Abstract
Background
Adherence has significantly affected the efficacy of a randomized clinical trial (RCT) to test exercise interventions.
Objective
To analyze exercise-related adherence patterns among patients receiving active cancer treatment and to identify factors related to exercise adherence and contamination in both the intervention and control groups.
Methods
This is a secondary analysis of data from a RCT of a home-based walking intervention for patients receiving active cancer treatment. Hierarchical Poisson regression analysis was used to identify factors related to exercise adherence and exercise contamination in the exercise intervention and control groups.
Results
A total of 126 patients finished the study. Exercise adherence rate in the intervention group was 32.35%, while exercise contamination rate in the control group was 12.07%. Independent predictors of adherence for the exercise group were baseline physical fitness, pre-treatment fatigue level, treatment-related mood disturbance, and marital status (p < 0.01); past exercise history significantly predicted exercise contamination (p < 0.00) in the control group.
Conclusions
Adherence remains an issue in an exercise RCT among patients on active cancer treatment. Adherence is related to symptom, physical function, and exercise history.
Implications for Practice
Exercise researchers should consider stratifying samples based on pre-treatment variables found to be significantly associated with outcome variables in this study to reduce confounding effects. Oncology clinicians can use the study findings to appropriately tailor strategies to encourage exercise adherence among patients receiving active cancer treatment so that these patients can receive the known benefits of exercise.
Keywords: Exercise, adherence, cancer, treatment, randomized clinical trial
Background and Significance
Cancer and cancer treatment are associated with unfavorable symptoms and a decline in physical function1,2. Exercise has been proposed as a way to manage symptoms and improve physical function. Across an increasing number of exercise studies conducted with cancer populations, the randomized controlled trial (RCT) has been primarily used, because of its potential to test the effectiveness of exercise intervention. However, poor adherence within exercise and control groups can seriously counteract the effectiveness of exercise on outcome variables, potentially skewing the results of such RCTs 3–5.
Adherence, generally understood as the level of participation achieved in a behavioral regimen once an individual has agreed to undertake it 6, has alternate meanings in the case of exercise-related RCTs: In the intervention group when the participants are instructed to exercise, those who reached prescribed exercise levels are referred to as “exercise adherents”. However, among sedentary controls, those who remain inactive are referred to as “control group adherents”. Exercise engaged in by control group participants is then referred to as “exercise contamination”.
To effectively assess the efficacy of an exercise intervention in a RCT, a high level of adherence by each study group is desirable. Realistically, this presents a challenge. For example, Mock et al. 5 reported that only 72% of breast cancer patients in a home-based exercise program achieved the minimum requirement of their exercise regimen in the intervention group, while 39% of the control group patients exercised. Similarly, Courneya et al. 3 reported a 75.8% adherence rate in the intervention group and a 51.6% contamination rate in the control group among colorectal cancer survivors in a home-based exercise study. More specifically, in a RCT of home-based exercise program among cancer patients, from which this analysis is drawn, intent-to-treat analysis yielded no differences between the exercise group and control group on multiple outcomes of interest such as fatigue, and physical function, possibly due to issues encompassing adherence 4, which warrants a further analysis on adherence in this sample.
Exercise adherence/contamination rates do not provide information regarding how exercise levels change over time. The long-term effects and accompanying benefits can be different for two patients with the same overall adherence rates but different exercise patterns. However, only very few investigators have explored adherence patterns in exercise RCT within cancer populations with conflicting results. Some investigators have reported a decline in adherence in the exercise intervention group 7–9, whereas others have found an increase in exercise adherence over time 10,11. No studies on exercise patterns in control group participants could be found.
Given the importance of adherence to appropriately evaluate exercise outcomes in RCTs, it is necessary to monitor and improve adherence in both the exercise intervention and control groups. A critical first step is to understand the determinants of exercise adherence and exercise contamination in RCTs. The few studies that have focused on this issue have produced conflicting results. In one study, researchers found no significant difference in age, smoking status, treatment type, physical activities, BMI, and psychosocial factors between exercise adherents and non-adherents among women 12 to 36 months after breast cancer treatment in a supervised exercise program 12; another group found that age, gender, employment status, cancer treatment type, exercise history, and some selected psychological factors were significantly related to exercise adherence in a supervised resistance training program involving cancer survivors 13–16. While most researchers have focused on cancer survivors after their cancer treatment, a few investigators have studied the cancer patients on active cancer treatments. One researcher found that patients’ baseline inactivity and days on chemotherapy related to decrease of exercise adherence 17, two investigators have observed that cancer patients experiencing cancer and treatment-related symptoms often failed to follow their exercise prescription 9,18. However, the relationship between symptoms and exercise adherence was not statistically tested.
A few investigators have examined the determinants of exercise contamination in the control group. In a control group, men were more likely to exercise than women, and past exercise history was significantly and positively associated with exercise contamination 14,15. These findings were corroborated by another researcher who discovered that control group non-adherents had actively engaged in regular exercise before study enrollment 9.
Thus, adherence is a critical issue that can weaken the efficacy of RCTs in assessing the effects of exercise. Although a small number of investigators have previously addressed the adherence issue in RCTs involving cancer populations and have explored factors related to exercise adherence and contamination, several important gaps remain: 1) The literature lacks information regarding exercise patterns for both exercise and control groups over time. 2) Most studies have focused on cancer survivors after treatment, and few have examined patients receiving active cancer treatment who are likely to experience disease- and treatment-related symptoms that might affect exercise behavior.
To address these gaps and provide evidence for future exercise research, we examined adherence among RCT-enrolled patients receiving active cancer treatment and looked at potential factors related to the exercise adherence and contamination among both the intervention and control groups.
Methods
This study was a secondary analysis of data from a RCT designed to determine the effects of a home-based mild-to-moderate muscle strengthening and walking exercise program on the management of fatigue and other outcomes in cancer patients receiving either chemotherapy or radiation treatment. The study was approved by the Western Institutional Review Board (WIRB). Data collection for the RCT began in October 2002 and ended in October 2006. In the parent study 4, cancer patients from a university teaching hospital and a community cancer center were screened using the eligibility criteria: 1) 21 years of age or older, 2) newly diagnosed with staged 0, I, II, or III cancer, 3) scheduled to receive chemotherapy or radiation therapy as initial treatment, 4) no evidence of metastatic disease, 5) no concurrent health problems /disabilities that would limit their ability to participate, and 6) not currently exercising more than three times per week totaling 120 minutes. Eligible patient were contacted by the research staff. Those who agreed to participate in the study provided signed written consent, after which demographic information, baseline symptoms and physical function capacity were assessed in interviews conducted by research staff. After enrollment, subjects were randomly assigned to either the control group or the exercise group.
Patients in the control group were instructed to continue their usual levels of physical activity, while patients in the exercise group were given an individualized exercise prescription after the initial assessment of physical status and fitness levels. The exercise prescription was a brisk 10-minute walk that increased to 30 minutes for 5 days per week as training progressed. The intensity of this walking prescription was to reach approximately 50% to 70% of maximum heart rate, consistent with the American College of Sports Medicine guidelines for ill populations 19,20. Participants were instructed to reach the desired goal of continuous exercise for 20 to 30 minutes each session; however, very de-conditioned individuals were told to assume a slower pace of two sessions of 5 to 10 minutes per day. Exercise participants were asked to wear pedometers daily throughout the study and to complete a daily exercise log. The control group participants also completed a daily exercise log but only wore pedometers for the first and last 2 weeks of the study period, to prevent potential Hawthorne or measurement effects. The exercise log data for both groups were collected and returned at a weekly basis throughout the entire study period.
Participants in both groups were contacted through biweekly telephone calls by research nurses to discuss their physical activity, cancer treatment, side-effects, and any concerns occurring in the previous 2 weeks. For the exercise group, adjustments to the walking prescription were made according to participants’ condition. A telephone log was completed by study nurses after each phone call.
For each participant, a mid-treatment point was calculated when they were contacted by research nurses and assessed their symptoms in a previous month. In post cancer treatment period, the participants were interviewed again by the research nurses when their symptoms for the previous month and physical function capacity were reassessed.
Measurement
Physical activity during the intervention phase was measured by a daily exercise log, an instrument developed specifically for the parent study. The log significantly correlated to an objective measurement of exercise -- a pedometer 21, was used to measure exercise activity and fatigue level on a daily basis in both groups, and to compute the weekly exercise minutes and exercise sessions.
Based on ACSM guidelines of 85% of minimum adherence to exercise prescription 20 and consistent with a similar exercise protocol found in the literature,5 the individual week was defined as follows: first an adherent week for the exercise intervention group, or a contaminated week for the control group occurred if the participant engaged in more than 60 min of aerobic activities and in more than 3 sessions in that week. Next, the individual adherence rate (or contamination rate) was then calculated as the percentage of adherent weeks (or contaminated weeks) out of the total prescribed weeks for each (Adherent weeks/total prescribed weeks). Finally, the exercise adherents (or control group non-adherents) were defined as those participants who had an individual adherence rate (or individual contamination rate) great than 67%. In other words, participants who adhere to group assignments for more than 2/3 of the entire study period were defined as group adherents.
Symptoms that were studied include: Fatigue – measured by Piper Fatigue Scale, a 22-item, ten-point Likert-type self-report scale with internal consistency reliabilities from 0.83 to 0.93; 22 Mood disturbance – measured by a 30-item self-report scale with a high subscales correlation (r = 0.951 – 0.979); 23 Symptom distress – measured by a 14-item 5-point self-report scale often used in cancer population and with good validity and internal consistency reliability (0.79 & 0.89); 24 and sleep disturbance – measured by Pittsburgh Sleep Quality Index (PSQI), a 11-item instrument that assesses different aspects of sleep quality.25 In this adherence analysis, a single item from the PSQI was used to evaluate participants’ overall sleep quality in past month. The physical function measured by the physical function subscale in the well established Medical Outcome Study Short Form-36,26 and physical fitness. The physical fitness was measured by the Treadmill Test with Maximal Oxygen Uptake that was recorded by the highest observed value of VO2.27 For participants who could not do treadmill (for schedule difficulty with treadmill testing center), a 12-minute walking tested was offered and results were converted to VO2 max.28
Statistical Analysis
Descriptive statistics were used to report exercise adherence or contamination in both groups. Weekly exercise minutes and weekly exercise sessions, the individual adherence or contamination rate, and total number of exercise adherents (or control group non-adherents) were reported for both the exercise intervention and control groups. Exercise pattern was determined from the exercise logs. The weekly exercise minutes were calculated for each individual. Because of the participants’ differing cancer treatment protocols, the total study duration varied by participants, ranging from 5 to 35 weeks. In order to capture exercise trends for patients with different study durations, the total duration was standardized by being converted to 10, with 0 representing the beginning of the study, and 10 representing the end of the study.
Bivariate correlations were assessed between each independent variable and the outcome variables defined as the individual adherence (or contamination rate). Variables significantly (p < 0.10) correlated with outcome variables were retained in the Hierarchical Poisson regression analyses and were entered in the following order: 1) physiologic factors, 2) medical factors, and 3) sociodemographic factors. In this analysis, the individual adherence (contamination) rate was calculated as the number of adherent (or contaminated) weeks divided by the total number of weeks (exposure). The Poisson regression that is appropriate for rate data in which the rate is a count of events (in this study, the number of adherent/contaminated weeks) divided by exposure (the number of total treatment weeks), because the outcome variables, the individual adherence (or contamination) rates, were between 0 and 1 and skewed. Finally, the effect size was calculated by using Cohen’s f for each block of factors.
By using our daily exercise log, we further explored the relationship between exercise and fatigue on a weekly basis. We computed the weekly total exercise minutes and weekly average fatigue score and conducted linear regression models for the groups of exercise-adherents, exercise non-adherents, control group adherents, and control group non-adherents.
Results
Exercise Adherence and Contamination
A total of 138 patients were enrolled in the parent study. Twelve patients withdrew from the study for various reasons such as change in cancer treatment plan, or medical complication 4, leaving 126 patients who completed the study: 68 in the exercise group and 58 in the control group. Table 1 summarizes the characteristics of the sample and shows no significant differences between the groups, except that more participants in the control group than in the intervention group had completed graduate school (p = .041). Table 2 shows participants’ symptoms and function capacity before and after cancer treatment. Participants reported minor symptoms and few limitations in physical function before cancer treatment. By the end of the treatment, participants reported slightly worsened symptoms and a decrease in function capacity. No significant differences were found for any of the pre-treatment and post-treatment measures between the exercise and control groups.
Table 1.
Characteristics of the Study Sample (Demographic and Medical Information)
| Study Groups | ||||
|---|---|---|---|---|
| Total | Exercise | Control | p-value a | |
| (N = 126) | (N = 68) | (N = 58) | ||
| Age | 60.2 (10.6) | 59.8 (10.8) | 60.6 (10.8) | .70 |
| N (%) | N (%) | N (%) | ||
| Gender | .84 | |||
| Male | 77 (61.1) | 41 (60.3) | 36 (62.1) | |
| Marital Status | .10 | |||
| Partnered | 107 (84.9) | 61 (89.7)) | 46 (79.3) | |
| Education | .04 | |||
| High school | 15 (11.9) | 7 (10.3) | 8 (13.8) | |
| College | 52 (41.3) | 35 (51.5) | 17 (29.3) | |
| Grad school | 59 (46.8) | 26 (38.2) | 33 (56.9) | |
| Employment Status | .59 | |||
| Full time | 60 (55.1) | 31 (54.4) | 29 (55.8) | |
| Part time | 11 (10.1) | 5 (8.8) | 6 (11.5) | |
| Resigned | 30 (27.5) | 15 (26.3) | 15 (28.9) | |
| Disabled | 8 (3.9) | 6 (10.5) | 2 (3.9) | |
| Leave of Absence | 4 (3.17) | 1 (1.7) | 3 (4.4) | |
| Other | 13 (10.3) | 8 (11.8) | 5 (8.6) | |
| Ethnicity/Race | .20 | |||
| Am. Indian | 1 (0.8) | 0 (0.0) | 1 (1.8) | |
| Asian/Pacific Islander | 2 (1.6) | 0 (0.0) | 2 (3.6) | |
| Black | 20 (16.4) | 9 (13.6) | 11 (19.6) | |
| White | 99 (81.2) | 57 (86.4) | 42 (75.0) | |
| Cancer Site | .55 | |||
| Breast | 41 (32.5) | 23 (33.8) | 18 (31.0) | |
| Colorectal | 7 (5.6) | 2 (2.9) | 5 (8.6) | |
| Prostate | 70 (55.6) | 38 (55.9) | 32 (55.2) | |
| Others | 8 (6.4) | 5 (7.4) | 3 (5.2) | |
| Treatment | .48 | |||
| Radiation therapy | 66 (52.4) | 38 (55.9) | 28 (48.3) | |
| Chemotherapy | 44 (34.9) | 24 (35.3) | 20 (34.5) | |
| Combined therapy | 9 (7.1) | 4 (5.9) | 5 (8.6) | |
| Brachytherapy | 7 (5.6) | 2 (2.9) | 5 (8.60) | |
t-tests were performed to compare ages between groups; chi-square contingency analyses were performed to compare categorical demographic characteristics
Table 2.
Symptoms, Physical Function, and Physical Fitness in the Study Sample
| Study Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total (N=126) | Exercise (N=68) | Control (N=58) | pa | |||||
| Study Variables | Measurement | Range | Higher Score Means |
Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | ||
| Fatigue | Piper Fatigue Scale | (0–10) | Very fatigued | Pre-trt | 2.42 (1.99) | 2.41 (1.84) | 2.42 (2.17) | .98 |
| Post-trt | 3.27 (2.18) | 3.39 (2.17) | 3.11 (2.19) | .46 | ||||
| Emotional disturbance | Profile of Mood States Scale Shortened | (0–100) | Extremely moody | Pre-trt | 7.68 (13.31) | 6.85 (11.15) | 8.66 (15.51) | .45 |
| Post-trt | 9.31 (15.74) | 10.47 (15.29) | 7.95 (16.27) | .37 | ||||
| Symptom distress | Symptom Distress Scale | (12–60) | A lots of symptoms | Pre-trt | 19.26 (4.67) | 19.51 (4.21) | 18.97 (5.19) | .51 |
| Post-trt | 21.01 (6.01) | 21.39 (5.49) | 20.55 (6.58) | .43 | ||||
| Sleep disturbance | Pittsburgh Sleep Quality Index | (1–4) | Worst sleep quality | Pre-trt | 2.04 (0.76) | 2.19 (0.10) | 1.98 (0.09) | .44 |
| Post-trt | 2.11 (0.80) | 2.20 (0.78) | 2 (0.82) | .15 | ||||
| Physical fitness b (VO2 max) | Treadmill testing | Good physical fitness | Pre-trt | 13.57 (4.22) | 13.92 (4.5) | 13.17 (3.87) | .33 | |
| Post-trt | 12.78 (4.62) | 12.92 (4.92) | 12.63 (4.30) | .74 | ||||
| Physical function | Medical Outcome Study Short Form – 36 | (0–100) | No limit | Pre-trt | 85.56 (14.43) | 84.26 (14.97) | 87.08 (13.74) | .28 |
| Post-trt | 78.63 (21.05.) | 77.15 (21.87) | 80.37 (20.10) | .39 | ||||
Abbreviations: VO2, oxygen uptake; trt, cancer treatment
t-tests were performed to compare differences between two groups;
121 participants finished pre-treatment treadmill-test or 12-minute walk test
In the exercise group, participants exercised an average of 4 sessions (S.D. = 1.77) and 113 minutes (S.D. = 61.07) per week. Nineteen participants (27.94%) adhered to the exercise assignments for the whole treatment period, and 3 participants (4.41%) did not adhere to the exercise prescription for any of the weeks in the entire treatment period. Using the cut-off point of 67% adherent weeks out of the total study weeks, 46 (67.65%) participants in the exercise group were exercise adherents, and 22 (32.35%) participants were exercise non-adherents. In the control group, participants exercised at an average of 1 session (S.D. = 1.63) and 48 minutes (S.D. = 64.79) per week. Four out of 58 (6.89%) participants in the control group had engaged in more than 60 minutes aerobic exercise and in more than 3 sessions weekly for the whole study period. Twenty-four (35.29%) participants had fully adhered to the group assignment, not exercising for the whole study period. Using the cut-off point of 67%, 51 (87.93%) control group participants were adherents, and 7 (12.07%) participants were non-adherents.
Exercise Patterns
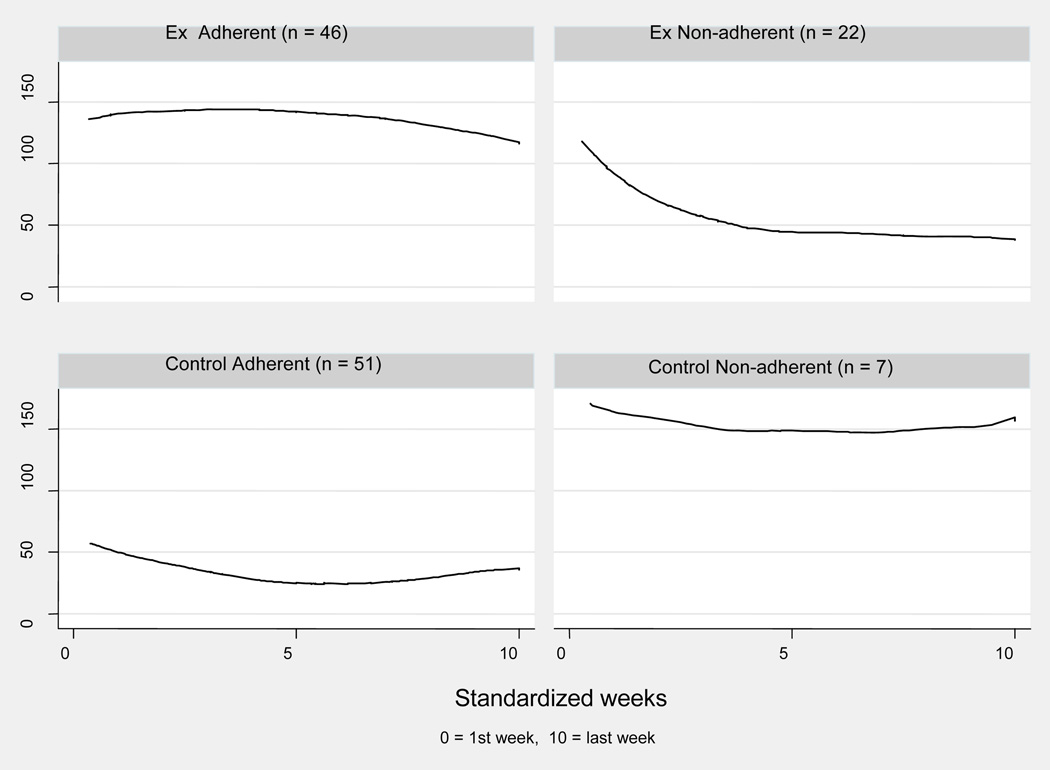
Figure 1 shows how weekly exercise level changed over the study period for exercise adherents, exercise non-adherents, control group adherents, and control group non-adherents. The exercise adherents (n = 46) began with adequate exercise minutes (> 120 minutes/week). Their weekly exercise minutes increased slightly (β = 2.72, p = .43) for about 1/3 of the study period and then gradually decreased (β = −3.74, p = .01), ending with an average of 115 minutes. The exercise non-adherents (n = 22) began with adequate exercise minutes (> 100 minute/week) but decreased their exercise level rapidly during the first half of the study (β = −14.51, p < .00) and maintained an average of < 50 weekly exercise minutes for the remainder of the study period. The control group adherents (n = 51) followed their group assignment: They exercised < 60 minutes/week over the entire study period and had slight changes in weekly exercise minutes throughout the whole study period. The control group non-adherents (n = 7) did not follow their group assignment. They began with a high level of exercise (about 170 minutes/week) and exercised > 145 minutes/week for the entire study period, with little fluctuation from week to week.
Figure 1.
Change of Weekly Exercise Minutes Over Standardized Exercise Duration by Groups
Bivariate analysis
Table 3 shows the bivariate relationship between the outcome variable – individual adherence (contamination) rates – and each of the independent variables. The variables that were correlated (p < 0.10) with individual adherence rate in the exercise group were: past physical activity (β = 0.22, p = .07), physical fitness (β = 0.19, p = .10), pre-treatment fatigue level (β = −0.26, p = .03), mid-treatment mood disturbance (β = −0.21, p = .09), cancer type (categorized as prostate cancer or non-prostate cancer) (t = −1.91, p = .06), and marital status (t = −2.30, p = .02). Variables that were related (p < .10) to individual contamination rate in the control group were: past exercise history (β = 0.45, p = .00), baseline physical function (β = 0.28, p = .03), mid-treatment symptom distress (β = −0.24, p = .06), and ethnicity (t = −1.69, p = .09).
Table 3.
Relationship Between Independent Variables and Exercise Adherence, and Exercise Contamination a
| Independent Variables | Exercise Adherence (N = 68) |
Exercise Contamination (N = 58) |
|
|---|---|---|---|
| Physiologic Variables | BMI | −0.17 | −0.11 |
| Past exercise history | 0.22 b | 0.45 d | |
| Physical Fitness | 0.19 b | 0.04 | |
| Pre-treatment physical function | 0.09 | 0.28 c | |
| Medical Variables | Cancer Type (prostate/non-prostate) | −1.91 b | −0.87 |
| Cancer Stage | 0.12 | 0.40 | |
| Treatment Type | 1.72 | 0.73 | |
| Pre-treatment fatigue | −0.26 c | 0.13 | |
| Pre-treatment mood disturbance | −0.12 | 0.02 | |
| Pre-treatment symptom distress | −0.13 | 0.01 | |
| Pre-treatment sleep disturbance | 0.05 | 0.04 | |
| Midterm mood disturbance | −0.21b | 0.21 | |
| Midterm symptom distress | −0.02 | −0.24b | |
| Socio-demographic Variables | Age | −0.06 | −0.11 |
| Gender | 1.65 | 0.89 | |
| Ethnicity (white/non-white) | 0.36 | −1.69 b | |
| Marital status | −2.30 c | −1.14 | |
| Educational level | 1.80 | 1.27 | |
| Employment status | 0.37 | 1.03 | |
Spearman correlations were conducted for continuous independent variables; t-tests were used for binominal variables, including: cancer type (prostate/non-prostate), treatment type (RXT/others), gender, marital status, ethnicity (white/non-white), employment status (employed/non-employed); one-way ANOVA was used for categorical variables with more than two levels, including: cancer stage, and educational level.
p<.10;
p<.05;
p<.01;
Hierarchical Poisson Regression Analysis
In the exercise group, physiologic factors counted for 35% of variance in exercise adherence, medical factors for 39%, and sociodemographic factors for 1%. Thus the final model (model 3 as shown in the tables 4) that included all three blocks of factors explained 75% of the variance in exercise adherence. The variables significantly associated with individual adherence rate in the final model were: physical fitness (β = 0.51, p < .01), past exercise history (β = 0.01, p < .01), pre-treatment fatigue level (β = −0.93, p < .01), lower mid-treatment mood disturbance (β = −0.27, p < .01), and being married (β = −3.56, p < .05). In the exercise intervention group, the medical factors had the largest effect size (f2 = 1.50) on exercise adherence, followed by physiologic factors, which had a large effect size (f2 = 0.54). Sociodemographic factors had a small effect size (f2 = 0.04) (Table 4).
Table 4.
Hierarchical Stepwise Regression of Exercise Adherence in the Exercise Group
| Steps | R 2 | R 2change | f2 | Variables | β 1 | β 2 | β 3 |
|---|---|---|---|---|---|---|---|
| Model 1 Physiologic factors |
0.35 | 0.35 | 0.54 | Physical Fitness | 1.13 b | 0.50 b | 0.51 b |
| Past exercise history | 0.02 b | 0.010 a | 0.010 b | ||||
| Model 2 Medical factors |
0.74 | 0.39 | 1.50 | Pre-trt fatigue score | −0.70 a | −0.93 b | |
| Mid-trt mood disturbance | −0.29 b | −0.27 b | |||||
| Cancer type | 0.62 | 1.57 | |||||
| Model 3 Demographic factors |
0.75 | 0.01 | 0.04 | Marital status | −3.56 a | ||
p<.05;
p<.01;
β 1–4 = standardized regression coefficients for equations #1 through #4; f2 = Cohen’s effect size
In the control group, physiologic factors counted for 27% of the variance in exercise contamination. Medical factors added 10% and demographic factors 9%. Thus, the final model explained 46% of the variance in exercise contamination in this group. Past exercise history (β = 0.01, p < .05), mid-treatment symptom distress (β =− 0.45, p < .01), and ethnicity (β = 4.47, p < .05) were significantly related to individual contamination rate. Physiologic factors had a large effect size (f2 = 0.37) on exercise contamination, as did medical factors, with a median effect size (f2 = 0.16) and the demographic factors with a median effect size (f2 = 0.17) (Table 5).
Table 5.
Hierarchical Stepwise Regression of Exercise Contamination in the Control Group
| Steps | R 2 | R 2change | f2 | Variables | β 1 | β 2 | β 3 |
|---|---|---|---|---|---|---|---|
| Model 1 Physiologic factors |
0.27 | 0.04 | 0.37 | Past exercise history | 0.02 b | 0.01 b | 0.01 a |
| Pre-trt physical function | 0.22 b | 0.20 a | 0.16 | ||||
| Model 2 Medical Factors |
0.37 | 0.10 | 0.16 | Mid-trt symptom distress | −0.24 b | −.45 b | |
| Model 3 Demographic Factors |
0.46 | 0.09 | 0.17 | Ethnicity | 4.47 b | ||
Abbreviations: trt, cancer treatment
p<.05;
p<.01;
β 1–3 = standardized regression coefficients for equations #1 through #3; f2 = Cohen’s effect size;
Relationship between weekly fatigue level and weekly exercise minutes
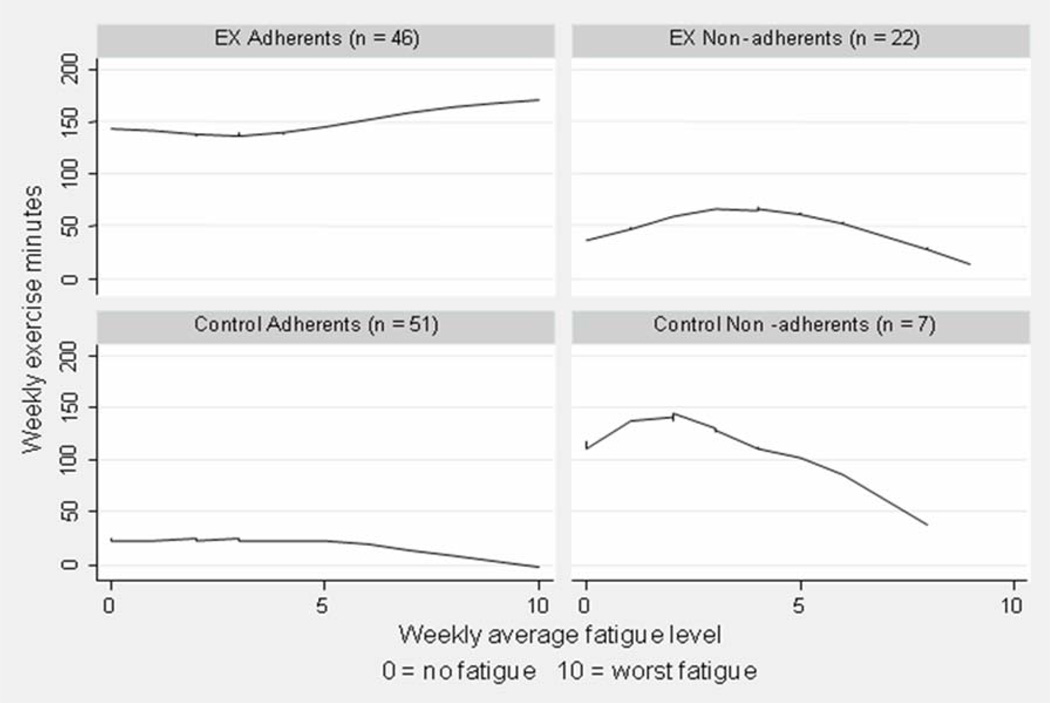
Figure 2 demonstrates how weekly exercise minutes changed with fatigue for the four groups. Exercise adherents were the only group of participants who maintained and even increased their exercise levels when experiencing increasing level of fatigue (β = 9.83, p = .01). Exercise non-adherents managed to increase their weekly exercise level when experiencing mid level of fatigue (fatigue < 4) (β = 10.04, p = .04). However, when experiencing a higher level fatigue (≥ 5), these participants dramatically reduced their exercise level (β = −11.78, p = .01). The control group adherents maintained a low level of exercise (< 50 minutes/week) and did not change much with fatigue (β = −0.86, p = .45). The control group non-adherents had a decrease of exercise level when their fatigue level increased (β = −12.20, p = .01). The findings suggest that fatigue affected most cancer patients’ exercise adherence. However, the continuous increase of exercise level among the exercise adherents implies that other factors (that are identified in the regression models as listed previously) play important roles in their exercise.
Figure 2.
Correlation between Weekly Exercise Minutes and Weekly Average Fatigue Level
Discussion
The present study examined the determinants of exercise adherence and contamination and their correlates among patients on active cancer treatment. Our findings are consistent with a home-based exercise program involving breast cancer patients on active cancer treatment exercised 5, but it was on the low end in terms of exercise duration when compared to other studies 8,10,13,29,30 of cancer survivors, in which average weekly exercise times of 141 to 160 minutes and adherence rates of 67% to 97% were reported. The differences in exercise level may reflect differences in the respective study populations and study duration. These previous studies with higher adherence rates and more exercise minutes usually involved cancer survivors post-treatment, who therefore had less severe symptoms and would be more likely to exercise when compared to patients such as ours who were concurrently undergoing cancer treatment. In contrast to our study, which had a duration of 5 to 35 weeks with a mean of 12.67 weeks, another study had a 5-week exercise program and reported a 97% adherence rate 10. As the study’s duration increased, exercise adherence decreased 8. As compared to previous studies in which contamination rates were reported between 22% to 52% 5,13,29,31, our study had a lower contamination rate (12.07%), perhaps because we excluded those who had exercised more than 120 minutes per week on a regular basis prior to enrollment.
One novel contribution of our study is the identification of a strong relationship in the exercise group between exercise adherence and symptoms that included fatigue and mood disturbance. We studied patients on active cancer treatment, who often experience unpleasant symptoms that can affect their ability to exercise. Fatigue, the most often-reported adverse side-effect experienced by cancer patients, has been commonly studied in research on cancer-related symptoms. Since 2000, the National Comprehensive Cancer Network has emphasized the value of exercise in the management of fatigue. The ACSM 2010 new guidelines for cancer patients and cancer survivors strongly suggested avoid inactivity. 32 However, the negative relationship between exercise level and fatigue level identified here and in a previous study 32 suggests that, in spite of evidence supporting the benefits of exercise over energy conservation, for some cancer patients, energy conservation continues to be used as a primary strategy for fatigue management. Yet fatigue and insufficient exercise can be a vicious cycle. When fatigued, patients exercise less; the less they exercise, the more likely they are to lose physical capacity and experience more fatigue. The strong relationship between exercise adherence and fatigue indicates that patient education is still necessary to promote exercise among cancer patients.
Mood disturbance is another commonly reported symptom among cancer patients. For patients who are facing a life-threatening illness and unpleasant symptoms related to cancer treatment, some level of mood distress is inevitable and can affect patients’ participation in exercise. One researcher has reported a negative relationship between mood disturbance, especially depression, and cancer patients’ participation in exercise in a non-RCT study 33. In a series of qualitative interviews with exercise non-adherents in our study, patients noted that treatment-related side effects such as fatigue, depression, and other medical symptoms prevented them from exercising at the prescribed level 34. Our observation that among exercise non-adherents, the number of weekly exercise minutes dropped sharply from >100 at the beginning to <50 at the study mid-point indicates the importance of assessing patients for side effects after the first few doses of cancer treatment and evaluating how these side effects may affect their ability to exercise. In the case of patients experiencing severe symptoms, clinicians may need to increase the frequency of their contacts with patients, discuss strategies to effectively manage their symptoms, and either make exercise adjustments according to their specific medical condition, or refer patients to a qualified health professional (i.e., physical therapist or exercise physiologist).
Past exercise history had an effect on both exercise adherence in the exercise group and exercise contamination in the control group, as has been observed in previous studies 9,15,16. Exercise has the potential to improve bone remodeling and reduce the muscle weakness and wasting effects often caused by cancer treatment. It helps cancer patients maintain strength, endurance, and level of functioning 35; therefore they are more likely to tolerate cancer treatment and subsequently to adhere to their exercise assignment even when experiencing severe unpleasant symptoms. With increasing evidences supporting the benefit of exercise among the general public and cancer patients, larger numbers of national and local media have highlighted the importance of exercise in health promotion. Meanwhile, oncology health care professionals have also been promoting exercise among cancer patients. Thus, it is not surprising that cancer patients begin to exercise and maintain their exercise practice as a habit. For participants in the exercise intervention group, the effect of habit helped them to maintain their exercise level. However, in the control group, habit may have explained why regular exercisers continued their pre-existing level of exercise even after being assigned to a study control group.
Our analysis in subgroups (exercise adherent, exercise non-adherents, control adherents, and control non-adherents) reveals that exercise adherent and control non-adherents were able to maintain a sufficient level of exercise throughout the study (see figure 1). However, when experiencing high levels of fatigue, only the exercise adherents managed to keep their exercise level, while control non-adherents reduced their exercise (see figure 2). This difference reflects the significant supporting role by health care professional in promoting exercise among cancer patients. During the study-prescribed biweekly phone calls to the exercise group participants, the research nurses not only assessed their symptoms, readjusted their exercise assignments, but also gave encouragement and support to the participants. Actually some patients indicated that it was the research nurses’ phone calls making them exercise.
We also found that partnered participants were more likely to exercise than were non-partnered counterparts, consistent with the important role played by family members in exercise adherence, as reported in two previous studies 33,36. During study enrollment, participants in the exercise intervention group in our study were encouraged to exercise with a family member, to improve their adherence. Anecdotally, these patients reported that having a family member’s encouragement was helpful, especially when they felt tired or depressed. Some patients also indicated that exercising in the company of family was entertaining and could address concerns about safety when exercising outside close to dark.
An additional new finding from our study was that white participants exercised more than did non-whites in the control group, perhaps because of differences in access to exercise equipment or facilities. Even though our exercise prescription was walking, some participants reported difficulty in exercising in bad weather. Therefore, access to exercise equipment or facilities could help them maintain exercise adherence. Socioeconomically disadvantaged adults have previously reported environmental barriers to physical activity 37. However, information about access to exercise equipment and facilities was not captured in our study. This important disparity issue should be addressed in future research to ensure that minorities do not experience additional cancer disparities related to supportive management.
Study Limitations and Recommendations for Future Research
It should be noted that the nature of the secondary analysis of data limited our ability to explore other factors of interest, such as the psychosocial factors and environmental factors that have been shown to have a significant impact on exercise adherence and contamination in previous studies. In the qualitative interviews with exercise non-adherents, some patients indicated environmental factors such as lack of access to exercise equipment or venue in the winter 34. However, that information was not included in this dataset and could not be included in analyses. Future studies should include both psychological and environmental factors related to exercise adherence. Secondly, the regression analyses combined patients with different types of treatment that make study duration vary. Separating groups by cancer treatments is limited because of the sample size. In addition, the cancer type and treatment type was found not significantly related to both exercise adherence and contamination. Finally, the generalizability of this study may be somewhat restricted because of the relative uniformity of the sample; participants in this study were primarily Caucasian, well-educated, and receiving treatment for prostate or breast cancer. Thus, we suggest replicating this work in a sample of cancer patients with more diverse ethnic backgrounds and lower levels of education in order to increase the applicability of the findings to other populations with cancer.
Implications for research and clinical practice
In conclusion, our study has important implications for both research and clinical practice. The study contributes new knowledge about exercise for cancer patients among clinical health care providers who may better understand individual patient preferences for exercise during treatment, and appreciate the difficulty that patients may have adhering to prescribed exercise regimes.
It is important for exercise RCT researchers to maintain a low contamination rate in the control group, in other words, to keep control group participants from exercising. Researchers can achieve this by excluding regular exercisers in the beginning. Or, if that exclusion is not an option, that the sample be stratified based on participants’ previous exercise history. This approach may prove advantageous, even if an increase in the study sample size is required.
Based on our findings, health care professionals should help patients to establish an exercise habit at an early stage in their diagnosis; suggest an individualized exercise plan based on their physical activity level; encourage family support; and assess and reassess their patients’ conditions and exercise status throughout the treatment period and help patients adjust their exercise programs accordingly, or refer patients to an appropriate exercise professional for further consultation. More specifically, the following strategies might be applied to improve exercise adherence: 1) assess the patient’s baseline fatigue level and design an individual exercise prescription based on patient’s situation; 2) encourage the patient to identify a family member or friend who can serve as an exercise partner to provide support or encouragement; encourage social support groups; 3) teach patients to exercise actively when the side effects and symptoms are at a minimum level; 4) establish regular contact with patients; 5) assess patients’ situation and exercise condition, identify any problem which may serve as a barrier to keep patients from exercising, and adjust exercise according to patients’ situation; 6) engage patients in discussions surrounding symptoms such as fatigue and exercise.
Acknowledgments
The authors would like to acknowledge Drs. Jerilyn Allen and Haera Han for their instruction and guidance, and Carol Thompson and Hayley Hedlin for their assistance in data analysis.
This Study was supported by American Cancer Society (ACS) Doctoral Scholarship in Cancer Nursing [DSCN-07-138-01]. The funding sources had no role in the study design, data collection, analysis, or interpretation, or writing of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to disclose in this study
The content is solely the responsibility of the authors’ and does not necessarily represent the official views of the ACS.
References
- 1.Lindley CV S, Sawyer WT, Winer EP. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol. 1998;16(4):1380–1387. doi: 10.1200/JCO.1998.16.4.1380. [DOI] [PubMed] [Google Scholar]
- 2.Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain. 2001;93(3):247–257. doi: 10.1016/S0304-3959(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 3.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12(4):347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 4.Griffith K, Wenzel J, Shang J, Thompson C, Stewart K, Mock V. Impact of a walking intervention on cardiorespiratory fitness, self-reported physical function, and pain in patients undergoing treatment for solid tumors. Cancer. 2009;115(20):4874–4884. doi: 10.1002/cncr.24551. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, Stewart KJ, Cameron L, Zawacki K, Podewils LJ, Cohen G, McCorkle R. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005;14(6):464–477. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 6.Dishman . Human Kinetics. Champaign, IL: 1998. Exercise Adherence. [Google Scholar]
- 7.Headley JA, Ownby KK, John LD. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol Nurs Forum. 2004;31(5):977–983. doi: 10.1188/04.ONF.977-983. [DOI] [PubMed] [Google Scholar]
- 8.Matthews CE, Wilcox S, Hanby CL, Der Ananian C, Heiney SP, Gebretsadik T, Shintani A. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support Care Cancer. 2007;15(2):203–211. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- 9.Pickett M, Mock V, Ropka ME, Cameron L, Coleman M, Podewils L. Adherence to moderate-intensity exercise during breast cancer therapy. Cancer Pract. 2002;10(6):284–292. doi: 10.1046/j.1523-5394.2002.106006.x. [DOI] [PubMed] [Google Scholar]
- 10.Windsor PM, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101(3):550–557. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 11.Pinto BM, Rabin C, Dunsiger S. Home-based exercise among cancer survivors: adherence and its predictors. Psychooncology. 2009;18(4):369–376. doi: 10.1002/pon.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daley AJ, Crank H, Mutrie N, Saxton JM, Coleman R. Determinants of adherence to exercise in women treated for breast cancer. Eur J Oncol Nurs. 2007;11(5):392–399. doi: 10.1016/j.ejon.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Courneya KS, Segal RJ, Reid RD, Jones LW, Malone SC, Venner PM, Parliament MB, Scott CG, Quinney HA, Wells GA. Three independent factors predicted adherence in a randomized controlled trial of resistance exercise training among prostate cancer survivors. J Clin Epidemiol. 2004;57(6):571–579. doi: 10.1016/j.jclinepi.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. Predictors of adherence and contamination in a randomized trial of exercise in colorectal cancer survivors. Psychooncology. 2004;13(12):857–866. doi: 10.1002/pon.802. [DOI] [PubMed] [Google Scholar]
- 15.Courneya KS, Friedenreich CM, Sela RA, Quinney HA, Rhodes RE. Correlates of adherence and contamination in a randomized controlled trial of exercise in cancer survivors: an application of the theory of planned behavior and the five factor model of personality. Ann Behav Med. 2002 Fall;24(4):257–268. doi: 10.1207/S15324796ABM2404_02. [DOI] [PubMed] [Google Scholar]
- 16.Courneya KS SC, McNeely ML, Sellar CM, Peddle CJ, Friedenreich CM, Mazurek A, Chua N, Tankel K, Basi S, Reiman T. Predictors of adherence to supervised exercise in lymphoma patients participating in a randomized controlled trial. Annals of Behavioral Medicine. 2010;40(1):30–39. doi: 10.1007/s12160-010-9205-5. [DOI] [PubMed] [Google Scholar]
- 17.Swenson KK, Nissen MJ, Henly SJ. Physical activity in women receiving chemotherapy for breast cancer: adherence to a walking intervention. Oncol Nurs Forum. 2010;37(3):321–330. doi: 10.1188/10.ONF.321-330. [DOI] [PubMed] [Google Scholar]
- 18.Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006;29(2):156–165. doi: 10.1097/00002820-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Medicine ACoS. ACSM's exercise management for person with chronic disease and disabilities. Champaign, IL: Human Kinetics; 1997. [Google Scholar]
- 20.Medicine ACoS. ACSM's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 21.Shang J, Wenzel J, Griffith K. A Recommended Approach for Measuring Exercise in a Home-based Exercise Study. 11th National Conference on Cancer Nursing Research; Los Angeles, CA. 2011. [Google Scholar]
- 22.Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol.Nurs.Forum. 1998;25:677–684. [PubMed] [Google Scholar]
- 23.Shacham S. A shortened version of the Profile of Mood States. J Pers.Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 24.McCorkle RYK. Development of a symptom distress scale. cancer nursing. 1978;1(373–378) [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26(7):724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J Appl Physiol. 1955;8(1):73–80. doi: 10.1152/jappl.1955.8.1.73. [DOI] [PubMed] [Google Scholar]
- 28.Cooper KH. A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. JAMA. 1968;203(3):201–204. 15. [PubMed] [Google Scholar]
- 29.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12(4):347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 30.Segar ML, Katch VL, Roth RS, Garcia AW, Portner TI, Glickman SG, Haslanger S, Wilkins EG. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol Nurs Forum. 1998;25(1):107–113. [PubMed] [Google Scholar]
- 31.Mock V, Pickett M, Ropka ME, Muscari Lin E, Stewart KJ, Rhodes VA, McDaniel R, Grimm PM, Krumm S, McCorkle R. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9(3):119–127. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- 32.So WK, Tai JW. Fatigue and fatigue-relieving strategies used by Hong Kong Chinese patients after hemopoietic stem cell transplantation. Nurs Res. 2005;54(1):48–55. doi: 10.1097/00006199-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Pinto BM, Trunzo JJ, Reiss P, Shiu SY. Exercise participation after diagnosis of breast cancer: trends and effects on mood and quality of life. Psychooncology. 2002;11(5):389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- 34.Ruble K, Mock V, Rose L, Hall S. Adherence to exercise: a qualitative study. 9th National Conference on Cancer Nursing Research; Hollywood, CA. 2007. [Google Scholar]
- 35.Schwartz AL. ACSM's Exercise Management for Person with Chronic Diseases and Disabilities. Champaign, IL: Hman Kinetics; 1997. Cancer; pp. 166–172. [Google Scholar]
- 36.Satia JA, Walsh JF, Pruthi RS. Health behavior changes in white and African American prostate cancer survivors. Cancer Nurs. 2009;32(2):107–117. doi: 10.1097/NCC.0b013e3181982d4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark DO. Physical Activity and Its Correlates Among Urban Primary Care Patients Aged 55 Years or Older. the Journals of Gerontology. 1999;54B:S41–S48. doi: 10.1093/geronb/54b.1.s41. [DOI] [PubMed] [Google Scholar]