes.
Although lipid rafts have been proposed to perform a key role in the sorting of certain membrane proteins in polarized epithelial cells (19), little is known about the potential role played by these microdomains in the organization of the exocytotic machinery in cells specialized for secretion. Therefore, we have undertaken a comprehensive analysis of the association of components of the exocytotic machinery with lipid rafts isolated from Triton-solubilized PC12 cells. The results described show that lipid rafts interact specifically with SNARE proteins, with vesicle- and tSNAREs found in distinct types of raft. The association of SNAREs with lipid rafts is likely to be of functional importance to exocytosis, as syntaxin 1A-containing protein complexes were differentially distributed between raft and nonraft fractions, and cholesterol-depleted PC12 cells showed a decreased extent of regulated exocytosis.
Materials and Methods
Materials.
Antibodies against SNAP-25 (C terminus), Csp, and NSF were as previously described (21–23). Antibodies against nSec1, Sec8, munc13, SNAP-25 (N terminus), caveolin 1, and flotillin were purchased from Transduction Laboratories (Lexington, KY). Antibodies specific for VAMP2, αSNAP, rabphilin, Rab3A, Rab5, and secretory carrier membrane protein were from Synaptic Systems (Göttingen, Germany). Antibodies recognizing syntaxin 1A and synaptophysin were from Sigma. The anti-α1 subunit of the Na,K ATPase was purchased from Upstate Biotechnology (Lake Placid, NY). Anticomplexin was a gift from Harvey McMahon (Laboratory of Molecular Biology, Cambridge, U.K.), antisynaptotagmin (p65) was a gift from David Apps (Department of Biochemistry, University of Edinburgh, Edinburgh, U.K.). Ethyleneglycol-bis(succinimidylsuccinate) (EGS) and lovastatin were purchased from Calbiochem. Lubrol WX (Lubrol 17A17) was obtained from Serva. [7, 8-3H] dopamine was from Amersham Pharmacia. Triton X-100, saponin, 1-ethyl-3-(3-dimethylaminopropyl)carboiimide (EDAC), methyl-β-cyclodextrin, mevalonate, delipidated calf serum, Infinity cholesterol reagent, and all other reagents were of an analytical grade from Sigma.
PC12 Cell Culture.
PC12 cells were grown in RPMI 1640 media supplemented with 10% horse serum/5% FCS/100 units/ml of penicillin/100 μg/ml of streptomycin. Cells were incubated in a humidified atmosphere of 5% CO2.
Detergent Solubilization and Sucrose Gradient Fractionation.
PC12 cells (100–120 × 106) were used for each experiment. The cells were washed twice in ice-cold PBS and once in 25 mM Mes/150 mM NaCl, pH6.5 (MBS). The cells were then resuspended in 2 ml of 1% Triton X-100 or 1% Lubrol WX in MBS supplemented with a protease inhibitor mix (Boehringer Mannheim) and incubated at 4°C for 20 min. The solubilized cells were homogenized with 10 strokes of a Dounce homogenizer, and 1.5 ml of the homogenate was added to an equal volume of 80% (wt/vol) sucrose in MBS. The solubilized cells (in 40% sucrose) were overlayed successively with 6 ml of 30% sucrose and 4 ml of 5% sucrose. After centrifugation at 240,000 × g in a Beckman SW40 rotor for 18 h, 1-ml fractions were collected from the top of the gradient [designated fractions number 1 (top) through 13 (bottom)] and immediately supplemented with protease inhibitors. The pellet was resuspended by Dounce homogenization in 1 ml of MBS and designated fraction 14.
For saponin treatment, the protocol was the same as above, except that cells were solubilized either in 1% Triton or 0.5% Triton + 0.5% saponin.
Crosslinking Experiments.
Crosslinking experiments were performed on membranes isolated from PC12 cells. Membranes were prepared by homogenization of cells in 3 ml of 20 mM Hepes/1 mM EDTA/255 mM sucrose, pH7.4 (HES) with 20 strokes of a Dounce homogenizer, followed by centrifugation of the homogenate at 196,000 × g for 45 min. For chemical crosslinking, membranes were resuspended in 2 ml of HES either in the presence or absence of 10 mM EGS or 10 mM EDAC and incubated at room temperature for 30 min. Membranes were diluted 2-fold, recovered by centrifugation, washed in 5 ml of HES, and solubilized in 2 ml of 1% Triton. Gradients were prepared as above.
Analysis of SNARE Complex Levels.
Gradients were prepared from solubilized PC12 cells as above. Raft and nonraft (fraction number 12 was used) fractions were added to SDS-dissociation buffer and separated by SDS/PAGE with or without previous boiling. SDS-resistant complexes were identified by immunoblotting with an antibody against syntaxin 1A.
Cholesterol Depletion and Dopamine Release Assays.
Cholesterol depletion was based on that described in ref. 24. Cells were grown on collagen-coated 24-well trays in RPMI 1640 media supplemented with 10% delipidated calf serum, in the presence or absence of 4 μM lovastatin and 0.25 mM mevalonate for 4–5 days. The cells were incubated with 0.5μCi/ml [3H] dopamine in RPMI 1640 with 0.088 mg/ml ascorbic acid for 90 min at 37°C. Methyl-β-cyclodextrin (5 mM) was added to the lovastatin-treated cells for the last 10 min of this incubation. The cells were then washed twice in 145 mM NaCl/5 mM KCl/1.3 mM MgCl2/1.2 mM NaH2PO4/10 mM glucose/3 mM CaCl2/20 mM Hepes, pH 7.4 and release of tritiated dopamine measured in the presence or absence of 300 μM ATP for 10 min. The total amount of cellular [3H] dopamine was calculated for every sample, and dopamine release was expressed as a percentage of the total cell content. Total cellular cholesterol levels were measured by using Infinity cholesterol reagent (Sigma).
Results
Isolation of Lipid Rafts from PC12 Cells.
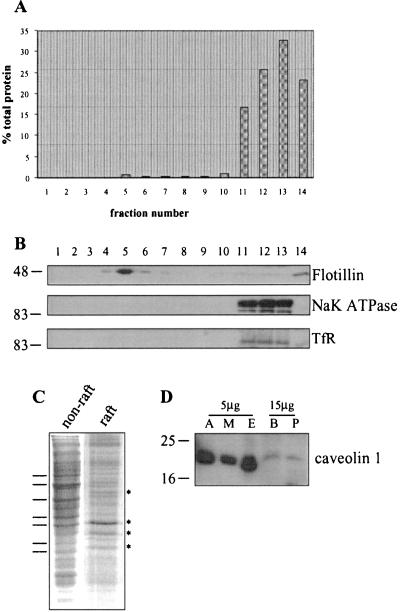
The biochemical characterization of lipid rafts from various cell types has been greatly facilitated by isolation procedures on the basis of relative insolubility of these membrane domains in Triton X-100. After solubilization in Triton, the cells are centrifuged in a 40/30/5% discontinuous sucrose gradient (25). Cytosolic proteins and solubilized membrane proteins remain in the 40% sucrose layer. Insoluble lipid rafts, on the other hand, have a lower buoyant density and float to the interface between the 30 and 5% sucrose layers. We used this well-established protocol to purify lipid rafts from PC12 cells. Fig. 1A shows that the majority of cellular protein is recovered in fractions 11–14, which represent proteins present in the 40% sucrose layer (fractions 11–13) and insoluble pelleted material (fraction 14). A small peak of protein is also centered at fraction 5, which contains the 30/5% interface, where lipid rafts are known to accumulate. The amount of protein recovered in fraction 5 accounts for around 0.7% of the total protein. The recovered gradient fractions were analyzed by immunoblotting with an antibody against flotillin, a protein known to be enriched in lipid rafts (26). Fig. 1B shows that the large majority of flotillin was recovered in fraction 5, confirming that this fraction is enriched in lipid rafts. In contrast, the α1 subunit of the Na,K ATPase and the transferrin receptor, proteins that do not associate with lipid rafts, were absent from fraction 5.
Figure 1.
Characterization of raft and nonraft fractions isolated from PC12 cells and caveolin expression in PC12 cells. PC12 cells were solubilized in 1% Triton X-100 and fractionated on a discontinuous sucrose gradient, as detailed in Materials and Methods. (A) The protein content of gradient fractions shown as a percentage of the total protein on the gradient. (B) Equal volumes of the gradient fractions were separated by SDS/PAGE and transferred to nitrocellulose for immunoblotting analysis by using antibodies specific for flotillin, α1 subunit of the NaK ATPase and the transferrin receptor (TfR). (C) Similar amounts of protein from fraction 12 (nonraft) and fraction 5 (raft) were separated by SDS/PAGE and stained with Coomassie blue. Differences between the protein profiles are highlighted by lines and asterisks. (D) Homogenates prepared from 3T3-L1 adipocytes (A), L6 skeletal muscle cells (M), endothelium (E), brain (B) and PC12 cells (P) were separated by SDS/PAGE and transferred to nitrocellulose for immunoblotting analysis by using a caveolin-1 specific antibody. Molecular weight markers are shown on Left in B and D.
The differential distribution of the proteins shown in Fig. 1B demonstrates that the procedure used successfully separates raft-associated and nonraft proteins. This separation is also highlighted by the distinct protein profiles of fraction 5 (raft) and protein remaining in the 40% sucrose (nonraft) (Fig. 1C).
It is well established that Triton-insoluble material recovered from the 30/5% sucrose interface contains both lipid rafts and caveolae (27, 28). These two membrane structures have similar lipid compositions; however, the formation of caveolae depends on the structural protein caveolin (29). Caveolin expression was found to be barely detectable in PC12 cells (Fig. 1D), in agreement with previous studies (30, 31). The very low levels of caveolin detected in PC12 cells suggest that fraction 5 mainly contains noncaveolar lipid rafts rather than caveolae.
Association of SNARE Proteins with Lipid Rafts.
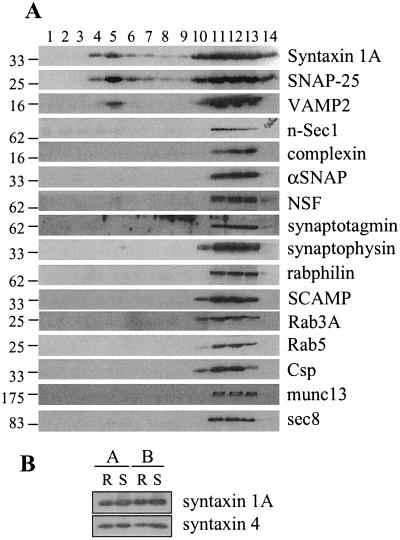
To establish what role lipid rafts play in organizing the secretory pathway in PC12 cells, we examined the distribution of components of this pathway in sucrose gradient fractions. Fig. 2A shows that significant amounts of syntaxin 1A, SNAP-25, and VAMP2 were present in fraction 5 (raft). Blot quantitation was used to estimate the amount of syntaxin 1A and SNAP-25 associated with rafts. Total immunoreactivity was calculated as the sum of the intensities of fractions 4, 5, and 10–14, as these fractions contained the vast majority of syntaxin 1A and SNAP-25. Assuming that protein in fractions 4 and 5 are raft associated, we estimated that 22.1 ± 1.35% of syntaxin 1A (n = 12) and 24.1 ± 1.2% of SNAP-25 (n = 10) are associated with rafts. This percentage approximates to a 25-fold enrichment of syntaxin 1A and SNAP-25 in raft relative to nonraft fractions. The recovery of VAMP2 in the raft fractions was found to be more variable than that of syntaxin 1A and SNAP-25 (4.7–23.9% raft associated). Although SNARE proteins were associated with lipid raft fractions, no other secretory proteins were significantly present in these domains (Fig. 2A). Syntaxin association with lipid rafts was not specific to syntaxin 1A, as syntaxin 4 was also associated with rafts (Fig. 2B).
Figure 2.
Analysis of raft-associated proteins in PC12 cells. Cells were solubilized in 1% Triton X-100 and fractionated on a discontinuous sucrose gradient, as detailed in Materials and Methods. (A) Equal volumes of the recovered fractions were separated by SDS/PAGE and transferred to nitrocellulose for immunoblotting analysis by using antibodies against the indicated proteins. Molecular weight markers are indicated on the Left of all blots. This analysis was repeated on four separate gradients with similar results. Shown is a representative experiment. (B) Comparison of syntaxin 1A and syntaxin 4 association with isolated raft (R) and solubilized (S) fractions from two separate experiments (A and B).
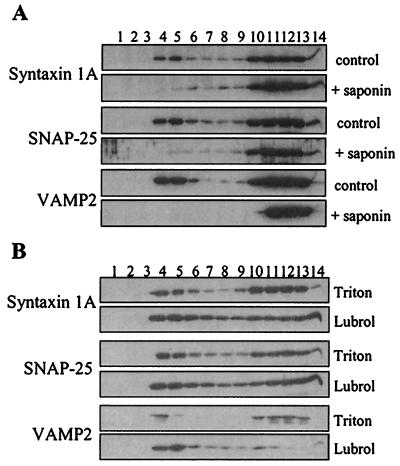
The observed flotation of SNARE proteins in sucrose gradients after Triton solubilization is good evidence that these proteins are associated with lipid rafts. However, as a more stringent test for raft association, we analyzed the effect of cholesterol disruption on the buoyant property of the SNAREs. It has previously been shown that the insolubility of lipid rafts in Triton depends on cholesterol. When PC12 cells were treated with a combination of saponin (to disrupt cholesterol) and Triton, the SNARE proteins were effectively solubilized and no longer exhibited buoyancy in sucrose gradients (Fig. 3A). It should be noted that 0.5% saponin effectively solubilized the SNARE proteins in the presence of 0.5% Triton (compared with 1% Triton used for control experiments). This cholesterol-dependent insolubility of SNARE proteins is strong evidence of their association with bona fide cholesterol-rich lipid rafts.
Figure 3.
Cholesterol dependence of raft association of SNARE proteins, and analysis of SNARE association with Lubrol-insoluble rafts. PC12 cells were solubilized in (A) either 1% Triton X-100 (control) or 0.5% Triton + 0.5% saponin (+ saponin); or (B) 1% Triton X-100 (Triton) or 1% Lubrol WX (Lubrol), and fractionated on a discontinuous sucrose gradient, as detailed in Materials and Methods. Equal volumes of the recovered fractions were separated by SDS/PAGE and transferred to nitrocellulose for immunoblotting analysis by using antibodies specific for syntaxin 1A, SNAP-25 and VAMP2.
A novel class of lipid raft has recently been identified that is soluble in Triton X-100 but insoluble in Lubrol WX (20). We examined the association of SNARE proteins with these “Lubrol rafts”. Fig. 3B shows that the large majority of VAMP2 was recovered in Lubrol-resistant rafts. In contrast, there was no difference in the recovery of SNAP-25 in rafts isolated by using Triton or Lubrol, whereas syntaxin 1A showed only a modest increase in rafts isolated by using Lubrol. These results suggest that VAMP2 is largely present in a distinct class of raft to syntaxin 1A and SNAP-25.
Chemical Crosslinking Analysis of Protein Complexes in Raft and Nonraft Fractions.
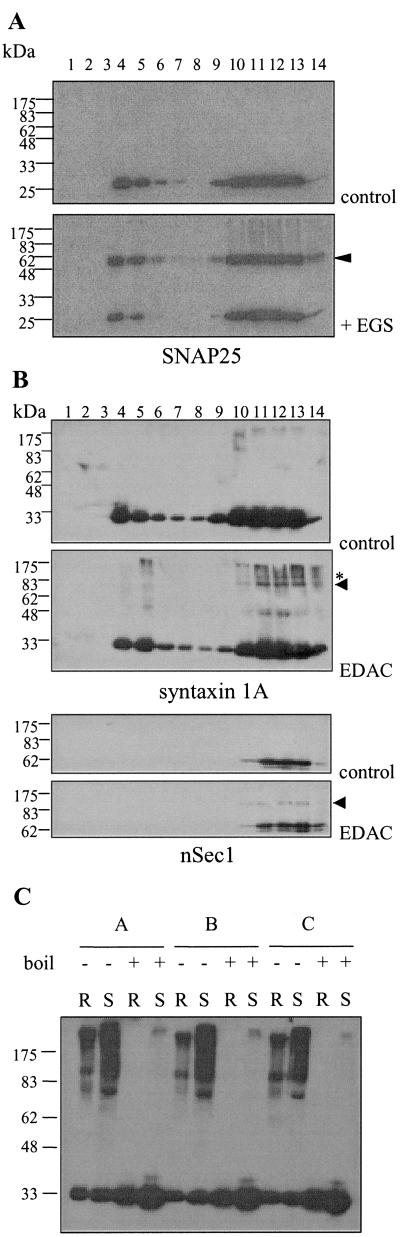
Syntaxin 1A and SNAP-25 interact to form a high-affinity VAMP2-binding site. We used chemical crosslinking to compare the presence of syntaxin 1A/SNAP-25 heterodimers in Triton-insoluble raft and nonraft fractions. Membranes were incubated in the presence or absence of the crosslinking reagent EGS before Triton solubilization. When samples were probed with an antibody recognizing the N terminus of SNAP-25, an adduct was clearly visible in EGS-treated samples at around 60 kDa (Fig. 4A, arrowhead), the size expected of a syntaxin 1A/SNAP-25 heterodimer. The relative amounts of this adduct were comparable between raft and nonraft fractions, implying that similar levels of the heterodimer are found in these domains. When the crosslinked samples were probed for syntaxin 1A, this 60-kDa adduct was also detected; however, the signal was significantly lower than that from the N-terminal SNAP-25 antibody (data not shown). This reduced signal is likely because of epitope masking, as this reduction was also observed when the samples were probed with an antibody recognizing the C terminus of SNAP-25 (data not shown).
Figure 4.
Analysis of protein complexes in raft and nonraft fractions. Membranes were prepared from PC12 cells and incubated in the presence or absence of 10 mM EGS (A) or 10 mM EDAC (B) for 30 min at room temperature. The membranes were washed, solubilized in 1% Triton X-100, and fractionated on sucrose gradients, as described in Materials and Methods. Equal volumes of the recovered fractions were separated by SDS/PAGE and transferred to nitrocellulose for immunoblotting analysis by using the antibodies indicated. Arrowheads indicate putative SNAP-25/syntaxin 1A heterodimers (A) and nSec1/syntaxin 1A complex (B). Asterisk in B highlights an unknown syntaxin 1A-containing complex. (C) Raft fractions (R) and solubilized fractions (S) were identified and separated by SDS/PAGE with (+) or without (−) previous boiling of the samples. Immunoblotting analysis with syntaxin 1A antibody detected monomeric syntaxin 1A and syntaxin 1A-containing SDS-resistant complexes in nonboiled samples. This analysis was performed on fractions prepared from three individual experiments (A, B, and C). Molecular weight markers are indicated on the Left of all blots.
nSec1 is an essential component of the exocytotic machinery. This protein binds to monomeric syntaxin 1A, forming a heterodimeric complex mutually exclusive of SNARE complex formation (6, 7). We performed additional crosslinking experiments to compare the level of nSec1/syntaxin 1A heterodimer in raft and nonraft fractions. For these experiments, EDAC was used, as this chemical has been shown to effectively crosslink nSec1/syntaxin 1A complexes (32). Fig. 4B shows that treatment of membranes with EDAC before Triton solubilization resulted in the formation of a higher molecular-weight band (arrowhead), which was recognized by both syntaxin 1A (Upper) and nSec1 (Lower) antibodies. This crosslinked adduct has a molecular weight of ≈120 kDa, strongly suggesting that it represents an nSec1/syntaxin 1A heterodimer. From Fig. 4B, it can be seen that this adduct was not detected in raft fractions, consistent with Fig. 2, in which nSec1 was not present in raft fractions. Note that EDAC did not crosslink syntaxin 1A/SNAP-25 complexes, as shown previously (32). The crosslinking experiments shown in Fig. 4 have successfully identified three pools of syntaxin 1A: (i) raft-associated, SNAP-25 complexed; (ii) nonraft, SNAP-25 complexed; and (iii) nonraft, nSec1 complexed. The organization of these protein complexes into distinct membrane domains is likely to be important for exocytosis.
Analysis of SNARE Complex Levels in Raft and Nonraft Fractions.
To analyze whether raft-associated syntaxin1A/SNAP-25 complexes interact with VAMP2, we examined purified raft and nonraft fractions for the presence of SDS-resistant SNARE complexes. The ternary SNARE complex is highly stable, such that it resists denaturation by SDS, and is disrupted only on boiling of samples (33). Therefore, it is possible to measure SNARE complex levels by comparing boiled and nonboiled samples. Fig. 4C shows that high-molecular-weight syntaxin 1A-containing protein complexes were detected in nonboiled samples. These high-molecular-weight bands disappeared on boiling the samples, and a corresponding increase in the levels of monomeric syntaxin 1A was observed. Fig. 4C shows that these SDS-resistant SNARE complexes were present in both raft and nonraft fractions, demonstrating that raft association of syntaxin 1A and SNAP-25 does not prevent their participation in ternary SNARE complex formation. Nevertheless, we did consistently observe that SNARE complex levels were greater in nonraft fractions (see Fig. 4C), supporting the conclusion, based on Lubrol insolubility, that VAMP2 is present in distinct rafts.
Effect of Cholesterol Depletion on Dopamine Release from PC12 Cells.
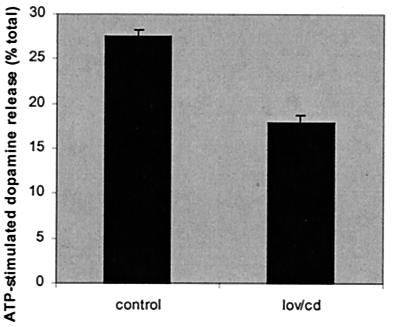
We have shown that the integrity of SNARE-associated lipid rafts depends on cholesterol (Fig. 3A). To examine the role of cholesterol-rich rafts in exocytosis, we treated cells with lovastatin and methyl-β-cyclodextrin to deplete cellular cholesterol (see Materials and Methods). This treatment reduced cellular cholesterol levels by an average of 35% (data not shown). To determine the effect of cholesterol depletion on exocytosis, release of [3H] dopamine was assayed from control and cholesterol-depleted PC12 cells. Fig. 5 shows that the extent of ATP-stimulated dopamine release was reduced by around 35% in the cholesterol-depleted cells, providing good functional evidence that cholesterol and lipid rafts play an important role in regulated exocytosis.
Figure 5.
Effect of cellular cholesterol depletion on ATP-stimulated dopamine release from PC12 cells. Cells were treated without (control) or with lovastatin and methyl-β-cyclodextrin (lov/cd) as detailed in Materials and Methods. Cells were loaded with [3H] dopamine, and release was assayed in response to 300 μM ATP for 10 min. The total cellular [3H] dopamine content of each sample was also measured and dopamine release in response to ATP expressed as a percentage of total dopamine content. [3H] dopamine release in the absence of ATP was assayed and subtracted from the values shown. The data shown are averaged from three separate experiments (n = 18).
Discussion
Regulated exocytosis requires the specific and sequential interaction of a host of different proteins. Such a complex process is likely to require strict organization of the participating proteins. We have examined the potential role played by lipid rafts in organizing the exocytotic machinery in PC12 cells. Interestingly, of the large number of proteins examined in this study, only SNARE proteins were found to be significantly associated with lipid rafts. These proteins represent the “core” fusion machinery, and so their presence in lipid raft fractions raises interesting questions concerning the role of these microdomains in membrane fusion. Lafont et al. (34) recently reported that syntaxin 3 and toxin-insensitive-VAMP were raft-associated in Madin–Darby canine kidney cells, suggesting that raft association of SNAREs is not specific to PC12 cells.
Interestingly, we found that vesicle SNARE and tSNAREs were largely associated with distinct types of lipid raft. VAMP2 was mainly associated with Lubrol-insoluble rafts and in this regard is similar to the synaptic vesicle protein synaptophysin (20), which binds cholesterol directly (35). VAMP2 and synaptophysin have previously been shown to be associated in a protein complex present on synaptic vesicles and in PC12 cells (36), and Lubrol-insoluble domains may, therefore, play an important role in coordinating this interaction.
SNARE proteins bind to a large number of other (effector) proteins. It was, therefore, surprising that no other secretory proteins were significantly present in lipid raft fractions. The SNARE-binding protein αSNAP has highest affinity for the ternary SNARE complex but also binds to monomeric syntaxin 1A and syntaxin 1A/SNAP-25 heterodimers. Both syntaxin 1A and syntaxin 1A/SNAP-25 were detected in lipid rafts, and yet no αSNAP was present in these domains. Similarly, nSec1 was also not detected in purified raft fractions. Although this protein has been widely suggested to function as a negative regulator of membrane fusion, there is strong evidence that nSec1 is required for membrane fusion (8, 37, 38). Association of the syntaxin 1A/SNAP-25 tSNARE complex with lipid raft domains may facilitate spatial organization of vesicle trafficking events at the plasma membrane. Indeed, we found that reduction of cellular cholesterol levels (a key component of rafts) resulted in a decreased extent of evoked dopamine release from PC12 cells. This observation provides good evidence that cholesterol-rich lipid rafts perform an important function in regulated exocytosis and membrane fusion.
As discussed above, lipid rafts may function in exocytosis by organizing SNARE proteins at particular sites of the plasma membrane. Rafts could also play an active role in the process of membrane fusion. For example, fusion of vesicles with regions of the plasma membrane rich in cholesterol and glycosphingolipid may be more energetically favorable than fusion with nonraft domains. Also, the association of VAMP2 with Lubrol rafts implies that these poorly defined microdomains are important for membrane fusion. Recent work has shown that the transmembrane domains of both syntaxin 1A and VAMP2 play an active role in membrane fusion (39). The ordered nature of lipid raft domains may be important to allow efficient force transduction to the membrane anchors of these proteins.
This work has highlighted the likely role played by lipid rafts in organizing the secretory pathway in PC12 cells. The selective interaction of rafts with SNARE proteins should provide the cell with a mechanism to exert both functional and spatial control of regulated exocytosis.
Acknowledgments
We are grateful to Dr. Harvey McMahon and Dr. David Apps for anticomplexin and antisynaptotagmin antibodies. This work was supported by grants from the Biotechnology and Biological Sciences Research Council and The Wellcome Trust.
Abbreviations
- NSF
N-ethylmaleimide-sensitive factor
- SNAP
soluble N-ethylmaleimide-sensitive factor attachment protein
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- tSNARE
target SNARE
- SNAP-25
synaptosomal-associated protein of 25 kDa
- VAMP
vesicle-associated membrane protein
- EGS
ethyleneglycol-bis(succinimidylsuccinate)
- EDAC
1-ethyl-3-(3-dimethylaminopropyl)carboiimide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Weber T, Zemelman B V, McNew J A, Westermann B, Gmachl M, Parfiati F, Sollner T H, Rothman J E. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 2.Sudhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain L H, Roth D, Morgan A, Burgoyne R D. J Cell Biol. 1995;130:1063–1071. doi: 10.1083/jcb.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ungermann C, Nichols B J, Pelham H R B, Wickner W. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu T, Ashery U, Burgoyne R D, Neher E. EMBO J. 1999;18:3293–3304. doi: 10.1093/emboj/18.12.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pevsner J, Hsu S C, Braun J E, Calakos N, Ting A E, Bennett M K, Scheller R H. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Steegmaier M, Gonzalez LC, Jr, Scheller R H. J Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher R J, Pevsner J, Burgoyne R D. Science. 2001;291:875–877. doi: 10.1126/science.291.5505.875. [DOI] [PubMed] [Google Scholar]
- 9.Hata Y, Slaughter C A, Sudhof T C. Nature (London) 1993;366:347–350. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 10.Bennett M K, Calakos N C, Scheller R H. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 11.Betz A, Okamoto M, Benseler F, Brose N. J Biol Chem. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- 12.Wu M N, Fergestad T, Lloyd T E, He Y, Broadie K, Bellen H J. Neuron. 1999;23:593–605. doi: 10.1016/s0896-6273(00)80811-9. [DOI] [PubMed] [Google Scholar]
- 13.Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A, et al. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 14.Garcia E P, McPherson P S, Chilcote T J, Takei K, DeCamilli P. J Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 16.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto T, Schlegel A, Scherer P E, Lisanti M P. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 18.Smart E J, Graf G A, McNiven M A, Sessa W C, Engelman J A, Scherer P E, Okamoto T, Lisanti M P. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zegers M M P, Hoekstra D. Biochem J. 1998;336:257–269. doi: 10.1042/bj3360257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roper K, Corbeil D, Huttner W B. Nat Cell Biol. 2000;2:582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- 21.Roth D, Burgoyne R D. FEBS Lett. 1995;351:207–210. doi: 10.1016/0014-5793(94)00833-7. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain L H, Burgoyne R D. J Biol Chem. 1996;271:7320–7323. doi: 10.1074/jbc.271.13.7320. [DOI] [PubMed] [Google Scholar]
- 23.Morgan A, Burgoyne R D. EMBO J. 1995;14:232–239. doi: 10.1002/j.1460-2075.1995.tb06996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledesma M D, Simons K, Dotti C G. Proc Natl Acad Sci USA. 1998;95:3966–3971. doi: 10.1073/pnas.95.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown D A, Rose J K. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 26.Bickel P E, Scherer P E, Schnitzer J E, Oh P, Lisanti M P, Lodish H F. J Biol Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- 27.Fra A M, Williamson E, Simons K, Parton R G. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- 28.Schnitzer J E, McIntosh D P, Dvorak A M, Liu J, Oh P. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- 29.Rothberg K G, Heuser J E, Donzell W C, Ying Y-S, Glenney J R, Anderson R G W. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt A, Hannah M J, Huttner W B. J Cell Biol. 1997;137:445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilderback T R, Gazula V-R, Lisanti M P, Dobrowsky R T. J Biol Chem. 1999;274:257–263. doi: 10.1074/jbc.274.1.257. [DOI] [PubMed] [Google Scholar]
- 32.Haynes L P, Morgan A, Burgoyne R D. Biochem J. 1999;342:707–714. [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Sudhof T C, Niemann H. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Proc Natl Acad Sci USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiele C, Hannah M J, Fahrenholz F, Huttner W B. Nat Cell Biol. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- 36.Calakos N, Scheller R H. J Biol Chem. 1994;269:24534–24537. [PubMed] [Google Scholar]
- 37.Harrison S D, Broadie K, van de Goor J, Rubin G M. Neuron. 1994;13:555–566. doi: 10.1016/0896-6273(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 38.Verhage M, Maia A S, Plomp J J, Brussaard A B, Heeroma J H, Vermeer H, Toonen R F, Hammer R E, van den Berg T K, Missler M, et al. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 39.McNew J A, Weber T, Parlati F, Johnston R J, Melia T J, Sollner T H, Rothman J E. J Cell Biol. 2000;150:105–117. doi: 10.1083/jcb.150.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]