Abstract
Interleukin-36 (IL-36) is the common name for the three IL-1 family members IL-36α, IL-36β and IL-36γ, formerly known as IL-1F6, IL-1F8 and IL-1F9, respectively. IL-36 appears to have pro-inflammatory activities; however, the physiological function of these cytokines remains unknown. Expression of IL-36 by keratinocytes implies its possible involvement in innate immune responses in the skin. We observed that, of the three IL-36 isoforms, human keratinocytes express high levels of IL-36γ. IL-36γ mRNA expression was dramatically induced by the Toll-like receptor ligands poly(I:C) and flagellin. Surprisingly, the IL-36γ protein was released by cells treated with poly(I:C) but remained intracellular in cells treated with flagellin only. Poly(I:C), but not flagellin, induced cell death and caspase-3/7 activation. Inhibition of caspase-3/7 and caspase-1 blocked extracellular release of IL-36γ from poly(I:C) treated cells. Furthermore, caspase-1 inhibition prevented poly(I:C)-induced caspase-3/7 activation. Interestingly, transcription of the gene IL36G was dependent upon caspase-1, but not caspase-3/7, activation. This demonstrates that the pathways leading to IL36G transcription and caspase-3/7 activation branch after caspase-1. This divergence of the pathways allows the cells to enter a state of de novo protein synthesis before committing to pyroptosis. Overall our observations suggest that IL-36γ may be an alarmin that signals the cause, e.g. viral infection, of cell death.
INTRODUCTION
The skin is continuously exposed to potential pathogens and plays an active role in initiating immune responses to eliminate these microorganisms. Keratinocytes express functionally active pathogen associated molecular pattern recognition receptors such as the Toll-like receptors (TLRs) and retinoic acid-inducible gene-I-like receptors (RLRs) ((Kalali et al., 2008) and refs. therein). Different TLRs bind a diverse range of structural motifs associated with bacteria, fungi and viruses. In contrast, RLRs appear to be specific for viral double stranded RNA (Takeuchi and Akira, 2010). Engagement of TLRs and RLRs activates intracellular signaling pathways which regulate expression of pro-inflammatory cytokines (Takeuchi and Akira, 2010). Release of these cytokines from keratinocytes subsequently orchestrates the recruitment and activation of immune cells.
Interleukin-1 (IL-1) and IL-18 are among the many pro-inflammatory cytokines which are expressed following TLR and/or RLR engagement. IL-18 is considered to be an IL-1 family member due to sequence and structural similarity to IL-1. There are two forms of IL-1; IL-1α is the foremost form produced by keratinocytes, whereas IL-1β is primarily expressed by myeloid cells (Dinarello, 2009). With the recent discovery of the inflammasomes, which are intracellular protein complexes involved in initiation of inflammation (van de Veerdonk et al., 2011), the process whereby myeloid cells secrete IL-1β and IL-18 has gained renewed interest. Both cytokines lack signal peptides and are initially produced as pro-proteins. Assembly of the inflammasomes activates the protease caspase-1 which in turn cleaves the pro-IL-1β and pro-IL-18 (van de Veerdonk et al., 2011). This process permits extracellular release of the mature cytokines through a process called pyroptosis (Franchi et al., 2009). Pyroptosis is a highly inflammatory form of programmed cell death. The process is dependent upon caspase-1 activation and has so far only been described in myeloid cells (Franchi et al., 2009). Although it is known that keratinocytes have inflammasomes (Feldmeyer et al., 2010), how IL-1 cytokines are released from keratinocytes is poorly understood.
IL-36α (formerly known as IL-1F6), IL-36β (IL-1F8) and IL-36γ (IL-1F9) are three recently discovered novel members of the IL-1 family (Dinarello et al., 2010). IL-36 binds to IL-1 receptor like 2 (IL-1RL2) to initiate intracellular activation of MAP kinase and NF-κB signal transduction (Debets et al., 2001; Towne et al., 2004). IL-1RL2 is primarily expressed in epithelial cells of tissues such as the skin, lungs and digestive tract. Recent studies have demonstrated that transgenic overexpression of IL-36α in basal keratinocytes leads to a psoriasis-like phenotype (Blumberg et al., 2007), and intratracheal administration of recombinant IL-36γ in mice results in increased neutrophil influx (Ramadas et al., 2011). These studies suggest that IL-36 has pro-inflammatory activities. Epidermal expression of the IL-36 and IL-1RL2 mRNAs is up-regulated in many inflammatory skin conditions such as psoriasis and eczema (reviewed in (Jensen, 2010)). However, the specific physiological functions of the three IL-36 isoforms and how they may contribute to disease remain unclear.
The high degree of IL-36 and IL-1RL2 expression in tissues in direct contact with the environment may suggest that these proteins are involved in first line defenses against microorganisms colonizing these areas. To initiate studies of this hypothesis we explored the expression of IL-36 cytokines in keratinocytes. Our investigations presented here establish that keratinocytes respond differently depending on how they are challenged. Furthermore, we demonstrate for the first time that the double stranded RNA analogue poly(I:C) induces pyroptosis in keratinocytes thereby facilitating the extracellular release of IL-36γ.
RESULTS
TLR ligands induce dramatic changes in IL-36γ expression
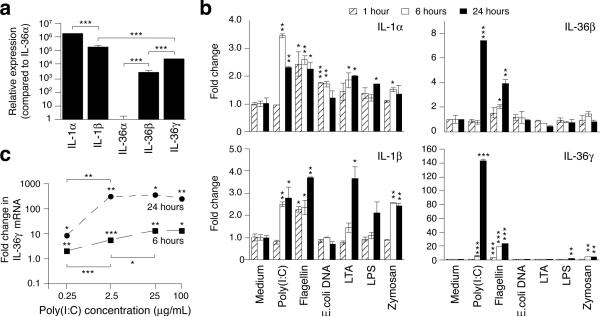
Since IL-36 is expressed in skin (Jensen, 2010) we speculated that these cytokines play a role in the homeostatic processes regulating the continuous interaction between the skin and colonizing or invading microorganisms. We first compared expression of the novel IL-36 cytokines to the classical IL-1 family members IL-1α and IL-1β in unstimulated cells. Levels of the IL-36α mRNA were the lowest observed. Relative to the IL-36α mRNA, levels of IL-36β, IL-36γ, IL-1β, and IL-1α mRNAs were 3,100-, 25,000-, 190,000-, and 1,800,000-times higher, respectively (Fig. 1a). No discernable differences were noticed between adult and neonatal keratinocytes (data not shown).
Figure 1. Expression of IL-1 and IL-36 in human primary keratinocytes.
Keratinocytes were left untreated (a), treated (b) with medium only, poly(I:C) (25 μg/mL), flagellin (1 μg/mL), E. coli. DNA (10 μg/mL), LTA (5 μg/mL), LPS (5 μg/mL) or zymosan (25 μg/mL), or treated (c) with medium only or poly(I:C) at the indicated concentrations. Total RNA was isolated after 1, 6 or 24 hours and reverse transcription and real-time PCR performed. IL-1 family mRNA levels were normalized against GAPDH and fold differences (mean ± SD, n=2 per independent time-point and treatment) calculated relative to the IL-36α mRNA (a) or medium only (b and c) treated samples. Only IL-36γ mRNA expression was examined in (c). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (comparing treatment with medium only at the same time-point (b and c) or as indicated (a and c)). Data shown are from one representative experiment of at least three independent experiments.
Keratinocytes were challenged with a panel of TLR ligands to examine their capacity to stimulate IL-1 and IL-36 mRNA expression (Fig. 1b). While the IL-1α and IL-1β mRNA levels were modestly increased (1.5-4-fold, P < 0.05) by most TLR ligands, the IL-36β and IL-36γ mRNAs were most significantly (P < 0.05) regulated by flagellin (4- and 24-fold, respectively) and poly(I:C) (8- and 143-fold, respectively). The dramatic induction of the IL-36γ mRNA by poly(I:C) was concentration dependent (Fig. 1c) and resulted in mRNA levels similar to that of the IL-1α mRNA (compare Fig. 1a and Fig. 1b). The very clear differential expression patterns of IL-1 versus IL-36 suggest that these proteins have distinct functions.
Similar to previous reports (Debets et al., 2001; Kumar et al., 2000) we also observed increased IL-36β and IL-36γ mRNA expression in response to IL-1 and TNFα (data not shown). As observed with the TLR ligands (Fig. 1b), IL-36γ was the most noticeably regulated (approx 25-fold increases versus maximum 12-fold increases in IL-36β mRNA levels (data not shown)). Other cytokines such as IFN-γ and IL-6 did not affect the degree of IL-36 mRNA expression (data not shown). Several additional cytokines have been shown to regulate IL-36 expression including IL-17A, IL-22 and IL-36 itself (Carrier et al., 2011; Muhr et al., 2011). Although we observed no indication of a biphasic expression profile in the dose-response curves or time-courses (Fig. 1b and c), it is plausible that the dramatic induction of the IL-36γ mRNA is, in part, driven by an autocrine mechanism involving IL-36 (see below regarding extracellular localization of IL-36).
IL-36γ is only secreted in response to poly(I:C)
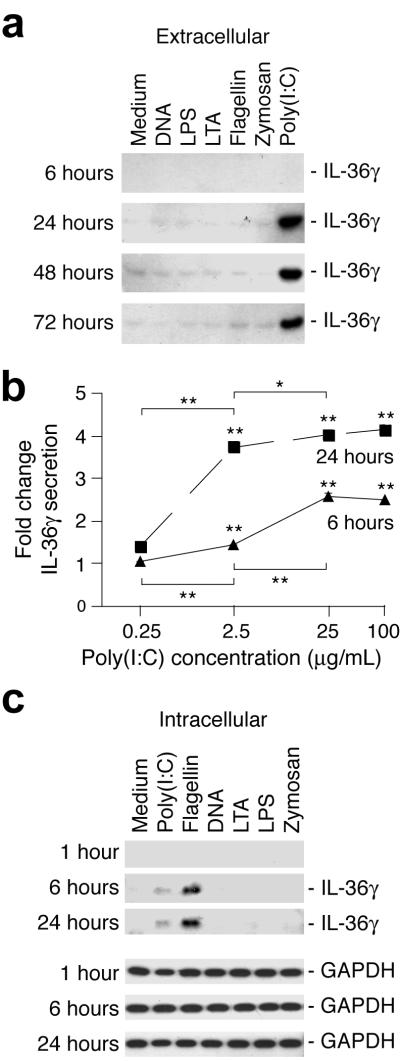
Similar to IL-1β, the IL-36 cytokines lack signal peptides required for conventional protein secretion ((Martin et al., 2009) and refs. therein). Interestingly, unlike IL-1β, which is synthesized as a pro-protein that is cleaved prior to extracellular release, the IL-36 proteins appear to lack pro-domains. Hence they are predicted to be transcribed directly into mature cytokine forms (Martin et al., 2009). We therefore examined whether IL-36β and IL-36γ were present in the medium or cellular fractions of cells treated with TLR ligands. Since both poly(I:C) and flagellin significantly increased IL-36β and IL-36γ mRNA levels (Fig. 1b), we predicted that these TLR ligands would trigger a corresponding rise in IL-36β and IL-36γ production. Furthermore, cellular localization was anticipated to be similar. Surprisingly, we found that IL-36γ could only be detected in medium from cells stimulated with poly(I:C) (Fig. 2a). The level of IL-36γ secretion was dependent upon the poly(I:C) concentration (Fig. 2b). We were unable to detect IL-36β in the same samples (data not shown). In contrast, flagellin activated cells contained increased levels of intracellular IL-36γ (Fig. 2c). A low level of intracellular IL-36γ could also be detected in poly(I:C) treated cell fractions. No discernable size differences between the intracellular and extracellular IL-36γ were detected.
Figure 2. Extracellular and intracellular localization of IL-36γ from TLR-ligand activated keratinocytes.
Cells were treated as described in Fig. 1b and c. Medium and cells were collected at the indicated time-points and extracellular (medium, a and b) and intracellular (cells, c) IL-36γ levels examined by Western blotting (a and c) or ELISA (b). GAPDH was used as a loading control for intracellular protein fractions. *, P < 0.05; **, P < 0.01 (comparing treatment with medium only at the same time-point or as indicated).
It is well known that ATP stimulates the release of intracellular IL-1β by macrophages (Franchi et al., 2009). Overexpression of a murine green fluorescence protein-IL-36α fusion protein in mouse macrophages results in intracellular protein buildup. This cytokine fusion protein is secreted when cells are treated with ATP (Martin et al., 2009). Similar to this we found that a 20 min pulse with 5 mM ATP stimulated the extracellular release of IL-36γ (data not shown), which accumulated in cells treated with flagellin. Similar observations have recently been reported by (Johnston et al., 2011).
Poly(I:C) causes cell death and caspase-3/7 activation
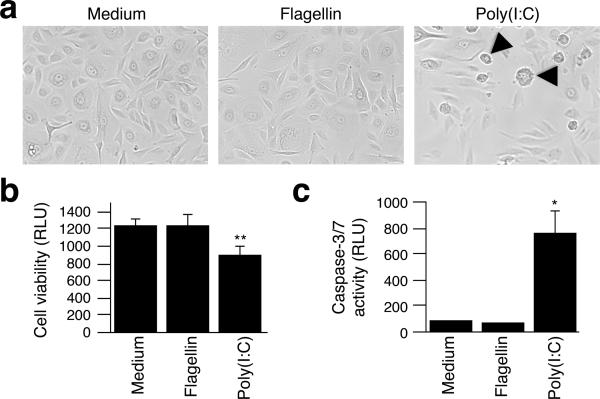
During our analyses of IL-36 expression in response to TLR ligands, we observed lower cell density 24 hours after poly(I:C) treatments compared to other agent applications (Fig. 3a). Staining with Trypan blue indicated that 40–60% of the cells remained alive 24 hours after poly(I:C) addition (data not shown). The poly(I:C) treated cultures further revealed cell morphologies suggestive of cells at different stages of cell death (Fig. 3a, arrowheads). Activation of caspases 3 and 7 commits cells to undergo programmed cell death such as apoptosis (Lakhani et al., 2006). We therefore speculated that pro-apoptotic caspase activity would be present at earlier time-points. Preliminary microscopic observations of cultures revealed that around 6 hours post-treatment a few single dispersed cells, which appeared to be dying, could be observed only in cultures treated with poly(I:C) (data not shown). Measurements of intracellular ATP levels revealed significant reduction in the number of viable cells when cultures were treated with poly(I:C) compared to those treated with medium only or flagellin (Fig. 3b). Further measurements of active caspase-3/7 demonstrated a significant 8-fold (P < 0.05) increase in caspase activity compared to cells treated with medium only (Fig. 3c). In comparison, caspase-3/7 was not significantly activated in cells treated with flagellin (Fig. 3c).
Figure 3. Poly(I:C) induces cell death and pro-apoptotic caspase-3/7 activation.
Keratinocytes were treated with medium, poly(I:C) or flagellin for 6 (b–c) or 24 (a) hours. Phase contrast images were acquired at 20× magnification (a). Arrowheads indicate cells at different stages of cell death. The number of viable cells (b) and levels of active caspase-3/7 (c) were determined using luminescence assays as described in Materials and Methods. Relative luminescence units (RLU) are represented as means ± SD. *, P < 0.05; **, P < 0.01 (compared to medium only).
Poly(I:C) induced IL-36γ extracellular release is caspase-3 dependent
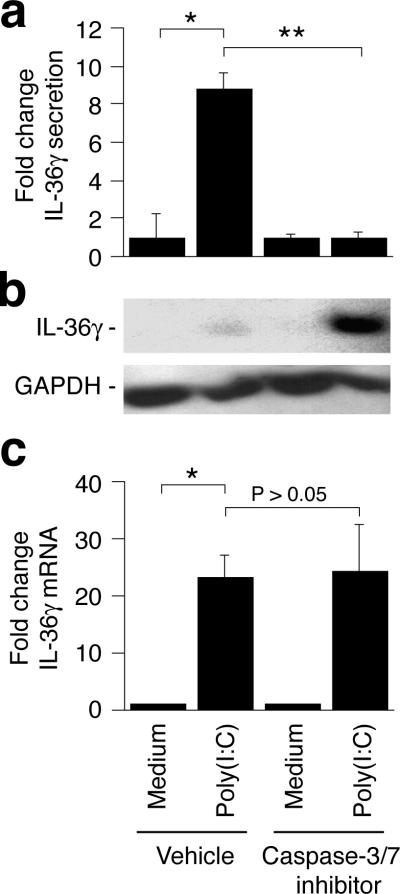
Since poly(I:C), but not flagellin, caused cell death, caspase-3/7 activation, and extracellular release of IL-36γ, we speculated that the poly(I:C)-induced release of IL-36γ was dependent upon the caspase-3/7 mediated cell death. Inclusion of the target specific caspase-3/7 inhibitor III significantly (P < 0.01) blocked the extracellular release of IL-36γ (Fig. 4a). Furthermore, in the presence of the caspase-3/7 inhibitor, the de novo synthesized IL-36γ instead accumulated inside the cells (Fig. 4b). The caspase-3/7 inhibitor had no effect on the poly(I:C)-induced IL-36γ mRNA expression (Fig. 4c).
Figure 4. Extracellular release of IL-36γ is caspase-3/7 dependent.
Cells were treated with medium only or poly(I:C) in the presence or absence of the caspase-3/7 inhibitor III. Extracellular (a) and intracellular (b) levels of IL-36γ protein were determined using ELISA and Western blotting, respectively. IL-36γ mRNA levels (c) were determined after 3 hours as described in Fig. 1. ELISA and real-time PCR results are represented as fold changes (mean ± SD) compared to medium only samples treated with the equivalent vehicle or caspase-3/7 inhibitor. *, P < 0.05; **, P < 0.01.
Caspase-1 is an upstream activator of caspase-3/7
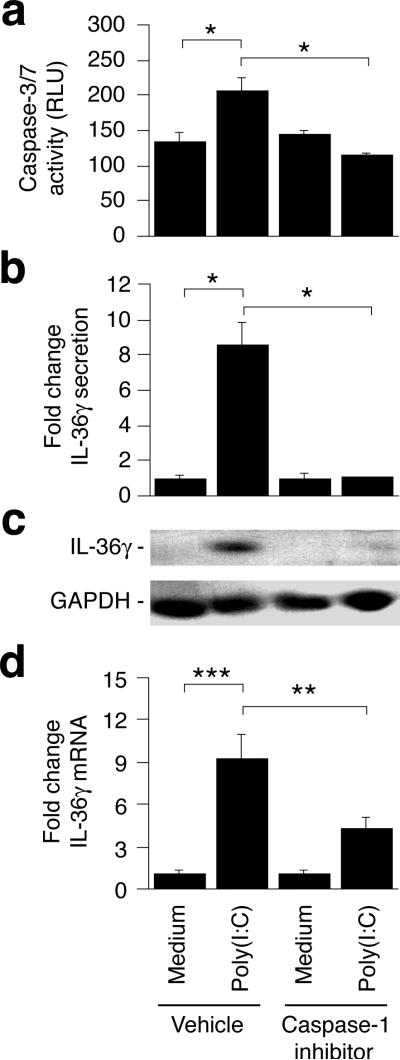
In myeloid cells the pyroptosis associated release of IL-1β and IL-18 requires activation of caspase-1 (Franchi et al., 2009). Furthermore, caspase-1 can activate caspase-7 (Lamkanfi et al., 2008). To examine whether caspase-1 is an upstream activator of caspase-3/7 in keratinocytes, we treated cells with poly(I:C) in the presence and absence of the caspase-1 inhibitor VI and then examined caspase-3/7 activity. Inclusion of the caspase-1 inhibitor prevented caspase-3/7 activation (Fig. 5a, P < 0.05), demonstrating that poly(I:C)-mediated caspase-3/7 activation requires prior caspase-1 activation.
Figure 5. Caspase-1 activation is required for caspase-3/7 activation and IL-36γ expression.
Cells were treated with medium only or poly(I:C) in the presence or absence of the caspase-1 inhibitor VI. Caspase-3/7 activity (a) was determined using a luminescence assay as described in Materials and Methods and relative luminescence units (RLU) represented as means ± SD. Extracellular IL-36γ (b), intracellular protein (c) and IL-36γ mRNA (d) levels were determined as described in Fig. 4. ELISA and real-time PCR results are represented as fold changes (mean ± SD) compared to medium only samples treated with the equivalent vehicle or caspase-1 inhibitor. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IL-36γ expression is caspase-1 dependent
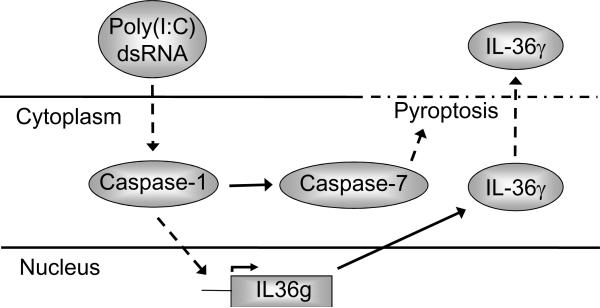
In analogy with the above studies on the role of caspase-3/7 in the release of IL-36γ, we next examined whether IL-36γ secretion was caspase-1 dependent. Inclusion of the target specific caspase-1 inhibitor blocked (p < 0.05) extracellular accumulation of IL-36γ (Fig. 5b) in a manner similar to that observed when caspase-3/7 activation was prevented (Fig. 4a). However, while caspase-3/7 inhibition resulted in intracellular IL-36γ accumulation (Fig. 4b), IL-36γ did not build up in the cells when caspase-1 was specifically inhibited (Fig. 5c). We therefore examined IL-36γ mRNA expression in the presence and absence of the caspase-1 inhibitor. Interestingly, we found that the poly(I:C)-mediated increase in IL-36γ mRNA expression was prevented (P < 0.01) by blocking caspase-1 activity (Fig. 5d). This demonstrates that the poly(I:C)-induced IL-36γ gene expression is caspase-1 dependent (Fig. 6). Since expression of the IL-36γ encoding gene (IL36G) is not caspase-3/7 dependent (Fig. 4c) our data further establish that the pathways leading to IL36G transcription and activation of cell death pathways branches after caspase-1 (Fig. 6).
Figure 6. Schematic representation of pathways whereby poly(I:C) and double stranded RNA may activate IL-36γ expression and secretion.
Dashed and dotted line indicates the disintegration of the cell during pyroptosis. Dashed arrow lines indicate pathways that may involve several intermediates.
DISCUSSION
Preliminary studies indicate that IL-36 has pro-inflammatory properties (Blumberg et al., 2007; Debets et al., 2001; Ramadas et al., 2011; Towne et al., 2004); however, the actual physiological function(s) remain unknown. To explore the possibility that IL-36 plays a role in immune responses in the skin, we examined IL-36 expression in keratinocytes challenged with a panel of TLR ligands. Poly(I:C), a double stranded RNA analogue, proved to be a very potent activator of IL-36γ expression and extracellular release. Double stranded RNA is a molecular pattern associated with viral infections, and IL-36γ mRNA expression is known to be up-regulated in proliferating keratinocytes during in vivo skin infections with HSV-1 (Kumar et al., 2000). The direct implication of our observations is that IL-36γ may be involved in regulating innate or adaptive immune responses aimed at eliminating viral infections in keratinocytes.
Alarmins are endogenous danger signals released by pathogen activated and/or injured cells (Oppenheim and Yang, 2005; Yang et al., 2004). The functions of alarmins are to promote both chemotaxis and activation of antigen-presenting cells (Oppenheim and Yang, 2005; Yang et al., 2004). IL-1α and IL-33, an additional member of the IL-1 family, have previously been characterized as alarmins largely due to their extracellular release by damaged cells (Bianchi, 2007; Haraldsen et al., 2009). The observed release of IL-36γ only by dying cells (Fig. 2–4) suggest that extracellular IL-36γ may also act as a danger signal to alert surrounding cells about the cell death. Combined with the reported involvement of IL-36γ in promoting inflammation (Blumberg et al., 2007; Debets et al., 2001; Ramadas et al., 2011; Towne et al., 2004) and activation of dendritic and T cells (Vigne et al., 2011) our observations support the notion of IL-36γ as an alarmin involved in activating the adaptive immune system in response to pathogen challenges such as viruses infecting the skin.
Interestingly, both IL36G transcription (Fig. 5d) and caspase-3/7 activation (Fig. 5a) are dependent upon caspase-1 activation; however, only the secretion of IL-36γ is caspase-3/7 dependent (Fig. 4). This demonstrates that the two pathways branch after caspase-1 (Fig. 6). The branching of the pathways appears to allow the cells to enter a stage of de novo protein synthesis before committing to self-termination. This protein synthesis stage may last more than 6 hours as we observed increased mRNA expression and protein release between the 6 and 24 hours time-points (Fig. 1 and 2). The subsequent extracellular release of the alarmin IL-36γ from dying cells therefore may not only alert surrounding cells about the associated tissue damage, but also about the cause (double stranded RNA/viral infection) of the cell death. This may further ensure that a specific immune response towards viruses is initiated.
Cell death can occur through necrosis or apoptosis. While the latter does not cause inflammation, the former is highly inflammatory because the ruptured cell releases cellular components. Research in recent years have revealed that there are many other forms of cell death mechanisms, one of which is pyroptosis (Fink and Cookson, 2005). Pyroptosis is defined as a form of programmed cell death that is caspase-1 dependent and pro-inflammatory. The latter is mediated through IL-1β and IL-18. Pyroptosis has so far only been described in myeloid cells such as macrophages, monocytes, and dendritic cells (van de Veerdonk et al., 2011). We observed that poly(I:C) induced keratinocyte cell death and caspase-3/7 activation (Fig. 3). Furthermore, the caspase-3/7 activation was caspase-1 dependent (Fig. 5a). By definition the observed keratinocyte cell death should therefore be described as pyroptosis. This nomenclature is further supported by the anticipated pro-inflammatory actions of IL-36 (Blumberg et al., 2007; Debets et al., 2001; Ramadas et al., 2011; Towne et al., 2004) and our data demonstrating extracellular release of IL-36γ.
Dermatological studies often examine cell death, e.g. when studying the effects of UV light on the skin (Weatherhead et al., 2011). UV light has been demonstrated to activate the inflammasomes and caspase-1 in keratinocytes (Feldmeyer et al., 2007). Whether the UV induced apoptosis reported by Weatherhead et al. is caspase-1 dependent, and hence could be characterized as pyroptosis, is yet to be determined. However, distinguishing the different types of cell death is important, as these have not only distinct mechanisms of activation and execution, but also have important differential physiological consequences, e.g. inflammation versus no inflammation. Adaptation of the pyroptosis terminology in the dermatological field is in our opinion warranted and timely.
Caspase-1 can directly cleave and activate caspase-7, but not caspase-3 (Lamkanfi et al., 2008). Caspase-3 and caspase-7 have redundant functions in the execution of apoptosis, i.e. both must be deleted to prevent apoptosis (Lakhani et al., 2006). During myeloid pyroptosis caspase-7 but not caspase-3 activation has been observed; however, cell lysis appears to be independent, at least in some situations, of caspase-7 (Lamkanfi, 2011). Due to the redundancies between caspase-3 and caspase-7, the substrate and the inhibitor used in our studies cannot distinguish caspase-3 and -7 activity. However, given the previously reported activation of caspase-7 only in myeloid pyroptosis, our data (Figs. 3–5) likely reflect caspase-7 activity (Fig. 6). Interestingly, although myeloid pyroptotic cell lysis has been reported to be independent of caspase-7 activation (Lamkanfi, 2011), our data demonstrate that in keratinocytes the extracellular release of IL-36γ is dependent upon caspase-3/7 activity (Fig. 5). This could suggest that the process of pyroptosis is regulated slightly differently in keratinocytes than in myeloid cells. The pharmaceutical implication of this may be that pyroptosis can be specifically inhibited in keratinocytes without affecting the functions of myeloid cells.
The observed differential accumulation of IL-36γ inside and outside of the cells in response to flagellin and poly(I:C) (Fig. 2) may indicate that IL-36γ has both intra- and extracellular functions, depending on the context in which it is expressed. Other IL-1 family members such as IL-1α, IL-33, and IL-37 have been reported to localize in the nucleus. While the nuclear function of IL-1α is unclear, the presence of IL-33 or IL-37 in the nucleus appears to suppress pro-inflammatory gene expression (reviewed in (Luheshi et al., 2009)). IL-37 mediates, at least in part, this effect by interacting with the signaling factor Smad3 (Nold et al., 2010). Further studies of the intracellular localization of IL-36γ, using for example confocal microscopy, could provide valuable support for, or against, the hypothesis that IL-36γ may have an intracellular function.
Our studies have demonstrated that expression of IL-36γ is highly complex. Strong induction of IL-36γ by TLR ligands suggests that IL-36γ plays a role in immune responses against microorganisms. Furthermore, TLR specific differential cellular localization indicates that IL-36γ may have distinct functions dependent upon the context in which it is expressed. Extracellular release of IL-36γ during pyroptosis may imply that IL-36γ acts as an alarmin. The specific function of this danger signal activity may be to inform surround cells about the nature of the pathogen causing cell damage and thereby ensuring engagement of an appropriately specific immune response. Further studies of the role(s) this exciting new cytokine may play in skin infections and chronic inflammatory conditions will advance our knowledge of innate and adaptive immune responses in the skin.
MATERIALS AND METHODS
Cell cultures and cell viability
Pooled human neonatal primary keratinocytes (Invitrogen, Carlsbad, CA) were maintained in Defined Keratinocyte Serum Free Medium supplemented with 20 μg/mL gentamicin (Invitrogen). Cells were treated with medium only or a panel of TLR ligands as previously described (Olaru and Jensen, 2010a). Poly(I:C) (Sigma-Aldrich, St. Louis, MO) and recombinant flagellin (Invivogen, San Diego, CA) were used at 25 μg/mL and 1 μg/mL, respectively. All treatments were performed in duplicate.
Cell viability was evaluated by measuring intracellular levels of ATP. Medium was removed from treated cells. Cells were rinsed with PBS and then lysed in CellTiter-Glo (Promega, Madison, WI) diluted 1/4 in PBS. The luminescent signal was allowed to stabilize for 10 min before measurement.
RNA isolation and real-time RT-PCR
RNA isolation and real-time RT-PCR was performed as previously described (Sanmiguel et al., 2009). IL-1α, IL-1β and GAPDH cDNA specific primers are described elsewhere (Olaru and Jensen, 2010b). The following additional gene specific primers were used: IL1F6-F2, 5'-GACCGTATGTCTCCAGTCAC-3'; IL1F6-R, 5'-CTTTAGCACACATCAGGCAG-3'; IL1F8-F2C, 5'-CAGCATTAAGCCTGTCACTC-3'; IL1F8-R2C, 5'-GCACAGAAGAGACAGAGATC-3'; IL1F9-F2, 5'-GGGCCGTCTATCAATCAATG-3'; IL1F9-R2, 5'-TGATAACAGCAACAGTGACTG-3'.
Western blotting
Medium was collected from treated cultures. Cells were rinsed with PBS and lysed in M-PER (Thermo Fisher Scientific, Rockford, IL) supplemented with 10 mM EDTA and Mammalian Cells Protease Inhibitor Cocktail (Sigma-Aldrich). Proteins were separated by SDS-PAGE and transferred to PVDF membranes. Levels of IL-36β, IL-36γ and GAPDH were examined using goat anti-IL1F8, goat anti-IL1F9 (R&D Systems, Minneapolis, MN) and rabbit anti-GAPDH (FL-335) (Santa Cruz Biotechnology, Santa Cruz, CA) as primary antibodies, respectively, appropriate HRP-secondary antibodies (Santa Cruz Biotechnology), and enhanced chemiluminescence reagents (GE Healthcare, Piscataway, NJ).
ELISA
Levels of extracellular IL-36γ protein were determined using a direct ELISA. Medium from treated cells was collected and centrifuged at 200 × g for 5 min. Supernatants were diluted in PBS and coated overnight onto high-binding ELISA plates. Remaining protein-binding sites on the ELISA plates were blocked with 1% (wt./vol.) bovine serum albumin in PBS. IL-36γ was detected using sequential incubations with 1 μg/mL rat anti-IL-36γ (R&D Systems), 250 ng/mL goat anti-rat IgG-HRP (Santa Cruz) and ABTS chromogen (Invitrogen). The direct ELISA was confirmed to be specific for IL-36γ using recombinant IL-1α, IL-1β, IL-36α, and IL-36β (PeproTech, Rocky Hill, NJ and R&D Systems). None of these IL-1 family members were detected.
Caspase activation and inhibition
Activity of caspase-3/7 was measured using the Caspase-Glo 3/7 Assay (Promega) as described above for the CellTiter-Glo assay. Caspase-1 and caspase-3/7 were inhibited with Caspase-1 Inhibitor VI and Caspase-3 Inhibitor III (EMD Biosciences, La Jolla, CA). Inhibitors were solubilised in DMSO (vehicle control) and added to cultures 30 min prior to stimulation with medium only or poly(I:C). Final concentrations were 20–50 μM (Caspase-1 Inhibitor) and 100–250 μM (Caspase-3 Inhibitor). Time-points for examining caspase activation and inhibition were designed to evaluate activity in the early induction phases and further optimisation performed empirically.
Statistical analyses
Data are shown as arithmetic mean values and standard deviations from one representative experiment of at least three independent experiments (n=2 per independent time-point and treatment within each individual experiment). Data were analyzed using the Student's t test when appropriate.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grant AR053672 to Dr. Jensen.
Abbreviations
- IL
interleukin
- IL-1RL
IL-1 receptor like
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- PGN
peptidoglycan
- poly(I:C)
polyinosinic-polycytidylic acid
- RLR
retinoic acid-inducible gene-I-like receptor
- TLR
Toll-like receptor
Footnotes
CONFLICT OF INTEREST The authors state no conflicts of interest.
REFERENCES
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Dinh H, Trueblood ES, Pretorius J, Kugler D, Weng N, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier Y, Ma H-L, Ramon HE, Napierata L, Small C, O'Toole M, et al. Inter-Regulation of Th17 Cytokines and the IL-36 Cytokines In Vitro and In Vivo: Implications in Psoriasis Pathogenesis. J Invest Dermatol epublished ahead of print. 2011 doi: 10.1038/jid.2011.234. [DOI] [PubMed] [Google Scholar]
- Debets R, Timans JC, Homey B, Zurawski S, Sana TR, Lo S, et al. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol. 2001;167:1440–1446. doi: 10.4049/jimmunol.167.3.1440. [DOI] [PubMed] [Google Scholar]
- Dinarello C, Arend W, Sims J, Smith D, Blumberg H, O'Neill L, et al. IL-1 family nomenclature. Nat Immunol. 2010;11:973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer H-D. The inflammasome mediates UVB-induced activation and secretion of interleukin-1[beta] by keratinocytes. Curr Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Feldmeyer L, Werner S, French LE, Beer H-D. Interleukin-1, inflammasomes and the skin. Eur J Cell Biol. 2010;89:638–644. doi: 10.1016/j.ejcb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Küchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11:1211–1220. [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Xing X, Guzman AM, Riblett M, Loyd CM, Ward NL, et al. IL-1F5, -F6, -F8, and -F9: A novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011;186:2613–2622. doi: 10.4049/jimmunol.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalali BN, Köllisch G, Mages J, Müller T, Bauer S, Wagner H, et al. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J Immunol. 2008;181:2694–2704. doi: 10.4049/jimmunol.181.4.2694. [DOI] [PubMed] [Google Scholar]
- Kumar S, McDonnell PC, Lehr R, Tierney L, Tzimas MN, Griswold DE, et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem. 2000;275:10308–10314. doi: 10.1074/jbc.275.14.10308. [DOI] [PubMed] [Google Scholar]
- Lakhani SA, Masud A, Kuida K, Porter GA, Booth CJ, Mehal WZ, et al. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11:213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti T-D, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi N, Rothwell N, Brough D. Dual functionality of interleukin-1 family cytokines: implications for anti-interleukin-1 therapy. Br J Pharmacol. 2009;157:1318–1329. doi: 10.1111/j.1476-5381.2009.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin U, Scholler J, Gurgel J, Renshaw B, Sims JE, Gabel CA. Externalization of the leaderless cytokine IL-1F6 occurs in response to lipopolysaccharide/ATP activation of transduced bone marrow macrophages. J Immunol. 2009;183:4021–4030. doi: 10.4049/jimmunol.0803301. [DOI] [PubMed] [Google Scholar]
- Muhr P, Zeitvogel J, Heitland I, Werfel T, Wittmann M. Expression of interleukin (IL)-1 family members upon stimulation with IL-17 differs in keratinocytes derived from patients with psoriasis and healthy donors. Br J Dermatol. 2011;165:189–193. doi: 10.1111/j.1365-2133.2011.10302.x. [DOI] [PubMed] [Google Scholar]
- Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaru F, Jensen LE. Chemokine expression by human keratinocyte cell lines after activation of Toll-like receptors (TLRs) Exp Dermatol. 2010a;19:e314–316. doi: 10.1111/j.1600-0625.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaru F, Jensen LE. Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1[alpha] signaling loop. J Invest Dermatol. 2010b;130:1866–1876. doi: 10.1038/jid.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Current Opinion in Immunology. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Ramadas RA, Ewart SL, Medoff BD, LeVine AM. Interleukin-1 family member 9 stimulates chemokine production and neutrophil influx in mouse lungs. Am J Respir Cell Mol Biol. 2011;44:134–145. doi: 10.1165/rcmb.2009-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmiguel JC, Olaru F, Li J, Mohr E, Jensen LE. Interleukin-1 regulates keratinocyte expression of T cell targeting chemokines through interleukin-1 receptor associated kinase-1 (IRAK1) dependent and independent pathways. Cell Signal. 2009;21:685–694. doi: 10.1016/j.cellsig.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-{kappa}B and MAPKs. J Biol Chem. 2004;279:13677–13688. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LAB. Inflammasome activation and IL-1[beta] and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood epublished ahead of print. 2011 doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- Weatherhead SC, Farr PM, Jamieson D, Hallinan JS, Lloyd JJ, Wipat A, et al. Keratinocyte apoptosis in epidermal remodeling and clearance of psoriasis induced by UV radiation. J Invest Dermatol. 2011;131:1916–1926. doi: 10.1038/jid.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chen Q, Rosenberg HF, Rybak SM, Newton DL, Wang ZY, et al. Human Ribonuclease A Superfamily Members, Eosinophil-Derived Neurotoxin and Pancreatic Ribonuclease, Induce Dendritic Cell Maturation and Activation. J Immunol. 2004;173:6134–6142. doi: 10.4049/jimmunol.173.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]