Abstract
Objectives
Increasing evidence suggests that depression is a risk factor for cognitive impairment, but it is unclear if this is true among the oldest old. We determined whether elevated depressive symptoms predicted five-year incident mild cognitive impairment (MCI) or dementia, and neuropsychological test performance among oldest-old women.
Design
Prospective
Setting
Three study sites
Participants
302 women ≥85 years (mean, 87 ±2)
Measurements
Depressive symptoms were measured with the 15-item Geriatric Depression Scale (GDS); scores ≥6 indicated elevated symptoms. Five years later, participants completed neuropsychological testing and clinical cognitive status was adjudicated.
Results
In analyses of MCI vs. normal cognition, 70% of women with GDS ≥6 at baseline developed MCI vs. 37% with GDS <6. After adjustment for age, education, alcohol and benzodiazepine use, and study site, GDS ≥6 remained independently associated with much greater likelihood of developing MCI (multivariable odds ratio (MOR) = 3.71, 95% confidence interval (CI) 1.30, 10.59). In analyses of dementia vs. normal cognition, 65% of women with GDS ≥6 developed dementia compared to 37% of those with GDS <6 (MOR = 3.15, 95% CI 1.03, 9.65). Only 19% of women with GDS ≥6 had normal cognitive status five years later, compared to 46% of those with GDS <6 (MOR = 0.28, 95% CI 0.11, 0.73). Women with elevated depressive symptoms had worse scores on tests of global cognition and working memory.
Conclusion
Elevated depressive symptoms are an important risk factor for cognitive disorders and lower cognitive performance among women living to their ninth and tenth decades.
Keywords: oldest-old, women, depression, mild cognitive impairment, dementia
Introduction
Depression is common in older adults. Estimates from community-based studies suggest that 3 to 26% of older adults have significant levels of depressive symptomatology.(1) Findings from population-based samples indicate that the twelve-month prevalence of major depression ranges from approximately 2 to 4% in adults aged 65 and older,(2, 3) and a study of adults over age 70 reported a one-month prevalence of 11%.(4) Sex differences have been observed in the prevalence of depression; older women may be more than three times more likely to have major depressive disorder than older men.(2)
Cognitive impairment also is common among elders; 14% of adults over 70 years and 37% of those over 90 have dementia.(5) At least as many older adults are thought to have mild cognitive impairment (MCI).(6) An emerging body of evidence supports an association between depression or depressive symptoms and cognitive impairment and decline in older adults. Studies have demonstrated that depressive symptoms are associated with lower performance or decline on cognitive tests.(7-11) An investigation in the Study of Osteoporotic Fractures (SOF) found an association between elevated depressive symptoms and both decline in cognitive test performance and patient-reported dementia diagnosis,(12) and other studies have reported an association between depressive symptoms and subsequent MCI diagnosis.(13, 14)
Adults aged 85 and older, the “oldest old,” are one of the fastest-growing segments of the US population; by 2050, 1 in 4 elders will be 85 or older.(15) Currently, however, our knowledge of risk or protective factors for dementia among the oldest old is limited,(16) and due to unique biological and social characteristics that influence survival into very old age, traditional risk factors for MCI and dementia might not apply to the oldest adults.(17, 18) To maximize quality of life and maintain independence, identification of risk factors for adverse cognitive outcomes in this burgeoning age group is critical. Evidence that depression increases the risk of MCI or dementia in the oldest old could have important implications for the prevention or treatment of depression as a means of preserving cognitive health and quality of life in the oldest adults. In this study, also in the SOF cohort, we determined the association between elevated depressive symptoms and adjudicated diagnoses of MCI and dementia in a large sample of oldest-old women, and identified the neuropsychological profile of elevated depressive symptoms in this population.
Methods
Participants
Participants were women aged 85 and older who were actively participating in the Study of Osteoporotic Fractures (SOF). SOF is a prospective study that recruited 9,704 mostly white women age 65 and greater from the Monongahela Valley (near Pittsburgh), PA, Portland, OR, Baltimore, MD, and Minneapolis, MN between September 1986 and October 1988 using population-based listings. To be eligible for participation in SOF, women were required to be community dwelling, able to ambulate without assistance from another, and to have no history of bilateral hip replacement at baseline. Since the initial study visit, the women have completed study visits every two to four years.
Participants in the present study were from an ancillary investigation, Women, Cognitive Impairment Study of Exceptional Aging (WISE). SOF WISE enrolled 1,534 women from three of the four sites during the 2006 to 2008 (Year 20 of SOF) study visit. These women completed an expanded battery of seven neuropsychological tests. We studied 302 women from the original SOF cohort who were ≥85 years of age, responded to all items on the 15-item Geriatric Depression Scale,(19) had complete data for adjudication screening as part of SOF WISE (see below), and reported being free of dementia and not taking Alzheimer’s disease medication at the 2002 to 2004 SOF study visit. For our study, this visit was our “baseline” and the Year 20 visit, our 5-year follow-up.
Women’s demographic data including age, race, and educational attainment were collected upon enrollment in SOF. At each visit, women provided information about their residence type and if they lived alone. They also reported whether a healthcare provider ever had told them that they have numerous medical conditions, including hypertension, myocardial infarction, diabetes, stroke, and dementia. History of angina or myocardial infarction was categorized as coronary artery disease. Participants’ height and weight were measured, and they provided information about functional status. In addition, participants were asked to bring all medications taken over the prior 30 days. An informant (e.g., family member, caregiver) was asked to complete the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE),(20) on which they reported the extent to which participants’ cognition and function had changed over the last several years.
Depressive Symptoms
Depressive symptoms were measured at baseline and five-year follow-up with the 15-item Geriatric Depression Scale (GDS), a self-report measure of depressive symptoms designed to rapidly identify older adults with significant depressive symptomatology.(19) Respondents indicate whether or not they have experienced particular depressive symptoms (e.g., dropping activities/interests, feeling hopeless) or states suggesting an absence of depression (e.g., feeling energetic, thinking that being alive is wonderful) using a yes/no format. Responses suggesting depression are summed, and scores ≥6 suggest probable depression.(19) The 15-item GDS has been shown to have acceptable internal consistency reliability (i.e., Cronbach’s α ≥0.70) in older adults from a range of populations.(21, 22) A systematic review of the criterion validity of the GDS reported that studies with the 15-item version using various cut-points had a mean sensitivity of 0.805 and mean specificity of 0.750.(23)
Neuropsychological Test Battery
At five-year follow-up, participants completed an expanded cognitive battery that included the Modified Mini-Mental State Examination (3MS),(24) an expanded version of the Mini-Mental State Examination(25) that measures global cognition; digit span, a test of attention (digits forward) and working memory (digits backwards)(26); the California Verbal Learning Test-II-Short Form (CVLT-SF),(27) a measure of verbal learning and memory; and verbal fluency tests, in which participants were given one minute to name as many words beginning with ‘f’ (phonemic fluency), and one minute to name as many vegetables (category fluency), as they could. They also completed Trails B,(28) a timed test of executive function and psychomotor speed, on which shorter completion times indicate better cognitive function. Participants were given up to 180 seconds to complete the measure. These tests were selected for the expanded neuropsychological battery because they cover a broad range of cognitive domains.
Adjudication of Clinical Cognitive Status
Clinical cognitive adjudication was conducted in two phases following the five-year follow-up visit. The first was a screening phase in which participants screened positive if they: (a) reported physician diagnosis of dementia; (b) resided in a nursing home; (c) scored <88 on the 3MS; (d) recalled <4 words after a delay on the CVLT; or (e) had an IQCODE score ≥3.6 indicating significant cognitive and functional decline.(29) In the second phase, participants who screened positive for impairment were adjudicated by a member of a multidisciplinary team (neuropsychologists, neurologist, and geropsychologist), who determined whether women were cognitively normal, or had MCI or dementia. MCI diagnoses were based on a modified version of the Petersen criteria,(30) and dementia diagnoses were based on DSM-IV-TR criteria.(31) Adjudication decisions were based on participants’ performance on the neuropsychological battery at the five-year follow-up visit, and on prior cognitive tests, medical history, medications, and functional status. In addition, although adjudicators did not receive data on GDS scores at baseline, they were informed of participants’ raw GDS scores at the follow-up visit. Women who did not screen positive were classified as cognitively normal.
Statistical Analyses
Analyses were conducted using Stata 10.1 (Statacorp). Our primary predictor was GDS score, which we analyzed dichotomously as GDS ≥6 or GDS <6. We first compared baseline characteristics between women with and without elevated depressive symptoms. We then compared participants’ performance on neuropsychological tests according to baseline depressive symptoms (GDS <6 vs. ≥6). We used t-tests to compare normally distributed variables (equal variances not assumed), Mann-Whitney or Kruskal-Wallis tests for skewed variables, and chi-square or Fisher’s exact tests for categorical variables. For women who did not complete Trails B within 180 seconds, we coded completion time as 181 seconds.
To determine the association between elevated depressive symptoms and incident cognitive impairment (MCI, dementia, or both), we conducted logistic regression analyses. To control for confounding variables, we identified covariates that were known correlates of cognitive decline or were associated with GDS score and cognitive status at the p <0.10 level. We included age, education (≤ high school vs. > high school), alcohol use, benzodiazepine use, and study site in multivariable models. History of stroke was associated with depression, but as it likely lies on the causal pathway linking depression and cognitive impairment, we conducted regression analyses with and without stroke in the models. Because results did not differ notably with or without stroke in models, we present results from the simpler models without stroke.
After fitting logistic regression models, we performed model diagnostics, including calculation of the Pregibon Delta-Beta statistic, a measure of the relative influence of observations on logistic regression model coefficients.(32) Examination of the multivariable-adjusted model with MCI vs. normal cognition as the outcome identified four high-influence observations, all of which appeared to have legitimate values for variables. All four women were aged 85 or 86, had ≤ high school education, received an MCI diagnosis, and were from the same study site. Three of them had less than a high school education, only one drank alcohol over the prior 30 days (average of two drinks per day), and three had elevated depressive symptoms. None took benzodiazepines. We present results with and without these four observations.
Results
Women were on average 86.9 ±2.1 years old at baseline (range 85 – 96), and 91.7 ± 2.2 (range 89 – 102) at follow-up. Of the 302 women, 301 were white and one reported “other” race/ethnicity. They had an average of 12.7 ±2.5 years of education; 106 (35.1%) had education beyond high school. None of the women resided in a nursing home at baseline. Their mean baseline GDS score was 2.3 ±2.2 (range 0 – 11) and 31 (10.3%) women had GDS scores ≥6, indicating elevated depressive symptoms. Compared to women without elevated depressive symptoms, those with GDS ≥6 were more likely to take benzodiazepine medications, less likely to drink alcohol, and had a more frequent history of stroke (Table 1). Women with and without elevated depressive symptoms did not differ by age, education, smoking status, antidepressant use, or history of diabetes, hypertension, coronary artery disease, or by MMSE score at baseline. Women from different study sites differed by educational attainment (χ2 (2, N = 302) = 12.0, p = 0.002), smoking status (p = 0.038), alcohol use (χ2 (2, N = 302) = 23.2, p <0.001), and history of diabetes (p = 0.009, Fisher’s exact test). They did not differ by level of depressive symptoms (χ2 (2, N = 302) = 2.6, p = 0.272).
Table 1.
Participant characteristics (mean ±SD or n (%)) by number of depressive symptoms.
| GDS <6 (n = 271) | GDS ≥6 (n = 31) | Test Statistic | p a | |
|---|---|---|---|---|
| Age | 86.9 ±2.0 | 87.2 ±2.4 | z = −0.6 | 0.571 |
| Education > high school | 99 (36.5) | 7 (22.6) | χ2 = 2.4 | 0.123 |
| MMSE score (0-30) | 28.2 ±1.5 | 28.2 ±1.5 | z = 0.2 | 0.876 |
| Current smoker | 4 (1.5) | 1 (3.2) | -- | 0.420b |
| # of alcoholic drinks/week | 0.9 ±2.5 | 0.4 ±1.0 | z = 1.9 | 0.057 |
| Antidepressant use | 36 (13.3) | 5 (16.1) | -- | 0.589b |
| Benzodiazepine use | 22 (8.1) | 7 (22.6) | χ2 = 6.7 | 0.010 |
| Diabetes | 25 (9.2) | 4 (12.9) | -- | 0.518b |
| Hypertension | 162 (59.8) | 22 (71.0) | χ2= 1.5 | 0.226 |
| Coronary artery disease | 60 (22.1) | 7 (22.6) | χ2 = 0.003 | 0.955 |
| Stroke | 33 (12.2) | 8 (25.8) | χ2 = 4.4 | 0.036 |
Note: N = 302 for all except MMSE (N = 282).
p-values are from Mann-Whitney tests, χ2 tests with 1 degree of freedom, or
Fisher’s exact test. GDS = 15-item Geriatric Depression Scale.
Coronary artery disease = history of angina or heart attack. MMSE = Mini-Mental State Examination.
Adjudicated cognitive status
Following screening, the data from 212 (70.2%) of the 302 women in our sample at five-year follow-up were referred for adjudication; the remaining 90 (29.8%) women were categorized as cognitively normal. Of the 212 women referred for adjudication, 88 of the women (41.5%) were judged to have MCI, 84 (39.6%) to have dementia, and 40 (18.9%) were categorized as cognitively normal.
The distribution of cognitive outcomes differed by level of depressive symptoms (χ2 = 8.3, p = 0.016) (Table 2). Among the 218 women with normal cognition or MCI at follow-up, those with elevated depressive symptoms were more likely to have MCI (70.0%) than those without elevated symptoms (37.4%) (χ2 (1, N = 218) = 8.0, p = 0.005). In unadjusted logistic regression analyses, women with elevated depressive symptoms had almost four times the odds of having MCI (vs. normal cognition), compared with women without elevated symptoms (odds ratio (OR) = 3.91, 95% confidence interval (CI) 1.44, 10.62; Wald-χ2 (1, N = 218) = 7.2, p = 0.008; see Figure 1). After multivariable adjustment, elevated depressive symptoms remained associated with more than a >3.5-fold increase in the odds of MCI (multivariable OR (MOR) = 3.71, 95% CI 1.30, 10.59; Wald-χ2 (1, N = 218) = 6.0, p = 0.014). When we removed four high-influence points from the model, the magnitude of the association between depressive symptoms and MCI decreased and became statistically non-significant (MOR = 2.61, 95% CI 0.87, 7.85; Wald-χ2 (1, N = 214) = 2.9, p = 0.087).
Table 2.
Clinical cognitive status (N (%)) at follow-up among 302 oldest-old women according to baseline depressive symptoms.
|
GDS <6 n = 271 |
GDS ≥6 n = 31 |
|
|---|---|---|
| MCI | 74 (27.3) | 14 (45.2) |
| Dementia | 73 (26.9) | 11 (35.5) |
| Normal | 124 (45.8) | 6 (19.4) |
N = 302. Overall χ2 (2, N = 302) = 8.3, p = 0.016. GDS = 15-item Geriatric Depression Scale; MCI = mild cognitive impairment.
Figure 1.
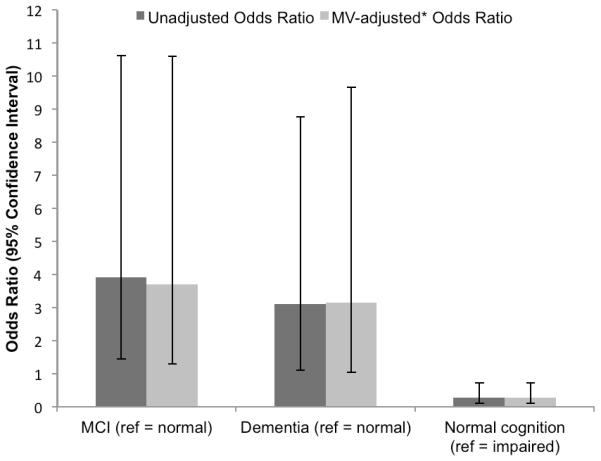
Association between elevated baseline depressive symptoms and clinical cognitive status five years later among oldest old women.
Note: For outcomes of MCI and dementia, reference = normal cognition; for normal cognition, reference = cognitive impairment (either MCI or dementia). Vertical lines represent 95% confidence intervals. *Adjusted for age, education (≤ vs. > high school), alcohol use, benzodiazepine use, and study site. MCI = mild cognitive impairment; MV = multivariable.
Among the 214 women with normal cognition or dementia at follow-up, women with elevated depressive symptoms were more likely to have dementia (64.7%) than those without elevated symptoms (37.1%) (χ2 (1, N = 214) = 5.0, p = 0.025). In unadjusted analyses, women with elevated depressive symptoms at baseline had a greater odds of dementia at five-year follow-up than those without elevated symptoms (OR = 3.11, 95% CI 1.11, 8.77; Wald-χ2 (1, N = 214) = 4.6, p = 0.032). This association remained after multivariable adjustment (MOR = 3.15, 95% CI 1.03, 9.65; Wald-χ2 (1, N = 214) = 4.04, p = 0.044).
We also investigated the association between depressive symptoms at baseline and maintenance of cognitive function five years later. Only 19.4% of oldest-old women with elevated depressive symptoms remained cognitively normal compared to 45.8% of these women without elevated symptoms (χ2 (1, N = 302) = 7.9, p = 0.005). GDS scores ≥6 were associated with more than a 70% decrease in the odds of remaining cognitively normal in unadjusted analyses (OR = 0.28, 95% CI 0.11, 0.72; Wald-χ2 (1, N = 302) = 7.1, p = 0.008). This association remained after adjustment (MOR = 0.28, 95% CI 0.11, 0.73; Wald-χ2 (1, N = 302) = 6.8, p = 0.009). Removal of the four high-influence points did not qualitatively affect results (MOR = 0.34, 95% CI 0.13, 0.89; Wald-χ2 (1, N = 298) = 4.83, p = 0.028).
Neuropsychological Test Performance
Participants with elevated baseline depressive symptoms performed more poorly on most of the cognitive tests, five years later (Table 3). Compared to women without elevated symptoms, they had lower performance in global cognition (3MS; 79.9 ±13.4 vs. 84.3 ±11.6) and working memory (digits backwards; 4.4 ±1.3 digits vs. 5.4 ±2.0). They also generated fewer ‘f’-words (8.8 ±3.7 vs. 10.4 ±4.1), and had poorer performance on Trails B (167.0 ±26.2 vs. 152.9 ±34.1) compared to women without symptoms, but these differences were not statistically significant. Their performance did not differ on CVLT delayed recall, digits forward, or category fluency tests.
Table 3.
Neuropsychological test performance (mean ± SD) by level of depressive symptoms.
| Test | GDS <6 n = 259-270a |
GDS ≥6 n = 28-31b |
Test Statistic | p c |
|---|---|---|---|---|
| 3MS | 84.3 ±11.6 | 79.9 ±13.4 | z = 2.2 | 0.031 |
| CVLT delayed recall | 4.3 ±2.9 | 3.5 ±2.6 | z = 1.6 | 0.109 |
| Digits forward | 7.4 ±2.2 | 7.1 ±2.1 | z = 0.5 | 0.583 |
| Digits backward | 5.4 ±2.0 | 4.4 ±1.3 | z = 2.9 | 0.003 |
| Phonemic fluency | 10.4 ±4.1 | 8.8 ±3.7 | z = 1.8 | 0.065 |
| Category fluency | 9.6 ±3.3 | 9.4 ±3.3 | t = 0.4 | 0.692d |
| Trails B time (sec) | 152.9 ±34.1 | 167.0 ±26.2 | z = −1.8 | 0.069 |
Note: Ranges of n apply to all tests except Trails B (n = a190, b18).
p-values are from Mann-Whitney tests or t-tests assuming unequal variances (dSatterthwaite’s df = 33.1).
CVLT = California Verbal Learning Test; GDS = 15-item Geriatric Depression Scale; SD = standard deviation; verbal fluency = naming words beginning with ‘f’; category fluency = naming vegetables. GDS scores are from the baseline visit, and cognitive test scores from the follow-up visit
Discussion
We investigated the association between elevated depressive symptoms, incident MCI and dementia, and neuropsychological test performance in a cohort of oldest-old women. We found that, compared to oldest-old women without elevated depressive symptoms, those with elevated symptoms had more than 3 times the odds of dementia and 3.7 times the odds of MCI five years later, after accounting for potential confounders. We also found that elevated depressive symptoms were associated with more than a 70% decrease in the odds of being cognitively normal five years later. On neuropsychological tests, we found that oldest-old women with elevated depressive symptoms performed more poorly on tests of global cognitive function and working memory, five years later, than women without elevated symptoms. Taken together, our results indicate that depression remains an important risk factor for cognitive impairment and cognitive disorders in oldest-old women—a population in which this association has been understudied.
Our finding that elevated depressive symptoms predicted incident dementia in oldest-old women is generally consistent with results of previous studies in younger samples of older adults,(33) (34) including an early (1999) study in women from SOF.(12) Although not all studies have found this association,(35, 36) results from meta-analyses of case-control and prospective studies support the association between depression and dementia.(37, 38) Nonetheless, the exact nature of the relationship between the two conditions (e.g., whether depression causes dementia, is a prodrome of dementia, etc.) remains to be determined.(37, 38)
Depressive symptoms are among the most common neuropsychiatric symptoms in MCI, which is commonly thought of as a transitional status between normal cognition and dementia.(39) Investigations in two population-based studies, the Cardiovascular Health Study and the Mayo Clinic Study of Aging, found that depressive symptoms occur in 20% and 27% of individuals with MCI, respectively.(40, 41) We know relatively little, however, about depression as a predictor of incident MCI, and results in this area have been mixed. Some studies in population-based and clinical samples have found that depressive symptoms predict subsequent MCI,(13, 14) and that older adults with a history of depression often meet criteria for MCI even after successful treatment and remission of depression.(42) Others have found that the association between depression and incident MCI only occurs in specific subgroups,(43) and still others have found no association of depression with incident MCI.(44) We found, in a sample of oldest-old women, that elevated depressive symptoms were associated with a 3.7-fold increase in the odds of MCI five years later. Although this association decreased and became statistically non-significant after removal of four influential observations, we believe that evidence of an association between elevated depressive symptoms and MCI is fairly strong, given consistent findings with all outcomes (i.e., dementia, MCI, and cognitively normal vs. impaired) and the fact that this association remained elevated (though not statistically significant) even after removal of four high-influence points.
Many other studies have investigated the association between depressive symptoms and neuropsychological test performance in younger samples of older adults, and our results are consistent with some, but not all of their findings. As in the present research, prior studies have found associations between depressive symptoms and decline in global cognition,(7, 12) and depression has been linked to reduced performance on digit span backward in adults over age 60.(45) We did not observe significant associations, however, between elevated depressive symptoms and other cognitive tests, including measures of delayed recall, attention, verbal fluency, or executive function and psychomotor speed. Although others have observed cross-sectional differences in these cognitive domains between older adults with elevated depressive symptoms or major depressive disorder and those without, findings regarding longitudinal associations between depressive symptoms and cognition have been inconsistent, perhaps due to between-study differences in measurement and statistical analysis.(8-10, 46)
Our findings of independent associations between elevated depressive symptoms and subsequent diagnoses of MCI and dementia raise the question of whether prevention or treatment of depression could, in turn, prevent cognitive decline in the oldest-old. Indeed, this question has been asked before, based primarily on results from younger samples of elders.(7, 12, 47) Ethical considerations preclude randomizing older adults to treatment vs. withholding treatment of depression to evaluate the impact on cognitive trajectories. However, trials aimed at preventing depression with prevention of cognitive decline as a long-term goal, would permit researchers to approach the answer to this question.
Several mechanisms have been hypothesized to link depression to poor cognitive outcomes. For example, it has been proposed that inflammatory processes associated with depression(48) mediate the association between depression and cognitive decline.(49) In addition, individuals of various ages with prolonged or repeated major depressive episodes have been shown to have smaller hippocampal volumes than controls, perhaps due to chronic exposure to elevated levels of stress hormones, such as cortisol.(50) Further, researchers are examining the role of depression in the pathogenesis of Alzheimer’s disease (AD). Results from postmortem research suggest that AD pathology is common among older adults with major depressive disorder who develop dementia.(51) In addition, some older adults with elevated depressive symptoms have been shown to have lower plasma levels of amyloid-β peptide 42 (Aβ42), and a lower ratio of plasma Aβ42 to Aβ40 than those without elevated symptoms; this has been called “amyloid-associated depression.”(52, 53) This depression subtype is being investigated as a potential prodrome of and risk factor for AD.(53, 54) Although these mechanisms could explain a causal link between depression and adverse cognitive outcomes in older adults, it also is possible that depression and cognitive decline both arise from a shared disease process. For example, cardiovascular disease is a known risk factor for dementia, and findings from epidemiologic and neuropathology studies support the vascular depression hypothesis, which implicates cardiovascular disease in the etiology of depression in a subset of older adults.(55, 56)
The present study has many strengths, including a sample of women from the largest ongoing cohort of oldest-old women, prospective study design, adjudication of clinical cognitive status, and multivariable adjustment for potential confounders; however, it also has several limitations. First, our primary predictor was elevated depressive symptoms on the 15-item GDS—a screening test for depression in older adults (19)—rather than a diagnosis of major (or minor) depressive disorder by structured psychiatric interview. The GDS items do not neatly correspond to symptoms listed in the DSM-IV major depressive episode criteria, the gold standard for depression assessment. In addition, our sample consisted mostly of white women; our findings might not generalize to non-white populations of oldest-old women, or to men. Also, because we did not collect neuroimaging data as part of this study, we are unable to assess the impact of elevated depressive symptoms on brain structure or function. This limits the extent to which we can evaluate the neural mechanisms by which depression affects cognition in the oldest old. Also, it is possible that participants classified as being free of elevated depressive symptoms at baseline developed elevated depressive symptoms later on, although such misclassification likely would have biased our results toward the null, reducing the strength of the associations we observed. Similarly, we did not investigate the association between persistent depression (i.e., elevated symptoms at both baseline and follow-up) and cognitive diagnoses. Further, like other studies in prospective cohorts, our results might have been affected by selection bias; women who died, left the study, or were lost to follow-up between baseline and follow-up might have differed from those who remained in the cohort in ways that affected our results. Finally, we lacked the power to evaluate the effect of study site X covariate interactions on our outcomes. Future studies with larger, more heterogeneous samples are needed to determine whether our findings generalize to diverse populations of oldest-old men and women, and whether persistence of depressive symptoms over time modifies the association between baseline symptoms and cognitive outcomes.
Conclusion
In the present prospective study of oldest-old women, elevated depressive symptoms were associated with more than a 3-fold increase in the odds of dementia or MCI, and more than a 70% reduction in the odds of being cognitively normal, five years later. In addition, elevated symptoms predicted lower scores on neuropsychological tests in this population. Our findings suggest that depression remains an important risk factor for cognitive impairment and cognitive disorders in this population. Further research is needed to clarify the precise role of depression on the pathway to MCI and dementia in the oldest old, and to determine whether treating depression can prevent cognitive decline and promote independence, even at the upper limits of the human lifespan.
Acknowledgments
This project was supported by National Institutes of Health Grants AG026720, AG05394, AG05407, AR35582, AR35583, AR35584, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, 2 R01 AG027574-22A1, R01 MH086498, NIA K24 AG031155, and Alzheimer’s Association award IIRG-08-88872.
Dr. Spira is supported by a Mentored Research Scientist Development Award (1K01AG033195) from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Yaffe is on the data safety monitoring board (DSMB) of the NIMH-funded CitAD trial and on DSMBs for Pfizer and Medivation trials; other authors have no disclosures to report. Data from this study were presented as a poster at the 2011 Annual Meeting of the American Association for Geriatric Psychiatry, San Antonio, TX.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19:663–696. v. doi: 10.1016/s0749-0690(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 2.Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17:769–781. doi: 10.1097/JGP.0b013e3181ad4f5a. [DOI] [PubMed] [Google Scholar]
- 3.Mojtabai R, Olfson M. Major depression in community-dwelling middle-aged and older adults: prevalence and 2- and 4-year follow-up symptoms. Psychol Med. 2004;34:623–634. doi: 10.1017/S0033291703001764. [DOI] [PubMed] [Google Scholar]
- 4.Steffens DC, Fisher GG, Langa KM, et al. Prevalence of depression among older Americans: the Aging, Demographics and Memory Study. Int Psychogeriatr. 2009;21:879–888. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chodosh J, Kado DM, Seeman TE, et al. Depressive Symptoms as a Predictor of Cognitive Decline: MacArthur Studies of Successful Aging. Am J Geriatr Psychiatry. 2007;15:406–415. doi: 10.1097/01.JGP.0b013e31802c0c63. [DOI] [PubMed] [Google Scholar]
- 8.Ganguli M, Du Y, Dodge HH, et al. Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch Gen Psychiatry. 2006;63:153–160. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- 9.Ganguli M, Snitz B, Vander Bilt J, et al. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry. 2009;24:1277–1284. doi: 10.1002/gps.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg PB, Mielke MM, Xue QL, et al. Depressive symptoms predict incident cognitive impairment in cognitive healthy older women. Am J Geriatr Psychiatry. 2010;18:204–211. doi: 10.1097/JGP.0b013e3181c53487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachs-Ericsson N, Joiner T, Plant EA, et al. The influence of depression on cognitive decline in community-dwelling elderly persons. Am J Geriatr Psychiatry. 2005;13:402–408. doi: 10.1176/appi.ajgp.13.5.402. [DOI] [PubMed] [Google Scholar]
- 12.Yaffe K, Blackwell T, Gore R, et al. Depressive Symptoms and Cognitive Decline in Nondemented Elderly Women: A Prospective Study. Arch Gen Psychiatry. 1999;56:425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 13.Barnes DE, Alexopoulos GS, Lopez OL, et al. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63:273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 14.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 15.He W, Sengupta M, Velkoff VA, et al. 65+ in the United States: 2005. U.S. Census Bureau; Washington, DC: 2005. [Google Scholar]
- 16.Kawas CH, Corrada MM. Alzheimer’s and dementia in the oldest-old: a century of challenges. Curr Alzheimer Res. 2006;3:411–419. doi: 10.2174/156720506779025233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawas CH. The oldest old and the 90+ Study. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2008;4:S56–59. doi: 10.1016/j.jalz.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West R, Beeri MS, Schmeidler J, et al. Better memory functioning associated with higher total and low-density lipoprotein cholesterol levels in very elderly subjects without the apolipoprotein e4 allele. Am J Geriatr Psychiatry. 2008;16:781–785. doi: 10.1097/JGP.0b013e3181812790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh JI, Yesavage JA. Geriatric Depression Scale: Recent evidence and development of a shorter version. Clinical Gerontologist. 1986;5:165–173. [Google Scholar]
- 20.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 21.Pomeroy IM, Clark CR, Philp I. The effectiveness of very short scales for depression screening in elderly medical patients. International Journal of Geriatric Psychiatry. 2001;16:321–326. doi: 10.1002/gps.344. [DOI] [PubMed] [Google Scholar]
- 22.Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15-item geriatric depression scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. Journal of the American Geriatrics Society. 2005;53:1570–1576. doi: 10.1111/j.1532-5415.2005.53461.x. [DOI] [PubMed] [Google Scholar]
- 23.Wancata J, Alexandrowicz R, Marquart B, et al. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta psychiatrica Scandinavica. 2006;114:398–410. doi: 10.1111/j.1600-0447.2006.00888.x. [DOI] [PubMed] [Google Scholar]
- 24.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Lezak M. Neuropsychological assessment. Oxford University Press; New York: 1995. [Google Scholar]
- 27.Delis D, Kramer J, Kaplan E, et al. California Verbal Learning Test--Second Edition. Pearson; San Antonio: 2000. [Google Scholar]
- 28.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 29.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 32.Vittinghoff E, Glidden DV, Shiboski SC, et al. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. Springer; NY: 2005. [Google Scholar]
- 33.Gatz JL, Tyas SL, St John P, et al. Do depressive symptoms predict Alzheimer’s disease and dementia? J Gerontol A Biol Sci Med Sci. 2005;60:744–747. doi: 10.1093/gerona/60.6.744. [DOI] [PubMed] [Google Scholar]
- 34.Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 35.Becker JT, Chang YF, Lopez OL, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry. 2009;17:653–663. doi: 10.1097/jgp.0b013e3181aad1fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geerlings MI, den Heijer T, Koudstaal PJ, et al. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70:1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 37.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 38.Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 40.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 42.Bhalla RK, Butters MA, Becker JT, et al. Patterns of mild cognitive impairment after treatment of depression in the elderly. Am J Geriatr Psychiatry. 2009;17:308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravaglia G, Forti P, Lucicesare A, et al. Prevalent depressive symptoms as a risk factor for conversion to mild cognitive impairment in an elderly Italian cohort. Am J Geriatr Psychiatry. 2008;16:834–843. doi: 10.1097/JGP.0b013e318181f9b1. [DOI] [PubMed] [Google Scholar]
- 44.Panza F, D’Introno A, Colacicco AM, et al. Depressive symptoms, vascular risk factors and mild cognitive impairment. The Italian longitudinal study on aging. Dement Geriatr Cogn Disord. 2008;25:336–346. doi: 10.1159/000119522. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien JT, Lloyd A, McKeith I, et al. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 46.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 47.Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000;46:219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- 48.Penninx BW, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 49.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 50.McKinnon MC, Yucel K, Nazarov A, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 51.Sweet RA, Hamilton RL, Butters MA, et al. Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology. 2004;29:2242–2250. doi: 10.1038/sj.npp.1300554. [DOI] [PubMed] [Google Scholar]
- 52.Sun X, Mwamburi DM, Bungay K, et al. Depression, antidepressants, and plasma amyloid beta (Beta) peptides in those elderly who do not have cardiovascular disease. Biol Psychiatry. 2007;62:1413–1417. doi: 10.1016/j.biopsych.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Sun X, Steffens DC, Au R, et al. Amyloid-associated depression: a prodromal depression of Alzheimer disease? Arch Gen Psychiatry. 2008;65:542–550. doi: 10.1001/archpsyc.65.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun X, Chiu CC, Liebson E, et al. Depression and plasma amyloid beta peptides in the elderly with and without the apolipoprotein E4 allele. Alzheimer Dis Assoc Disord. 2009;23:238–244. doi: 10.1097/WAD.0b013e31819cb3ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 56.Thomas AJ, O’Brien JT, Davis S, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]


