Abstract
OBJECTIVE
The rice rat (Oryzomys palustris) develops periodontitis-like lesions when fed a diet high in sucrose and casein (H-SC). We aimed to establish whether this model can accurately mimic the development of human periodontitis.
MATERIALS AND METHODS
For this purpose, 28 day-old rice rats (15/group) were assigned to standard (STD) or H-SC diets and sacrificed after 6, 12, and 18 wks. Jaws were processed for morphometric, histometric, histologic, histomorphometric, and microCT analyses.
RESULTS
We found a progressive increase in horizontal alveolar bone loss (ABL) with age in maxillae of rats fed the STD diet as determined by morphometry. The H-SC diet exacerbated horizontal ABL at the palatal surface at 12 and 18 wks. Furthermore, increased vertical ABL was detected in mandibles and maxillae of rats fed the H-SC diet for 12 and/or 18 wks by histometry and microCT. Remarkably, the H-SC diet significantly increased bone remodeling at the interproximal alveolar bone of mandibles from rats fed for 6 wks, but not in those fed for longer periods.
CONCLUSIONS
These findings indicate that the H-SC diet induced a transient increase in alveolar bone remodeling, which is followed by ABL characteristic of moderate periodontitis.
Keywords: periodontitis, bone loss, rice rats
Introduction
Numerous laboratory animal species, including non-human primates, dogs, rats (Rattus norvegicus), mice, guinea pigs, Syrian hamsters, ferrets, and rice rats (Oryzomys palustris) have been used to study the pathophysiology of periodontal disease or to test treatments for the prevention, control and resolution of periodontitis (Leonard 1979; Weinberg and Bral 1999). The criteria for selection of an ideal animal model are based on pathophysiological characteristics that mimic the human disease. Hence, a good animal model for human periodontal disease should exhibit pathological lesions such as progressive destruction of connective tissue attachment and alveolar bone loss accompanied by pocket formation, recession, or both.
The rice rat has been shown to be extraordinarily susceptible to the initiation and development of a spontaneous form of periodontitis (Gupta and Shaw 1956a; Gupta and Shaw 1956b; Gupta and Shaw 1956c; Ryder 1980). Periodontal lesions are accentuated by feeding the rats a diet high in sucrose and casein (H-SC) (Auskaps et al. 1957; Gotcher and Jee 1981a; Gupta & Shaw 1956a; Gupta & Shaw 1956b; Hattler et al. 1977; Ryder 1980). Importantly, periodontitis in rice rats appears to be similar to that observed in humans in its development, location and appearance (Gotcher & Jee 1981a; Gupta & Shaw 1956a; Gupta & Shaw 1956b; Leonard 1979). For instance, as observed in the human disease, marginal gingivitis appears to be the initial pathologic finding observed in rice rats (Leonard 1979). Furthermore, periodontal lesions are more severe in mandibular than maxillary molars (Gupta & Shaw 1956a). In addition, substantial alveolar bone loss, with or without comparable soft tissue involvement, and tissue destruction with loss of functional support for teeth, are the ultimate periodontal lesions observed in this animal species (Gotcher & Jee 1981a; Gupta & Shaw 1956a; Gupta & Shaw 1956b). Unlike the human disease, which takes years to develop, in the rice rat, periodontal lesions progress to a chronic destructive state within a short period of time (10–18 wks) (Gotcher & Jee 1981a; Gupta & Shaw 1956a; Leonard 1979).
To our knowledge, no studies involving rice rats, in the context of periodontal disease, have been reported in three decades. We reasoned that although the periodontal lesions described in rice rats (Auskaps, Gupta, & Shaw 1957; Gotcher & Jee 1981a; Gupta & Shaw 1956a; Gupta & Shaw 1956b; Hattler et al. 1977; Ryder 1980) have a similar histopathologic appearance to human periodontitis, the previous work suggests that it progresses too rapidly to advanced periodontitis with complete loss of alveolar bone to mimic human periodontitis that usually develops over several decades. A model with slower-developing periodontitis in the rice rat not only may be possible, but would also be highly desirable.
The purpose of this study was to reexamine the rice rat model of periodontitis and to characterize in more detail the periodontal lesions employing more recent advanced methodologies including morphometry, histomorphometry, histochemistry, and microCT techniques. We aimed to determine whether the rice rat model can accurately and conveniently mimic the development of human periodontitis.
Materials and methods
Animals and experimental groups
Rats received diet treatments at 4 weeks of age for periods of 6, 12, and 18 wks, corresponding to ages of 10-, 16- and 22 wks, respectively. There were a total of six groups, three fed the standard (STD) diet and three fed the H-SC diet. Each group was composed of 15–19 (10 males, 5–9 females) rats. Dietary treatments consisted of either a STD diet (Harlan 8604 Teklad Rodent Diet, Tampa, FL) or a pelleted H-SC diet (TestDiet, Purina Feed, Richmond, IN). The composition of the H-SC diet was based on the formula of the powdered Harvard high sucrose 700 diet and the ration 100 diet previously used to enhance periodontal disease in rice rats (Auskaps, Gupta, & Shaw 1957; Gotcher & Jee 1981a; Gupta & Shaw 1956a; Gupta & Shaw 1956b; Gupta & Shaw 1956c; Ryder 1980). The major components of the H-SC diet and their percentages are shown in Table 1.
Table 1.
Ingredients of the high sucrose and casein (H-SC) diet used in this study
| Ingredients of the H-SC diet | (%) |
|---|---|
| Sucrose | 67.3 |
| Casein-Vitamin Free | 20.0 |
| Corn Oil | 5.0 |
| AIN 93G Mineral Mix | 3.5 |
| Powdered Cellulose | 3.0 |
| AIN 93 Vitamin Mix | 1.0 |
| Chlorine Chloride | 0.2 |
Experimental animals were group-housed (2–5 animals per cage) in static filter top cages (area: 143 in2) with pine shavings bedding and continuous access to food and water. The housing room was maintained at 55–70 °F, an average humidity between 30–70%, and a 14L:10D light:dark cycle. The Animal Care Services resource at the University of Florida is an AAALAC-accredited animal care and use program. The animal protocol was approved by the University of Florida Institutional Animal Care and Use Committee. All animal care and experimental procedures were in accordance with federal policies and guidelines of the University of Florida Institutional Animal Care and Use Committee. Adequate measures were taken to minimize pain and discomfort in the animals.
Necropsy and tissue collection
Rats were injected SC with declomycin and calcein (Sigma) at a dose of 15 mg/kg on the 7th and 2nd days prior to sacrifice, respectively, to label sites of bone formation. Rice rats were then euthanized by CO2 inhalation followed by thoracotomy. Maxillae and mandibles were stripped of musculature, leaving the periosteum intact. All right maxillae were processed for horizontal alveolar bone loss (ABL) analysis. Left maxillae of males were placed in 10% buffered formalin for histology and vertical ABL analysis. Left mandibles of males were placed in 70% ethanol to assess static and dynamic histomorphometric parameters and vertical ABL. Left mandibles of females were placed in 4% paraformaldehyde for histology, histochemistry, and vertical ABL analyses. Right mandibles of females were placed in 70% ethanol for microCT analysis. Right mandibles of male rats and left maxillae of female rats were frozen at −80°C for RNA extraction (data not shown).
Determination of horizontal alveolar bone loss (ABL) and periodontal intrabony defects
Right maxillae were autoclaved at 121°C for 20 min, defleshed, immersed in 3% (vol/vol) hydrogenperoxide overnight, washed with deionized water, air dried, and stained for 1 min in an aqueous solution of 0.1% (wt/vol)methylene blue to delineate the cemento-enamel junction (CEJ) (Rajapakse et al. 2002; Verma et al. 2010a; Verma et al. 2010b). Digital images of both palatal and buccal root surfaces of all molar teeth were captured using a stereo dissecting microscope (SteReo Discovery V8; Carl Zeiss MicroImaging, Inc., Thornwood, NY), at 10x magnification, after superimposition of buccal and palatal cusps to maximize reproducibility and ensure consistency of alignment. The line tool was used to make horizontal bone resorption measurements from the CEJ to the alveolar bone crest (ABC). The surface perimeter of the CEJ and the ABC was calculated using the AxioVision LE 29A software version 4.6.3 (Carl Zeiss, Germany).
Periodontal intrabony defects on tooth surfaces were determined in right maxillae with a 10x stereo dissecting microscope (SteReo Discovery V8) by an experienced periodontist (JL). The maxillae were tilted and stabilized with dental wax to verify the presence of periodontal intrabony defects. Only the presence or absence of periodontal intrabony defects was detected because the crevasses in the rat jaw are too small to measure depth and width.
Vertical alveolar bone loss (ABL)
Maxillae of male and female rice rats were decalcified in 5% formic acid and processed as previously described with slight modifications (Verma et al. 2010a; Verma et al. 2010b). Briefly, tissues were dehydrated in increasing concentrations of ethanol, paraffin-embedded, sectioned in the mesio-distal plane at 5μm using a Microme HM 325 microtome (GMI Inc, Ramsey, MN), and stained with hematoxylin and eosin (H&E). Sectioning was performed from the palatal towards the buccal surface. The block was oriented so as to visualize the mesial and distal roots of all molars as an indicator of proper alignment. The first two sections obtained from each rat that were adequately aligned and contained alveolar bone processes from the palatal surface were used for the quantitative analysis (palatal surface sections). Fifty consecutive 10 μm sections were discarded eventually to obtain two consecutive sections more adjacent to the buccal surface (buccal surface sections). Loss of vertical alveolar bone height (ABH) was determined by measuring the distance from a line between the CEJs of adjacent teeth to the ABC of the interproximal alveolar bone between the first and second molars (M1–M2), and second and third molars (M2–M3) in the palatal and buccal surface sections using the OsteoMeasure System (OsteoMetrics, Inc., Atlanta, GA) at a magnification of 100X. Sections of mandibles from males that were stained enbloc with basic fuchsin and embedded in methyl methacrylate were used to assess vertical alveolar bone height, but only at the lingual surface (see bone histomorphometry below).
Region of interest (ROI) for histomorphometric and histochemical analyses
The ROI was located at the mandibular interproximal space between molar (M)-1 and M2, which is the site for the highest prevalence and severity of periodontal lesions in rice rats (Gotcher & Jee 1981a; Gupta & Shaw 1956a; Leonard 1979). The ROI was similar to that previously reported (Gotcher & Jee 1981a; Gotcher and Jee 1981b) except for minor modifications. The schematic view of the ROI is shown in Figure 1.
Figure 1.
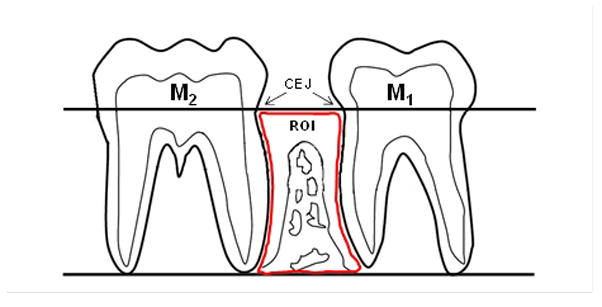
Schematic view of the region of interest (ROI) for the quantitative histomorphometric analysis. Histomorphometric analyses were performed within an area located at the interproximal alveolar bone between the first mandibular molar (M1) and the second mandibular molar (M2). The ROI boundaries are: coronally - a longitudinal line projected through the cementoenamel junction (CEJ) of M1 and M2; apically - a longitudinal line at the level of the apices of the roots of these molars; and mesially and distally - the distal root surface of M1 and the mesial root surface of M2, respectively.
Bone histomorphometry
Left mandibles from male rats (n=10/group) were processed by enbloc basic fuchsin staining and methyl methacrylate embedding to assess bone static and dynamic histomorphometric parameters within the ROI as previously described (Allen 2007; Allen and Burr 2007; Burr and Hooser 1995). Briefly, tissues were placed in 70% ethanol and bulk stained in 1% basic fuchsin in increasing concentrations of ethanol under vacuum and embedded undecalcified in modified methyl methacrylate (Baron et al. 1983). Samples were sawed into longitudinal sections of <200 μm thickness with an Isomet low-speed saw (Buehler, LakeBluff, IL, USA). These sections were then ground to a thickness of 80–100 μm for histomorphometric measurements within the ROI and for vertical ABL assessment using the OsteoMeasureSystem (OsteoMetrics, Inc., Atlanta, GA). Blocks were oriented so as to visualize the mesial and distal roots of M1 and M2 as an indicator of proper alignment. Sections obtained from each rat that were adequately aligned and contained alveolar bone processes from the lingual surface were used for the quantitative analysis. Variables assessed in the ROI included the % of alveolar bone present within the ROI (alveolar bone volume) and fluorochrome-based indices of bone formation, including mineralizing surface (% cancellous bone perimeter bearing double fluorochrome labels plus one half of the singly labeled surfaces), mineral apposition rate, and bone formation rate. Bone formation rate (surface referent, BFR/BS) was calculated by multiplying mineralizing surface by mineral apposition rate (Frost 1983). The terminology used, when applicable, was based on recommendations by the Histomorphometry Nomenclature Committee of the American Society of Bone and Mineral Research (Parfitt et al. 1987).
Osteoclast analyses
Left mandibles from female rats (n= 5/group)were decalcified, embedded in paraffin, sectioned at 5μm and stained for tartrate-resistant acid phosphatase (TRAP; Sigma) (Bainbridge et al. 2010). Bone resorption parameters, including eroded surface, osteoclast surface and osteoclast number, were measured on alveolar bone surfaces within the ROI using the OsteoMeasure System (OsteoMetrics, Inc., Atlanta, GA).
MicroCT Assessment
MicroCT assessment of mandibular alveolar bone was performed as previously described (Park et al. 2007). Right mandibles from females were trimmed with a disc to reduce the mesiodistal dimension to ~7.5mm and the coronoid process was removed. A microCT scan (8 μm pixel resolution [PR]) in an approximate parasagittal plane through the mandibular teeth was completed for each sample, producing a buccal-to-lingual view of the rat mandible that parallels the view customarily obtained in human periapical films. Ten evenly-spaced 8μm PR slices that contain a clear view of the interdental bone between molar (M) 1 and M2 were obtained from each rat. Vertical ABH loss was measured in the mandibular interproximal space M1-M2 in the customary fashion (distance from a line connecting the CEJs of adjacent teeth to the ABC) in up to ten 8μm slices that subtended the buccal-to-lingual dimension. Furthermore, a 3D reconstruction of the microCT scan of the whole rat mandible was performed to display horizontal alveolar bone on the buccal and lingual aspects of the first and second molars (Liu et al. 2008). In addition, bone mineral density (BMD) and bone volume (BV/TV) of alveolar bone were also assessed. All microCT measurements were performed in a blinded and randomized fashion.
Microscopic Analyses
Five μm decalcified paraffin-embedded sections of maxillae from males and mandibles from females rats were cut in a mesio-distal plane, stained with H&E, and examined in a blind-coded fashion. As described for the vertical ABL assessment, sectioning was performed from the palatal towards the buccal surface. The block was oriented so as to visualize the mesial and distal roots of all molars as an indicator of proper alignment. Ten consecutive sections obtained from each rat that were adequately aligned and contained alveolar bone processes from the palatal surface were used for microscopic analyses. An inflammation scoring system was used, ranging from 0–4, where 0 corresponded to no histological lesions, 1 to slight, 2 to mild, 3 to moderate and 4 to severe inflammatory lesions of the periodontium. Details regarding this scoring system are listed in Table 2.
Table 2.
Inflammation scoring system used to characterize periodontal lesions in maxillae and mandibles of rice rats.
| Score | degree | Lesions |
|---|---|---|
| 0 | absence | None |
| 1 | slight | Gingivitis: slight hyperplasia of GE, intraepithelial inflammatory cell infiltration. Accumulation of bacterial plaque. No substantial changes in the LP, PDL or ABC. |
| 2 | mild | Gingival hyperplasia, inflammatory cell infiltration of the GE and LP, accumulation of bacterial plaque. No substantial changes in the PDL or ABC. |
| 3 | moderate | Erosion/ulceration and hyperplasia of the GE and accumulation of bacterial plaque. Moderate inflammatory cell infiltration of the LP, disruption of the PDL, migration of the junctional epithelium, and ABC resorption. |
| 4 | severe | Ulceration and hyperplasia of the GE. Severe inflammatory cell infiltration of the LP, disruption of the PDL, migration of the junctional epithelium, and ABC resorption and osteolysis. |
GE: gingival epithelium, LP: lamina propria, PDL: periodontal ligament and ABC: alveolar bone crest.
Statistical analysis
Data are expressed as mean ± SD for each group. Data were evaluated with ANOVA followed by the Holm-Sidak test for multiple comparisons. When ANOVA assumptions regarding normality of data were not met, the non-parametric Kruskal-Wallis test was used. Differences in variables between rice rats fed the STD and H-SC diets were evaluated specifically at each time point using the unpaired Student’s t-test. Regardless of the test employed, P values less than 0.05 were considered to be statistically significant.
Results
We observed a rapid adaptation of the animals to their new environment and none of the animals developed any disease or underlying clinical condition. Rice rats fed the STD and H-SC diets consistently grew and increased body weight (BW) from weaned age (4 wks of age) to the end of the study (22 wks of age). Male and female rice rats had similar BW at weaning (38.1±3.6 and 34.8 ±1.78 grams, respectively). However, males became progressively larger in size and heavier than females after 8 wks of age. By age 22 wks, females were significantly smaller (about 40%) compared to males (54.2 ± 5.63 and 86.7 ± 7.97 grams, respectively). Importantly, there were no significant differences in BW between rats fed the H-SC diet compared to those fed the STD diet, in both males and females, at any time points (data not shown).
Maxillae
We found no gross changes in maxillae of rice rats fed the STD diet at any time point. The histologic examination revealed either total absence or presence of slight lesions in the periodontium of rats fed the STD diet for 6–18 wks (Figure 2A). Only one rat developed mild lesions after 12 wks. The H-SC diet generally induced slight periodontal lesions in rats fed for 6 wks, and slight to mild lesions in those fed for 12 and 18 wks. Moderate to severe changes were observed in 2 of 18 (11%) and in 2 of 19 (5.2%) rats, both genders represented, fed the H-SC diet for 12 and 18 wks, respectively. When present, moderate to severe changes were predominantly gingivitis, characterized by erosion/ulceration of the ginvival epithelium (GE), accumulation of bacterial plaque, moderate inflammatory cell infiltration of the lamina propria, disruption of the periodontal ligament (PDL), migration of the junctional epithelium, and ABC resorption (Table 2).
Figure 2.
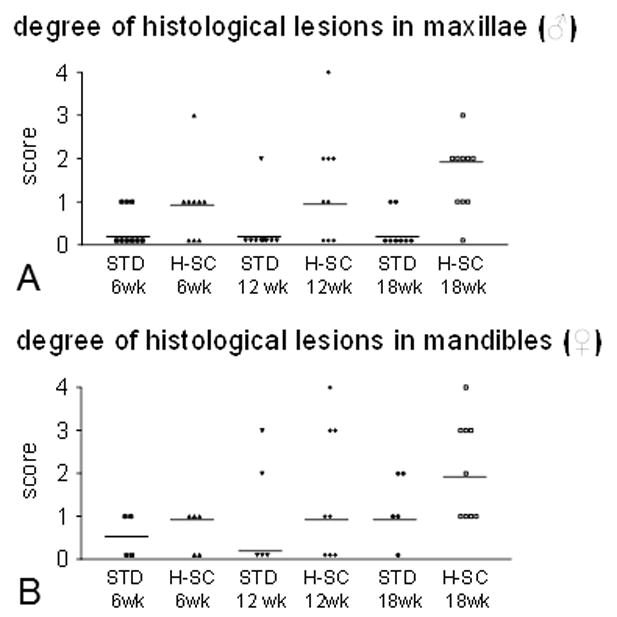
The high sucrose and casein (H-SC) diet induces predominantly slight to moderate periodontal lesions in maxillae and mandibles in rice rats after 12 and/or 18 wks. Degree of periodontal lesions in maxillae of male rats (A) and mandibles of female rats (B) fed the standard (STD) or the H-SC diets for 6, 12 and 18 wks. An inflammation scoring system was used, ranging from 0–4, where 0 corresponds to no histological lesions, 1 to slight, 2 to mild, 3 to moderate and 4 to severe inflammatory lesions affecting some or all of the periodontal structures. Details regarding this scoring system are described in Table 2. Each symbol represents an individual rat. Horizontal lines represent the median for each experimental group.
Morphometric analysis revealed a progressive increase in horizontal ABL with age in males and females at the palatal and buccal surfaces in rats fed the STD diet (Figures 3A–D and Figure 4). The H-SC diet further increased horizontal ABL at the palatal surface, at 12 and 18 wks in males but only at 12 wks in females (Figures 3A, 3C, and 4A–F). However, the H-SC diet did not induce horizontal ABL at the buccal surface in either gender at any time (Figures 3B and 3D). Furthermore, no significant differences were observed in periodontal intrabony defects between rats fed the H-SC and STD diets at any time (data not shown).
Figure 3.
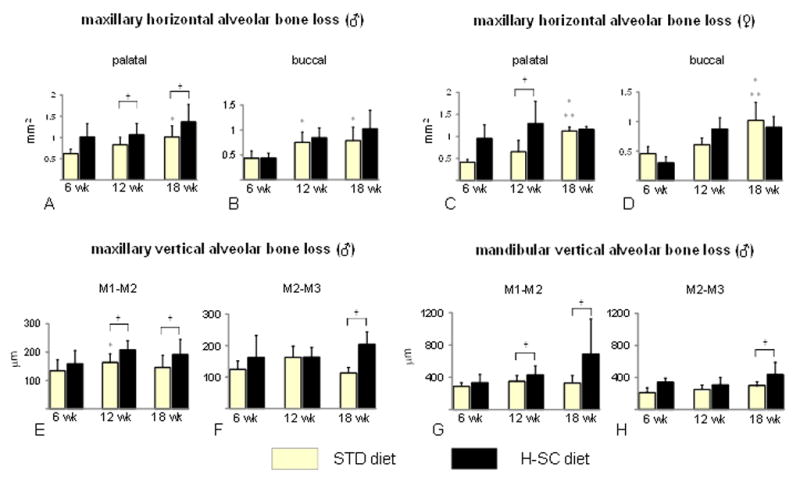
The high sucrose and casein (H-SC) diet induces increased horizontal and vertical alveolar bone loss (ABL) in rice rats after 12 and/or 18 wks. Maxillary horizontal ABL at the palatal surface of males (A), buccal surface of males (B), palatal surface of females, (C) and buccal surface of females (D). Maxillary vertical ABL at the interproximal space between the first and second molars (M1–M2) in males (E), and between the second and third molars (M2–M3) in males (F) fed the STD and the H-SC diets for 6, 12 and 18 weeks, respectively. Mandibular vertical ABL at the interproximal space of M1–M2 (G) and M2–M3 (H) in male rice rats fed the STD and the H-SC diets for 6, 12 and 18 weeks. An asterisk denotes a significant difference from the STD diet at 6 weeks. Two asterisks denote a significant difference from the STD diet at 12 weeks. A cross (†) above brackets indicates a significant difference from its respective time-matched STD control group. (P < 0.05).
Figure 4. The high sucrose and casein (H-SC) diet increases horizontal and vertical alveolar bone loss (ABL) in rice rats.
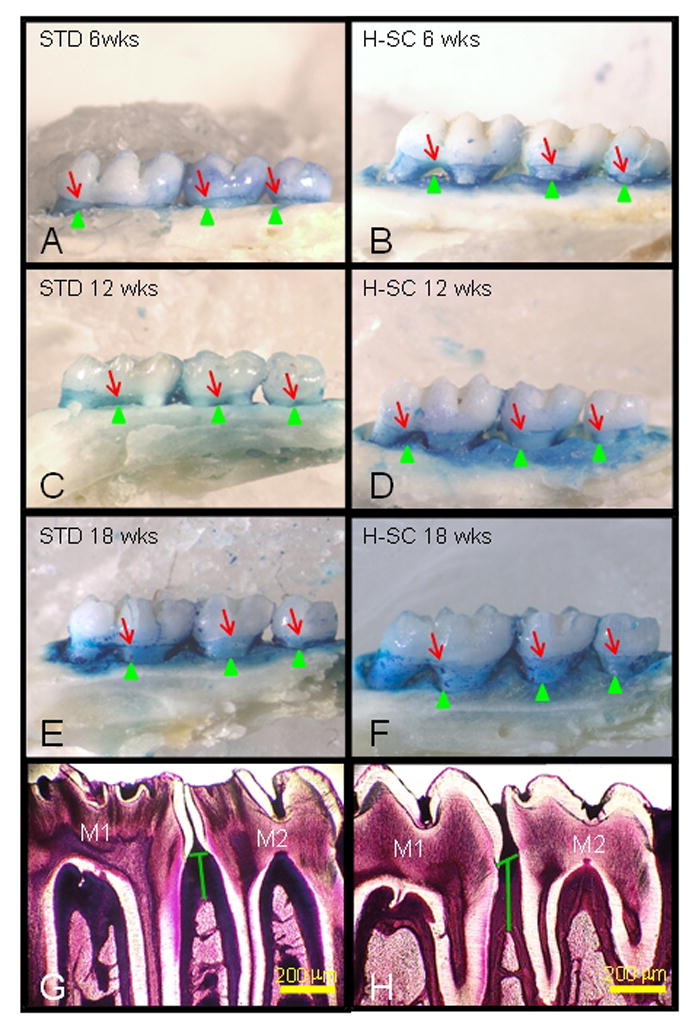
Comparative images stained with methylene blue to delineate the cementoenamel (CEJ) junction (red arrows) from the maxillary palatal surface of male rice rats fed the standard (STD) or H-SC diets for 6 wks (A and B), 12 wks (C and D) and 18 wks (E and F), respectively. The green arrowheads depict the borders of the alveolar bone crest (ABC). The blue-stained area corresponds to the area of exposed tooth root that can serve as an index of horizontal alveolar bone height. Note the progressive increase in the blue-stained area from rat fed the STD diet for 6 wks (A) to 12 wks (C) and 18 wks (E). Also note that rats fed the H-SC diet exhibited a further increase in the blue surface area, particularly after 12 (D) and 18 wks (F), compared to their time-matched controls (C and E, respectively). Figure H depicts increased mandibular vertical ABL (green vertical line) at the interproximal alveolar bone at molar (M) 1 and M2 in a male rat fed the H-SC diet for 18 wks compared to an age-matched control rat fed the STD diet (G). Methacrylate embedded section stained with en bloc basic fuchsin. Bars = 200 μm (G, H).
Maxillary vertical ABL was not observed in male rats fed the STD diet (Figures 3E and 3F). However, the H-SC diet increased maxillary vertical ABL at the interproximal alveolar bone between M1–M2 in rats fed for 12 wks and between M1–M2 and M2–M3 in rats fed for 18 wks (Figures 3E and 3F).
Mandibles
Mandibles from rice rats fed the STD diet had no gross changes at any time. In contrast, moderate to severe changes were observed in 3 of 16 rats (18.75%) and in 3 of 18 rats (16.7%) fed the H-SC diet for 12 and 18 wks, respectively. Moderate/severe changes were observed only in females and were characterized by erosion or ulceration of the gingiva at the lingual surface, particularly between M1–M2, molar furcation and loss of tooth support. Histologic examination revealed none or slight inflammation in rice rats fed the STD diet for 6–18 wks (Figure 5). Only a few rats from the STD diet groups developed mild to moderate inflammation at 12 and 18 wks. The H-SC diet induced predominantly slight inflammation in rats fed for 6 and 12 wks, and slight to moderate inflammation in those fed for 18 wks (Figure 2B, and 5). In only 1 of 8 and in 1 of 9 female rats, severe inflammation was found in their mandibles after feeding the H-SC diet for 12 and 18 wks, respectively (Figures 2B, 5G, and 5H).
Figure 5.
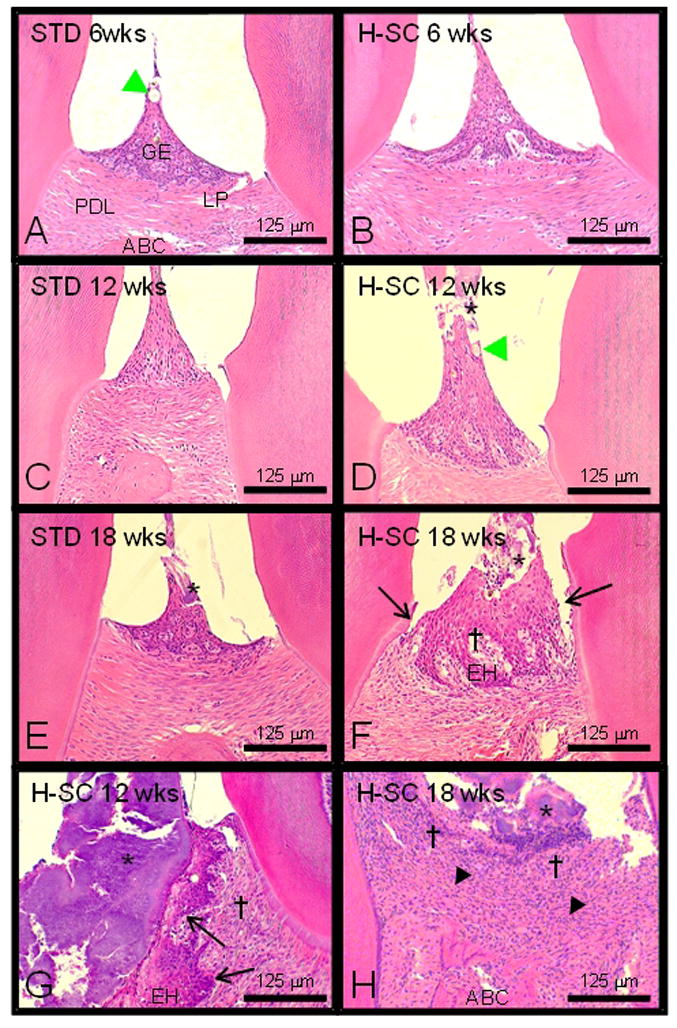
The high sucrose and casein (H-SC) diet induces slight to moderate periodontitis in the mandibles of rice rats fed for 12 wks and 18 wks. Comparative photos taken at the interproximal space between the first and second mandibular molars of rice rats fed standard (STD) or H-SC diets for 6 wks (A and B), 12 wks (C and D & G), and 18 wks (E and F & H), respectively. Periodontal lesions were absent or slight in rice rats fed the standard (STD) diet for 6–12 wks and the H-SC diet for 6 wks. A slight degree of inflammation of the periodontium is characterized by slight hyperplasia of the gingival epithelium (GE) with intraepidermal inflammatory cell infiltration and hair shafts or vegetable fibers impacted (green arrowhead) within the gingival epithelium. No substantial changes were observed in the lamina propria (LP), the periodontal ligament (PDL) or the alveolar bone crest (ABC). Periodontal lesions were predominantly slight to moderate (Table 2) in rice rats fed the H-SC diet for 12 wks and 18 wks. Bacterial plaques (*) were frequently observed in rats fed the H-SC diet for 12 and 18 wks, but also in those fed the STD diet for 18 wks (Figures D–H). Figure F illustrates accumulation of bacterial plaque (*), mild migration of the junctional epithelium (black arrow), GE hyperplasia (EH), and mild inflammatory cell infiltration in the LP (†) of a rat fed the H-SC diet for 18 wks. In only 1 of 8 and in 1 of 9 female rats, severe lesions were found in their mandibles after feeding the H-SC diet for 12 and 18 wks, respectively. Figure G illustrates a massive accumulation of bacterial plaque (*) in the interproximal area, disruption of the gingival papilla, EH with migration of the junctional epithelium (black arrow), and inflammatory cell infiltration in the LP (†) of a rat fed the H-SC diet for 12 wks. Figure H shows a severe disruption of the periodontium in one rat fed the H-SC diet for 18 wks. Bacterial plaque (*) is observed in the interproximal area, ulceration of the GE, abundant inflammatory cell infiltration in the LP (†), disruption of the PDL (▲), and alveolar bone osteolysis. Figures were taken at 200X magnification. H&E stain. Bars = 125 μm.
The histometric analysis revealed no progressive vertical ABL with age in males fed the STD diet (Figures 3G and 3H). The H-SC diet significantly enhanced vertical ABL at the interproximal alveolar bone at M1–M2 at 12 wks, and at M1–M2 and M2–M3 at 18 wks (Figures 3G, 3H, 4G and 4H).
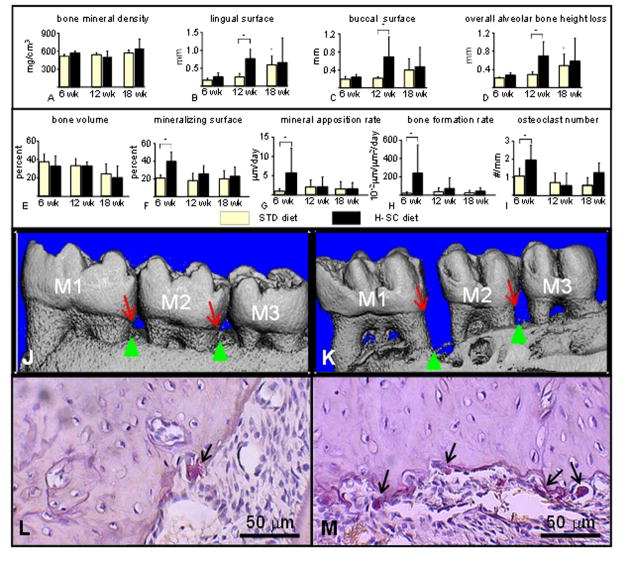
The microCT analysis performed in mandibles of female rats revealed no differences in alveolar bone mineral density between rats fed the H-SC and STD diets at any time points (Figure 6A). However, there was a spontaneous and progressive increase in vertical ABL with age at the lingual surface and overall decreased alveolar bone height at the interproximal surface, but not at the buccal surface, in rats fed the STD diet (Figures 6B–D). Remarkably, the H-SC diet further increased vertical ABL at the lingual and buccal surfaces, and decreased overall alveolar bone height at the interproximal region at 12 wks but not 18 wks (Figures 6B–D, 6J, and 6K). The histomorphometric analysis showed increased indices of alveolar bone turnover such as mineralizing surface (90%), mineral apposition rate (500%), bone formation rate (1080%), eroded surface (128%) and osteoclast number (84%), but no changes in alveolar bone volume in mandibles of male rats fed the H-SC diet at 6 wks, but not at 12–18 wks (Figures 6E–I, 6L, and 6M).
Figure 6.
The high sucrose and casein (H-SC) diet increases mandibular vertical alveolar bone loss (ABL) in female rice rats after 12 wks, and increases alveolar bone remodeling at 6 wks but not at 12 and 18 wks in male rice rats. A microCT analysis was performed in the mandibles of female rats fed the standard (STD) and H-SC diets for 6, 12 and 18 weeks (A–D). Bone mineral density (BMD) (A), mandibular vertical ABL at the lingual surface (B), mandibular vertical ABL at the buccal surface (C) and overall alveolar bone height loss (D). All measurements were performed at the interproximal space between the first and second molars (M1–M2). A histomorphometric analysis was performed in the mandibles of male rats fed the standard (STD) and H-SC diets for 6, 12 and 18 weeks (E–I). Bone volume (BV/TV) (E), mineralizing surface (F), mineral apposition rate (G), bone formation rate (H), and osteoclast number (I). All measurements were performed within the region of interest (ROI) described in Figure 1. An asterisk denotes a significant difference from the STD diet at 6 weeks. A cross (†) above brackets indicates a significant difference from its respective time-matched STD control group. (P < 0.05). Comparative reconstructed microCT images taken from mandibles of female rice rats fed the STD (J) or the H-SC diets (K) for 12 weeks, respectively. Note the increased vertical ABL at the lingual surface in the interproximal spaces between molar (M) 1 and M2 and M2–M3 in a rat fed the H-SC diet (K) compared to an age-matched control rat (J). Red arrows depict the cementoenamel junction (CEJ) and green arrows the alveolar bone crest (ABC). Representative photos of histological sections stained for tartrate-resistant acid phosphatase (TRAP) taken at the mandibular interproximal space M1–M2 of a female rice rat fed the STD (L) or H-SC (M) diets for 6 wks, respectively. Note the presence of numerous big multinuclear or mononuclear purple stained osteoclasts (TRAP positive; black arrows) on an scalloped alveolar bone surface in a rat fed the H-SC diet for 6 wks (M) compared to the presence of only one TRAP positive cell (black arrow) on a smoother alveolar bone surface of a rice rat fed the STD diet for 6 wks (L). Figures L and M were taken at 400X magnification. H&E stain. Bars = 50 μm.
Discussion
We have chosen to reexamine the rice rat model of periodontitis because past reports indicate that these rats appear to develop periodontitis that is similar in location and appearance to that in humans (Gotcher & Jee 1981a; Gupta & Shaw 1956a; Gupta & Shaw 1956b; Leonard 1979; Ryder 1980), without invasive procedures such as ligature placement or gingival lipopolysaccharide injection (Rovin et al. 1966; Sartori et al. 2009).
The primary focus of our study was to assess the irreversible damage induced by the H-SC diet to the alveolar bone. Previous studies (Gotcher & Jee 1981a; Gupta & Shaw 1956a; Gupta & Shaw 1956b) did not assess all the variables reported in the current study. Nevertheless, based on their descriptions and figures, we infer that they found more prominent periodontal lesions than those observed in our study. We propose that the following factors are responsible for the reduced severity observed in our study: 1) slight variations in the composition of the formulated diets, 2) change in the physical consistency of the diet (pelleted versus powdered diets), 3) genetic variation in the susceptibility to develop periodontitis between the rats in our colony and those used in previous studies, or 4) a combination of the above factors. Our findings are important since mild to moderate periodontal lesions, progressing at the rate observed in our study, may more closely mimic the periodontitis observed in humans, and permit various types of prevention or regeneration studies with therapeutic interventions.
Another important difference between the current and previous studies was that we reported data separately by gender. Our study showed that the H-SC diet induced horizontal and vertical ABL in both genders. However, we observed gender differences in the magnitude or time period when ABL occurred. Gender is important since rice rats exhibit a marked sexual dimorphism. Whereas at 4 wks of age, males and females have similar body weights, by age 22 wks, females are significantly smaller (about 40%) compared to males. Gender dimorphism affects not only the size of tissues and organs, particularly the size of jaw bones, but also the absolute values of variables that were analyzed at the alveolar crest and interproximal alveolar bone. For instance, male and female rice rats fed the STD pelleted diet experienced horizontal ABL with age, imparting a spontaneous element to the development of periodontitis in rice rats. This age-related feature was previously described in mice (Liang et al. 2010), but not in rice rats. However, only female rice rats had significantly increased horizontal and vertical ABL with the STD diet between week 12 and week 18 of treatments. Furthermore, the H-SC diet exacerbated maxillary horizontal ABL at the palatal surface at 12 wks in both genders but only at 18 wks in male rice rats as assessed by morphometry. Moreover, the H-SC diet accelerated maxillary and mandibular vertical ABL in male rice rats at 12 and 18 wks as assessed by morphometry. Although determined by a different method (microCT), the H-SC diet increased mandibular vertical ABL in female rats at 12 wks but not at 18 wks. These gender differences in the expression of ABL were unexpected, but at the same time interesting.
These data together suggest that female rice rats are more sensitive to ABL with age compared to males since females, but not males, spontaneously develop increasing ABL after 12 wks of treatment with the STD diet. These results also suggest that in female rats that had substantial vertical ABL with age (22 wks of age), the diet effect is null or minimal. These findings are significant since rice rats, particularly females, fed a STD diet may reproduce the natural development of ABL as a function of age, as observed in some humans (Muller and Ulbrich 2005; Persson et al. 1998; Shapira et al. 1995). These findings support the use of female rice rats fed a STD diet as a spontaneous rat model of horizontal ABL characteristic of slight to mild periodontitis. Our data also support the contention that rice rats fed a pelleted H-SC diet appear to be a good small animal model for mimicking mild to moderate periodontitis in humans since both species experience horizontal and vertical ABL (Newman et al. 2006; Persson et al. 1998).
Periodontal disease is a chronic immuno-inflammatory infectious disease induced by pathogenic biofilms containing numerous pathogens. Among the periodontal pathogens in humans, Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Provotella intermedia are the most frequent bacteria isolated from patients with periodontal disease (Clerehugh et al. 1997; Lopez 2000; Slots and Listgarten 1988). Conversely, the pathophysiology of periodontal disease in the rice rat is still to be defined. Nevertheless, several lines of evidence have strongly suggested an infectious etiology. For instance, early microbiological studies found a predominant bacterial flora composed of Enterococcus sp., Coli-Aerogenes sp., Actinomyces sp., Lactobacillus sp., aerobic diphteroids and Staphylococcus sp. in the oral cavity of rice rats fed a HSC diet (MacDonald et al. 1960). Furthermore, periodontal disease scores positively correlated with the Enterococci and Actinobacillus counts obtained from affected rice rats (Socransky et al. 1960). Moreover, periodontal disease was reproduced by oral inoculation of a fecal paste or oral inoculum of four specific microorganisms from periodontitis-susceptible rice rats to periodontitis-resistant animals (Dick et al. 1968; Dick and Shaw 1966). In addition, dietary supplementation with antibiotics results in reduction or elimination of soft tissue lesions and a delay in the progression of alveolar bone loss in rice rats (Shaw 1965; Shaw and Dick 1969).
Taken together these data permit one to advance the following pathophysiological mechanism for the rice rat model: 1) prolonged feeding with a H-SC diet creates an optimal microenvironment (pH, acidity, nutrients, metabolites, etc) favoring putative microorganism/s to proliferate, 2) pathogenic microorganisms produce multiple virulence factors that activate the host imflammatory response, 3) the host responds with recruitment of inflammatory cells in the periodontium, with generation of cytokines and prostanoids and induction of osteoclastogenesis, and 4) this is followed by destruction of soft tissues and alveolar bone loss. Further studies will be necessary to determine the etiology and better define the pathophysiology of periodontal disease in rice rats induced by the H-SC diet. However, it is clear, based on past and present studies, that rice rats fed a H-SC diet for 12–18 wks consistently exhibit progressive alveolar bone loss accompanied by gingival recession, which are distinctive pathological lesions observed in human periodontal disease.
In conclusion, our data support the use of rice rats, particularly females, fed a STD diet as a small animal model of spontaneous horizontal ABL observed in humans. They also suggest that rice rats fed a pelleted H-SC diet are a useful small animal model for accelerated horizontal and vertical ABL (moderate periodontitis) observed in humans with early or late onset periodontitis (Armitage and Cullinan 2010). With their low body weight and apparent ability to develop both spontaneous and induced periodontitis without a requirement for local mechanical intervention, the rice rat could be an attractive model for testing the effect of systemic medications on the progression and reversal of periodontal lesions. These findings also indicate that the H-SC diet induces a transient increase in alveolar bone remodeling that is followed by accelerated horizontal ABL and the initiation of vertical ABL that is characteristic of moderate periodontitis in humans.
Acknowledgments
This research was supported by NIH grant R03DE018924-01A1 from the National Institute of Dental and Craniofacial Research (NIDCR).
Footnotes
Conflict of interest
The authors have no conflicts of interest.
Contributor Information
J. I. Aguirre, Email: aguirrej@ufl.edu.
M. P. Akhter, Email: mohammedakhter@creighton.edu.
D. B. Kimmel, Email: kimmeldb@comcast.net.
J. Pingel, Email: jpingel@ufl.edu.
X. Xia, Email: xxia@ufl.edu.
A. Williams, Email: williamsaly@ufl.edu.
M. Jorgensen, Email: marda@ufl.edu.
K. Edmonds, Email: kedmonds@ius.edu.
J. Y. Lee, Email: heroine@pusan.ac.kr.
M. K. Reinhard, Email: reinhard@ufl.edu.
A. H. Battles, Email: abattles@ufl.edu.
L. Kesavalu, Email: kesavalu@dental.ufl.edu.
T. J. Wronski, Email: wronskit@ufl.edu.
References
- 1.Allen MR. Animal models of osteonecrosis of the jaw. J Musculoskelet Neuronal Interact. 2007;7 (4):358–360. [PubMed] [Google Scholar]
- 2.Allen MR, Burr DB. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res. 2007;22 (11):1759–1765. doi: 10.1359/jbmr.070720. [DOI] [PubMed] [Google Scholar]
- 3.Armitage GC, Cullinan MP. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:12–27. doi: 10.1111/j.1600-0757.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- 4.Auskaps A, Gupta O, Shaw J. Periodontal disease in the rice rat. III. Survey of dietary influences. J Nutr. 1957;63 (3):325–343. doi: 10.1093/jn/63.3.325. [DOI] [PubMed] [Google Scholar]
- 5.Bainbridge B, et al. Role of Porphyromonas gingivalis phosphoserine phosphatase enzyme SerB in inflammation, immune response, and induction of alveolar bone resorption in rats. Infect Immun. 2010;78 (11):4560–4569. doi: 10.1128/IAI.00703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron RA, Vignery A, Neff L, et al. Processing of undecalcified bone specimens for bone histomorphometry. In: Recker RR, editor. Bone Histomorphometry: Techniques and Interpretation. 1. CRC Press; Boca Raton, FL: 1983. pp. 13–35. [Google Scholar]
- 7.Burr DB, Hooser M. Alterations to the en bloc basic fuchsin staining protocol for the demonstration of microdamage produced in vivo. Bone. 1995;17 (4):431–433. doi: 10.1016/s8756-3282(95)00241-3. [DOI] [PubMed] [Google Scholar]
- 8.Clerehugh V, et al. The detection of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia using an ELISA in an adolescent population with early periodontitis. J Clin Periodontol. 1997;24 (1):57–64. doi: 10.1111/j.1600-051x.1997.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 9.Dick DS, Shaw JH, Socransky SS. Further studies on the microbial agent or agents responsible for the periodontal syndrome in the rice rat. Arch Oral Biol. 1968;13 (2):215–228. doi: 10.1016/0003-9969(68)90053-8. [DOI] [PubMed] [Google Scholar]
- 10.Dick DS, Shaw JR. The infectious and transmissible nature of the periodontal syndrome of the rice rat. Arch Oral Biol. 1966;11 (11):1095–1108. doi: 10.1016/0003-9969(66)90167-1. [DOI] [PubMed] [Google Scholar]
- 11.Frost HM. Bone Histomorphometry: Analysis of trabecular bone dynamics. In: Recker RR, editor. Bone Histomorphometry: Techniques and Interpretation. 1. CRC Press; Boca Raton, FL: 1983. pp. 109–132. [Google Scholar]
- 12.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat. I. Morphometric and autoradiographic studies. J Periodontal Res. 1981a;16 (3):275–291. doi: 10.1111/j.1600-0765.1981.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 13.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat. II. The effects of a diphosphonate on the periodontium. J Periodontal Res. 1981b;16 (4):441–455. doi: 10.1111/j.1600-0765.1981.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta O, Shaw J. Periodontal disease in the rice rat. I. Anatomic and histopathologic findings. Oral Surg Oral Med Oral Pathol. 1956a;9 (6):592–603. doi: 10.1016/0030-4220(56)90319-x. [DOI] [PubMed] [Google Scholar]
- 15.Gupta O, Shaw J. Periodontal disease in the rice rat. II. Methods for the evaluation of the extent of periodontal disease. Oral Surg Oral Med Oral Pathol. 1956b;9 (7):727–735. doi: 10.1016/0030-4220(56)90249-3. [DOI] [PubMed] [Google Scholar]
- 16.Gupta O, Shaw J. The relation of a chelating agent to smooth-surface lesions in the white rat. J Nutr. 1956c;60 (3):311–322. doi: 10.1093/jn/60.3.311. [DOI] [PubMed] [Google Scholar]
- 17.Hattler AB, et al. The lack of pulpal pathosis in rice rats with the periodontal syndrome. Oral Surg Oral Med Oral Pathol. 1977;44 (6):939–948. doi: 10.1016/0030-4220(77)90038-x. [DOI] [PubMed] [Google Scholar]
- 18.Leonard EP. Periodontitis. Animal model: periodontitis in the rice rat (Oryzomys palustris) Am J Pathol. 1979;96 (2):643–646. [PMC free article] [PubMed] [Google Scholar]
- 19.Liang S, et al. Periodontal inflammation and bone loss in aged mice. J Periodontal Res. 2010;45 (4):574–578. doi: 10.1111/j.1600-0765.2009.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu XM, et al. Osteotropic beta-cyclodextrin for local bone regeneration. Biomaterials. 2008;29 (11):1686–1692. doi: 10.1016/j.biomaterials.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez NJ. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia in progressive adult periodontitis. J Periodontol. 2000;71 (6):948–954. doi: 10.1902/jop.2000.71.6.948. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald JB, Socransky SS, Sawyer SJ. Pathogenicity experiments with the flora of the periodontium in rice rats. J Dent Res. 1960;39:861–867. doi: 10.1177/00220345600390041601. [DOI] [PubMed] [Google Scholar]
- 23.Muller HP, Ulbrich M. Alveolar bone levels in adults as assessed on panoramic radiographs. (I) Prevalence, extent, and severity of even and angular bone loss. Clin Oral Investig. 2005;9 (2):98–104. doi: 10.1007/s00784-005-0303-x. [DOI] [PubMed] [Google Scholar]
- 24.Newman MG, Takei HH, Klokkevold PR, et al. In: Epidemiology of gingival and periodontal diseases. 10. Newman MG, et al., editors. Saunders Elsevier; St. Louis, MO: 2006. pp. 110–131. [Google Scholar]
- 25.Parfitt AM, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2 (6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 26.Park C, et al. Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. J Periodontol. 2007;78 (2):273–281. doi: 10.1902/jop.2007.060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persson RE, et al. Horizontal alveolar bone loss and vertical bone defects in an adult patient population. J Periodontol. 1998;69 (3):348–356. doi: 10.1902/jop.1998.69.3.348. [DOI] [PubMed] [Google Scholar]
- 28.Rajapakse PS, et al. Immunization with the RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis protects against periodontal bone loss in the rat periodontitis model. Infect Immun. 2002;70 (5):2480–2486. doi: 10.1128/IAI.70.5.2480-2486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rovin S, Costich ER, Gordon HA. The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. J Periodontal Res. 1966;1 (3):193–204. doi: 10.1111/j.1600-0765.1966.tb01860.x. [DOI] [PubMed] [Google Scholar]
- 30.Ryder MI. Histological and ultrastructural characteristics of the periodontal syndrome in the rice rat. I. General light microscopic observations and ultrastructural observations of initial inflammatory changes. J Periodontal Res. 1980;15 (5):502–515. doi: 10.1111/j.1600-0765.1980.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 31.Sartori R, Li F, Kirkwood KL. MAP kinase phosphatase-1 protects against inflammatory bone loss. J Dent Res. 2009;88 (12):1125–1130. doi: 10.1177/0022034509349306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapira L, et al. The relationship between alveolar bone height and age in the primary dentition. A retrospective longitudinal radiographic study. J Clin Periodontol. 1995;22 (5):408–412. doi: 10.1111/j.1600-051x.1995.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 33.Shaw JH. Further studies on the sensitivity of the periodontal syndrome in the rice rat to dietary antibiotics. J Dent Res. 1965;44(2):431–438. doi: 10.1177/00220345650440022101. [DOI] [PubMed] [Google Scholar]
- 34.Shaw JH, Dick DS. Antibiotics and the periodontal syndrome in rice rats. Arch Oral Biol. 1969;14(2):227–230. doi: 10.1016/0003-9969(69)90066-1. [DOI] [PubMed] [Google Scholar]
- 35.Slots J, Listgarten MA. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15 (2):85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 36.Socransky SS, et al. Quantitative studies of the bacterial flora of the periodontium in rice rats. Arch Oral Biol. 1960;2:104–110. doi: 10.1016/0003-9969(60)90058-3. [DOI] [PubMed] [Google Scholar]
- 37.Verma RK, et al. Virulence of major periodontal pathogens and lack of humoral immune protection in a rat model of periodontal disease. Oral Dis. 2010a;16 (7):686–695. doi: 10.1111/j.1601-0825.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 38.Verma RK, et al. Porphyromonas gingivalis and Treponema denticola Mixed Microbial Infection in a Rat Model of Periodontal Disease. Interdiscip Perspect Infect Dis. 2010b;2010:605125–605131. doi: 10.1155/2010/605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinberg MA, Bral M. Laboratory animal models in periodontology. J Clin Periodontol. 1999;26 (6):335–340. doi: 10.1034/j.1600-051x.1999.260601.x. [DOI] [PubMed] [Google Scholar]