Abstract
Retinoic acid decreases proteinuria and glomerulosclerosis in several animal models of kidney disease by protecting podocytes from injury. Our recent in vitro studies suggest that all-trans retinoic acid induces podocyte differentiation by activating the retinoic acid receptor-α (RARα)/cAMP/PKA/CREB pathway. When used in combination with all-trans retinoic acid, an inhibitor of phosphodiesterase 4 further enhanced podocyte differentiation by increasing intracellular cAMP. Additionally, we found that Am580, a specific RARα agonist, has similar renal protective effects as all-trans retinoic acid in a rederived colony of HIV-1 transgenic mice with rapidly progressive renal failure (HIV-Tg) that mimics human HIV-associated nephropathy. Treatment with either the inhibitor of phoshodiesterase 4, roflumilast, or Am580 significantly reduced proteinuria, attenuated kidney injury, and improved podocyte differentiation in these HIV-Tg mice. Additional renal protective effects were found when roflumilast was combined with Am580. Consistent with the in vitro data, glomeruli from HIV-Tg mice treated with both Am580 and roflumilast had more active phosphorylated CREB than with either agent alone. Thus, phosphodiesterase 4 inhibitors could be used in combination with RARα agonists to provide additional renal protection.
Introduction
Glomerular kidney disease is a major cause of End-Stage-Renal-Disease (ESRD) in the United States 1. HIV-associated nephropathy (HIVAN), characterized as collapsing focal segmental glomerulosclerosis (FSGS), is a leading cause of kidney disease in young African Americans 2. Although suppression of viral replication with antiretroviral therapy alters the course of the kidney disease, many patients with HIVAN still progress to ESRD 3. Podocyte injury is a major cause of glomerular disease. Podocytes undergo apoptosis and detachment in diabetic kidney disease and classic FSGS 4,5. Podocyte dedifferentiation and proliferation are considered unique features of HIVAN and idiopathic collapsing FSGS 6,7,8. In vitro, HIV infection causes podocyte proliferation and dedifferentiation 9. Transgenic mice with expression of HIV-1 in the podocytes develop kidney disease similar to HIVAN 10. Therefore, prevention or reversal of podocyte injury is an important strategy to treat HIVAN as well as other glomerular diseases. However, no drugs are available to specifically prevent or reverse podocyte injury.
Retinoids are derivatives of vitamin A and protect against renal injury in multiple experimental models of kidney diseases 11. We found that all trans retinoic acid (ATRA) reduces proteinuria and glomerulosclerosis in a colony of HIV-1 transgenic mice with rapidly progressive renal failure (HIV-Tg), which is an animal model of HIVAN 12. An ongoing phase II clinical trial is examining the effect of ATRA on patients with glomerular diseases with podocyte injury including steroid-resistant minimal change disease, FSGS, and HIVAN (NIDDK website). However, it remains unclear how retinoids improve kidney injury. Recent studies suggest that ATRA restores the expression of podocyte differentiation markers including nephrin, podocin, and synaptopodin both in vitro and in vivo 13. We found that ATRA inhibits cell proliferation and restores differentiation markers in HIV-infected podocytes by activating the cAMP/PKA/CREB pathway 12,14. These anti-proliferative and pro-differentiation effects of RA were further enhanced when used in conjunction with a phosphodiesterase 4 (PDE4) inhibitor, which increases intracellular cAMP concentration by blocking RA degradation 12. These studies suggest that retinoic acid may have additive or synergic effects when given with a PDE4 inhibitor for protection against podocytes injury and treatment of glomerular diseases. Consistent with these findings, it has been shown that ATRA can induce rapid cAMP production and increase PKA activity in acute myeloblastic leukemia cells leading to leukemia cell differentiation 9. A synergistic effect between atRA and a PDE4 inhibitor has also been observed for myeloid differentiation 15.
We had demonstrated previously that retinoic acid increases intracellular cAMP concentration by activating retinoic acid receptor-alpha (RARα) and treatment of HIV-Tg mice with Am580, a RARα specific agonist, induced podocyte differentiation and attenuated kidney injury in HIV-Tg mice 16. Furthermore, knockout of RARα aggravated kidney injury in HIV-Tg mice. Based on these findings, we tested the hypothesis that the combination of a RARα agonist and a PDE4 inhibitor provides additional renal protection by increasing intracellular cAMP and activating the cAMP/PKA/CREB pathway.
Results
1. Effects of Am580 and roflumilast on proteinuria and renal function in HIV-Tg mice
Roflumilast is an oral PDE4 inhibitor recently approved by the FDA for the treatment of asthma. Since proteinuria in HIV-Tg mice usually develops at 4 weeks of age and peaks at 8–10 weeks, we treated HIV-Tg mice with vehicle, Am580, roflumilast, or roflumilast with Am580 starting from 4 weeks of age to 9 weeks of age. No significant side effects were noted during the treatment. Total body weight was not significantly different among the four groups (Fig 1A). The kidney/body weight ratio, which is known to be increased in HIV-Tg, was significantly reduced in HIV-Tg mice treated with either roflumilast or Am580 alone or in combination (Fig 1B). Both Am580 and roflumilast treatments alone prevented the worsening of renal function significantly and reduced the amount of proteinuria compared to vehicle treatment (Fig 2). When Am580 and roflumilast were given together, additional reduction in BUN and urinary albumin/Cr ratio were observed compare to mice treated with either Am580 or roflumilast (Fig 2). The percentage of mice that developed renal failure, which was defined by elevation of BUN that is more than two standard deviations above the mean BUN in the wild-type (WT) group (BUN>30mg/dl) was calculated for each treatment group (Table 1). All of the vehicle-treated HIV-Tg mice developed renal failure. Treatment with either Am580 or roflumilast reduced the percentage of animals with renal failure to 30% and 40%, respectively. None of the HIV-Tg mice treated with both agents both drugs developed renal failure (Table 1). These results suggest that when a RARα agonist (Am580) is given with a PDE4 inhibitor (roflumilast) there is an additive effect on renal protection.
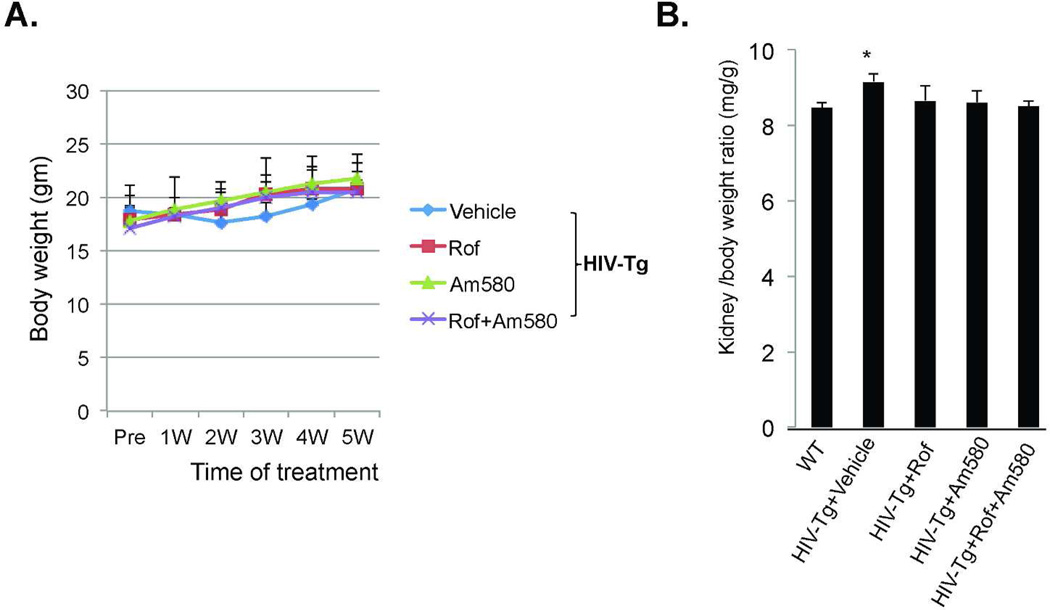
Figure 1.
A. Body weight. HIV-Tg mice were treated with vehicle, Am580, roflumilast (Rof) or Rof+Am580 from age of 4 weeks to 9 weeks for a total of 5 weeks. The body weight was recorded weekly. There was no significant difference among the 4 groups. B. Kidney/body weight ratio. At sacrifice, both body weight and kidney weight were recorded and kidney/body weight ratio was calculated. There is a significant difference among the groups (by ANOVA, p<0.0001). Pair analysis with Bonferroni correction detected a significant difference between the HIV-Tg+Vehicle group vs all other groups (*p<0.0001, n=10).
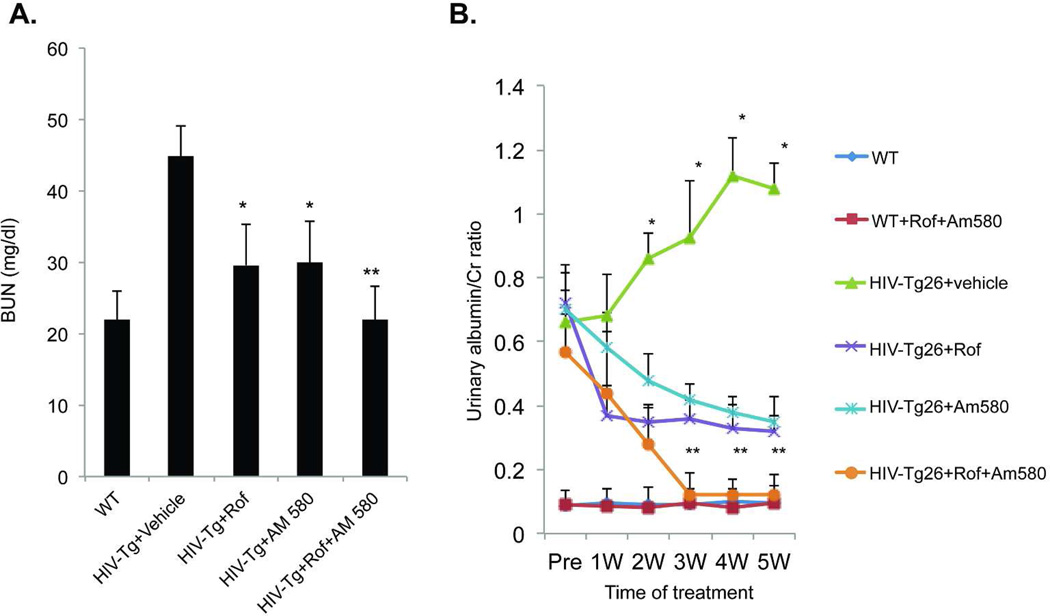
Figure 2. Proteinuria and BUN.
HIV-Tg mice were treated with vehicle, Am580, roflumilast (Rof) or Rof+Am580 from age of 4 weeks to 9 weeks. Serum was used for the determination of BUN (A) and urine was measured for albumin and creatinine to calculate the albumin/creatinine (Cr) ratio (mg/mg) (B). A significant difference in BUN and albumin/Cr ratio was detected among the groups by ANOVA (n=10). Pair-wise comparison with Bonferroni correction revealed significant difference between HIV-Tg+ Vehicle vs. all other groups (*p< 0.0001); HIV-Tg+Rof+Am580 vs HIV-Tg+Rof (**p<0.001); and HIV-Tg+Rof+Am580 vs HIV-Tg+Am580 (**p<0.001).
Table 1.
Effects of Am580 and roflumilast on the development of renal failure in HIV-Tg mice
| No-Renal failure |
Renal failure |
% of renal failure |
|
|---|---|---|---|
| WT | 10 | 0 | 0 |
| HIV-Tg+Vehicle | 0 | 10 | 100* |
| HIV-Tg+roflumilast | 6 | 4 | 40 |
| HIV-Tg+Am580 | 7 | 3 | 30 |
| HIV-Tg+roflumilast+Am580 | 10 | 0 | 0** |
The percentage of mice that developed renal failure in each group was determined. Renal failure was defined by elevation of BUN that is more than two standard deviations above the mean Bun in the wild type (WT) group (BUN>30mg/dl). Paired chi square analysis was used to calculate the difference between the groups.
p<0.05 for HIV-Tg+vehicle vs. all other groups;
p<0.05 for HIV-Tg+roflumilast+Am580 vs. HIV-Tg+roflumilast, and HIV-Tg+roflumilast+Am580 vs. HIV-Tg+Am580, n=10.
2. Effects of Am580 and/or roflumilast on kidney histology of HIV-Tg mice
To assess the effects of Am580 and/or roflumilast on kidney injury, histologic analysis was performed on H&E-stained kidney sections as described previously 16. We found that Am580 or roflumilast alone attenuated glomerulosclerosis, podocyte hypertrophy, and tubular cast/cyst formation. When given in combination, protection against renal injury in addition to what was observed for each agent alone was observed (Fig 3A and Table 2). Consistent with this, a reduction of foot process effacement was also observed by EM in HIV-Tg mice treated with both roflumilast and Am580 (Figure 3B).
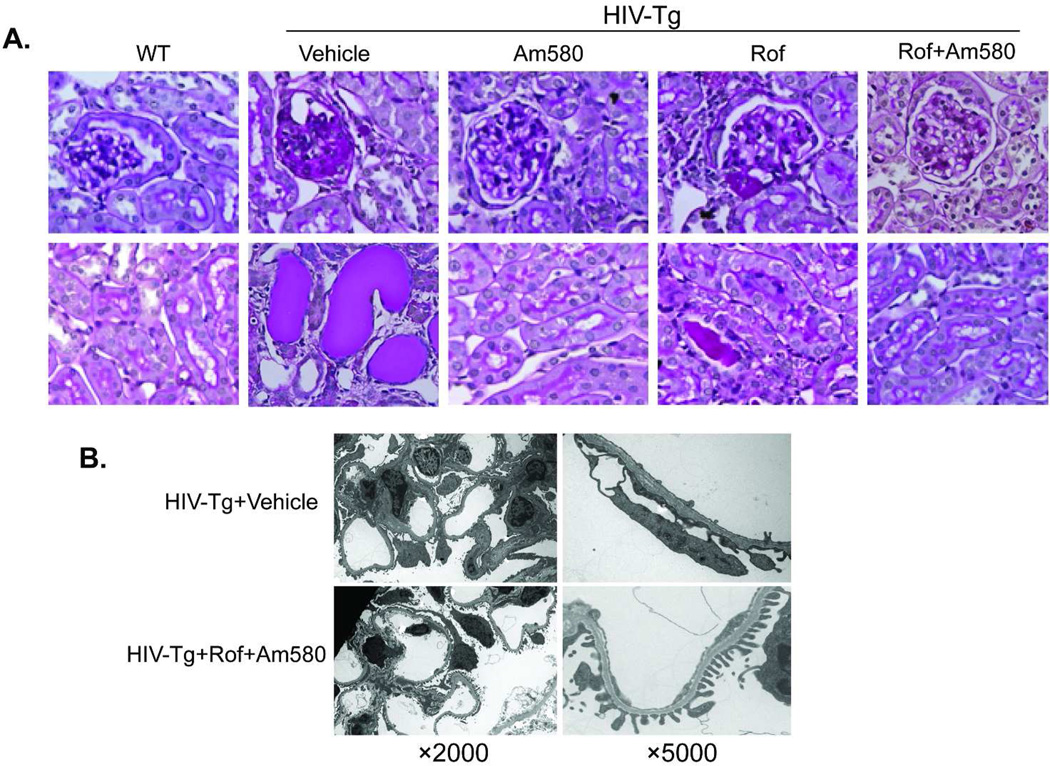
Figure 3. Kidney histology.
A. HIV-Tg mice were treated with vehicle, Am580, roflumilast (Rof), or Rof+Am580 from age of 4 weeks to 9 weeks. H&E staining of kidney sections at the end of the study performed. HIV-Tg+Vehicle had severe glomerulosclerosis, tubular microcysts and casts. These pathologic changes were less frequently observed in mice treated with Am580, Rof, or Rof+Am580. Some mice treated with Rof or Am580 alone deveoped mild glomerulosclerosis and tubular casts. B. Electron microscopy shows a significant improvement of foot process effacement in HIV-Tg+Rof+Am580 compared to HIV-Tg+vehicle. Representative pictures are shown here.
Table 2.
Effects of Am580 and roflumilast on kidney histology
| CG index | Podocyte Hypertrophy |
Tubular casts/cysts | |
|---|---|---|---|
| HIV-Tg+Vehicle | 15.6±6.2 | 1.5±0.9 | 10.4±4.2 |
| HIV-Tg+Am580 | 3.6±2.3* | 0.5±0.5* | 2.8±3.3* |
| HIV-Tg+roflumilast | 4.6±3.0* | 0.7±0.6* | 3.8±3.2* |
| HIV-Tg+Am580+roflumilast | 1.6±0.8** | 0** | 0** |
HIV-Tg mice were treated with Am580 or roflumilast alone or in combination for 5 weeks as compared to the mice treated with vehicle. Kidney histology of these mice was analyzed as described in the method, n=10. We performed non-parametric Mann-Whitney test between pairs and found
p<0.05 when we compared HIV-Tg mice treated with either Am580 or roflumilast alone to the mice treated with vehicle and
p<0.05 when we compared HIV-Tg mice treated with both drugs to the mice treated with Am580 or roflumilast alone. CG: collapsing glomerulosclerosis.
3. Effect of Am580 and/or roflumilast on the expression of podocyte differentiation markers in HIV-Tg mice
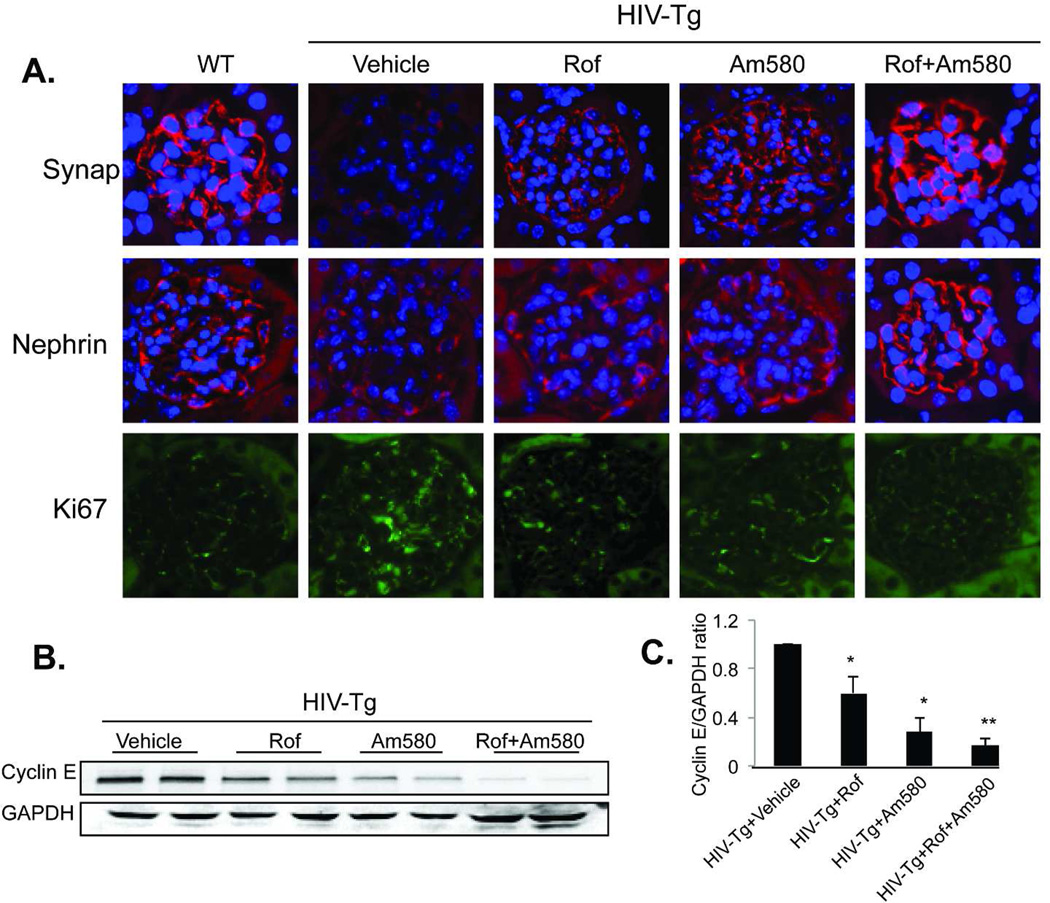
To determine the effects of Am580 and/or roflumilast on podocyte proliferation and differentiation, we examined the expression of genes related to podocyte proliferation and differentiated in isolated glomeruli by real-time PCR analysis as described previously 16. Treatment with either Am580 or roflumilast alone increased the expression of podocyte-specific markers (WT-1, nephrin and synaptopodin) (Fig 4A). Combination treatment with Am580 and roflumilast induced a further increase in podocyte differentiation marker expression. In contrast, mRNA levels of cell proliferation markers (cyclin E and Ki67) were suppressed in glomeruli by treating HIV-Tg mice with Am580 or roflumilast alone and a further reduction in proliferation markers was observed with Am580 and roflumilast combination treatment (Figure 4B). As a control, we also examined the mRNA levels of HIV nef gene and found that treatment of HIV-Tg mice with either Am580 or roflumilast or both did not affect glomerular nef expression (Figure 4B). These findings indicate that the beneficial effects of Rof/Am580 in HIV-Tg mice are likely independent of HIV viral gene expression. We also confirm the expression of these markers by immunostaining. The expression of synaptopodin and nephrin was upregulated in HIV-Tg mice by either Am580 or roflumilast and the expression was further increased in HIV-Tg mice treated with both Am580 and roflumilast (Figure 5A). In contrast, Ki67 expression was suppressed in mice treated with roflumilast or Am580 alone or in combination as compared to mice treated with vehicle (Figure 5A). By western blot, we also confirmed that glomerular Cyclin E level was suppressed in mice treated with either Am580 or roflumilast and further suppressed when treated with both agents (Figure 5B and 5C). We also noted that the suppression of Ki67 and cyclin E levels was less significant with roflumilast compared to Am580. Take together these data suggest that combination therapy of a RARα agonist with a PDE4 inhibitor could provide protection against podocyte injury in HIV-Tg mice beyond treatment with a single agent.
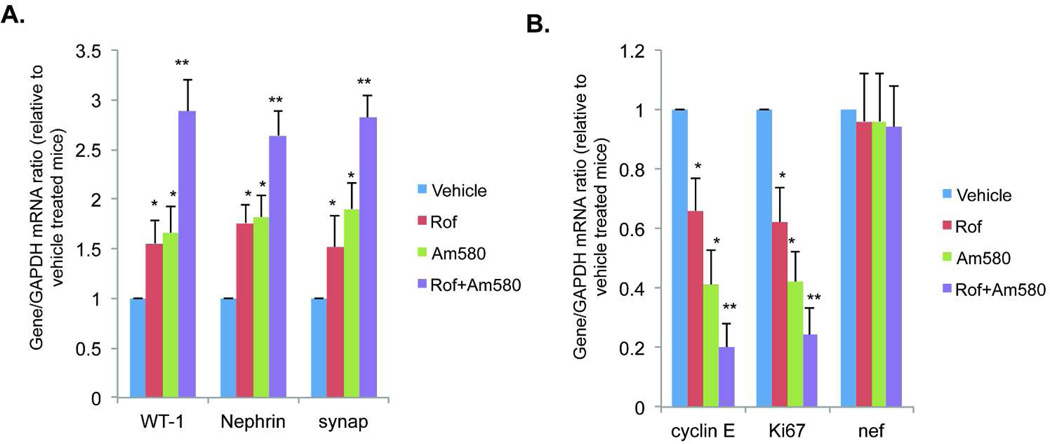
Figure 4. Real-time PCR analysis of podocyte differentiation markers.
Glomeruli were isolated from these mice treated with vehicle, Am580, roflumilast (Rof) or Rof+Am580 for 5 weeks. Total RNA was isolated from the glomeruli for real-time PCR analysis of podocyte differentiation markers (synaptopodin, nephrin, and WT-1), proliferation markers (Ki67 and cyclin E), and HIV nef. The ratio of these genes to GAPDH are presented (n=10). ANOVA followed by pair-wise analysis with Bonferron correction identified a significant difference between Vehicle vs Am580 (*p<0.01), Vehicle vs Rof (*p<0.01), Rof+Am580 vs Rof (**p<0.01), and Rof+Am580 vs Am580 (**p<0.01).
Figure 5.
A. Immunofluorescent staining of podocyte differentiation markers. Kidney sections from these mice were used for immunostaining of podocyte differentiation and proliferation markers as described in the method. DAPI staining was used to mark the nucleus. Representative pictures of five mice in each group are shown (x400). Since glomeruli with collapsing glomerulosclerosis are in the minority, selected pictures of non-sclerotic glomeruli, which are more representative of the overall histologic findings, are shown. B. Western blot analysis of cyclin E: Glomerular lysates from these mice were used for western blot analysis of cyclin E. The representative blots of two mice in each group were shown. Each lane represents one mouse. C. We performed western blot analysis for a total of six mice in each group and the average density of cyclin E and GAPDH in these mice was analyzed by densitometry. The ratio of cyclin E/GAPDH relative to vehicle-treated mice is shown. *p<0.05: HIV-Tg+Vehicle vs HIV-Tg+Rof and HIV-Tg+Vehicle vs HIV-Tg+Am580. **p<0.05: HIV-Tg+Am580 vs HIV-Tg+ Rof+Am580 and HIV-Tg+Rof vs HIV-Tg+Rof+Am580. N=6.
4. Effect of Am580 and/or roflumilast on CREB phosphorylation
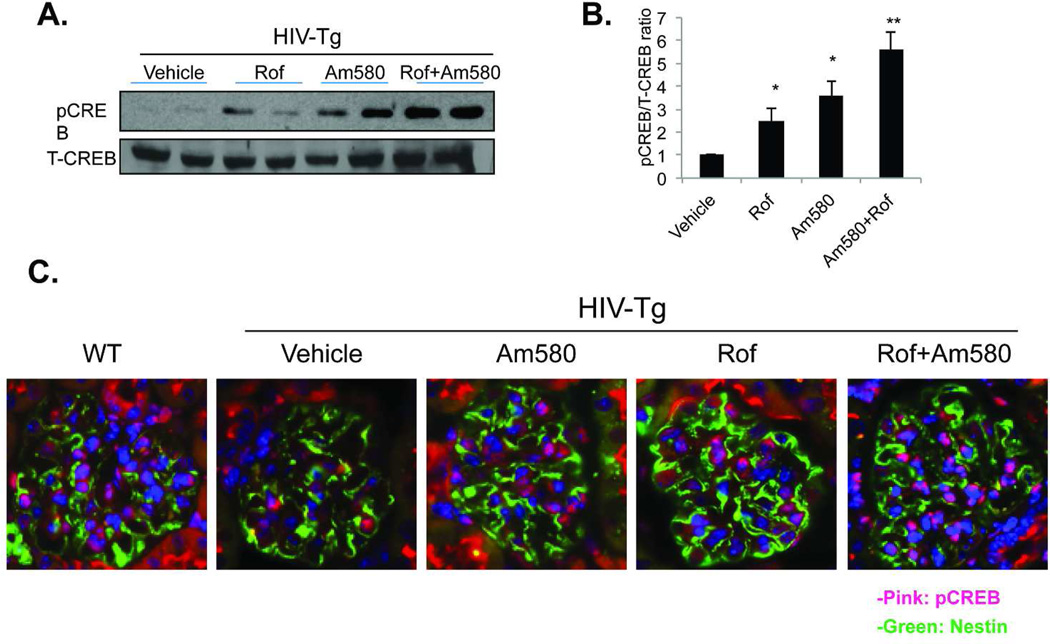
The addition of a PDE4 inhibitor to RA enhanced the differentiation of cultured podocytes by increasing the level of cAMP production and subsequent activation of the cAMP/PKA/CREB pathway 12,17. To determine whether the addition of roflumilast to Am580 also increases the level of cAMP and activation of the cAMP/PKA/CREB pathway we examined glomerular CREB phosphorylation. We found that combination treatment with Am580 and roflumilast caused an increase in CREB phosphorylation—as assessed by Western blotting and immunostaining of kidney tissue—more than treatment with either Am580 or roflumilast (Fig 6). Co-localization of pCREB and nestin staining was also observed in mice treated with Am580 or roflumilast alone or in combination (Figure 6C), indicating that phosphorylation of CREB is increased in the podocytes. We chose nestin as a podocyte marker because its expression is preserved in the kidney of HIV-Tg mice 18,19. These findings are consistent with our in vitro findings and support that cAMP/PKA/CREB pathway may play a role in mediating the effects of RA on podocyte differentiation in vivo.
Figure 6. Glomerular CREB phosphorylation.
Glomeruli were isolated from these mice treated with vehicle, Am580 or roflumilast (Rof) alone or in combination for 5 weeks. Nuclear lysates were obtained from glomeruli of these mice for western blot analysis for phosphor- (p-CREB) and total CREB (T-CREB). Representative blots of two mice in each group are shown in Fig 6A. We performed western blot analysis for a total of six mice in each group. The average density of p-CREB and T-CREB in these mice was analyzed by the densitometry. The ratio of p-CREB to T-CREB was calculated and the ratio of p-CREB/T-CREB related to the mice treated with vehicle is shown in Fig 6B, N=6, *p<0.01 for Vehicle vs Rof and Vehicle vs Am580, **p<0.01 for Am580 vs Rof+Am580 and or Rof vs Rof+Am580. Immunoflorescent staining for p-CREB was performed in kidney sections from six mice per group and the representative pictures are shown in Fig 6C. The green color indicates nestin and the pink color indicates the colocalization of p-CREB and DAPI (x400).
Discussion
Treatment of kidney glomerular disease is challenging. Many lines of evidence suggest that retinoic acid can improve kidney injury in animal models of kidney disease 20. Others and we find that ATRA improves kidney injury likely by protecting podocytes from injury 12,13. We previously demonstrated that ATRA restored podocyte differentiation markers in HIV-infected podocytes by activating the cAMP/PKA/CREB pathway 12. In vitro, we found that the effects of ATRA on podocyte differentiation were enhanced by the addition of a PDE4 inhibitor, which increased the accumulation of cAMP by blocking the degradation of cAMP 12. In addition, we demonstrated that RARα was required for ATRA-induced cAMP production and the effects of RA on podocyte differentiation 12. We also found that a RARα agonist (Am580) reduced protenuria, attenuated kidney injury, and induced podocyte differentiation in HIV-Tg mice 16. Here, we further extended our previous findings by studying the renal protective effect of combining a PDE4 inhibitor (roflumilast) with a RARα agonist (Am580) in HIV-Tg mice. The combination of roflumilast and Am580 further reduced proteinuria, improved renal function and kidney damage, and protected podocytes from injury in HIV-Tg mice compared to mice treated with roflumilast or Am580 alone. These findings indicate that PDE4 inhibitors and RARα agonists could be used in combination to treat patients with kidney disease.
Retinoids exert their effects by binding two families of nuclear receptors, the retinoic acid receptors (RAR) and the retinoid X receptors (RXR). The RARs and RXRs are expressed in a variety of tissues including the kidney 11. They affect gene transcription either directly by binding to the retinoic acid-response elements (RARE) of a promoter region 21 or indirectly by modulating transcription factors or intracellular signaling pathways 22,23. It has been shown that ATRA-induced gene expression is mostly independent of RAREs 21,24. Several studies indicate that ATRA induces leukemia cell differentiation through the activation of intracellular signaling pathways 25,26. Consistent with our findings in podocytes, ATRA can induce rapid cAMP production and increase PKA activity in acute myeloblastic leukemia cells to induce leukemia cell differentiation 9. A synergistic effect between ATRA and an inhibitor of phosphodiesterase has also been observed for myeloid differentiation 15.
Consistent with our findings, ATRA has been previously shown to induce neurite outgrowth in PC12 cells through CREB phosphorylation, which is independent of the retinoic acid response elements (RAREs) 27. CREB is phosphorylated by several signaling pathways including PKA and MAPK1,2, which could be activated by ATRA 28. CREB is a key transcription factor for neuronal differentiation. Our previous in vitro studies suggest that CREB plays an important role in podocyte differentiation 12,17. Here, our data suggest that CREB may also mediate the renal protective effects of ATRA in vivo.
Our studies suggest that PDE4 inhibitor enhances the effects of RARα agonist by increasing intracellular cAMP concentration. Components of the cAMP signaling pathway exist within the podocyte 29,30. Consistent with our findings, activation of the cAMP-PKA pathway in podocytes is known to influences cell morphology, actin assembly, and matrix production 30. In addition, cAMP seems to attenuate the detrimental effects of hormones that activate the Ca2+/Protein kinase C pathway 30. Overall, the cAMP pathway seems to exert a protective effect on podocytes survival.
The clinical use of retinoids in patients with kidney disease is limited by its significant side effects. Our studies suggest that adding a PDE4 inhibitor to a RARα agonist provides additional renal protection. In the future, we could test to see whether using PDE4 inhibitors in combination with a lower dose of RA or RARα agonist would offer the same renal protection while eschewing the side-effects associated with high dose RA and RARα agonists. FDA recently approved Roflumilast for the treatment of asthma and COPD. Its side effects are relatively minor. Therefore, we speculate that the combination of RARα agonist and roflumilast could be a potential new therapy regimen for patients with glomerular disease such as HIVAN.
HIV-Tg mouse is the best mouse model to study HIVAN because it mimics human kidney disease 31. However, it is known that the severity of kidney disease in HIV-Tg mice varies among the different colonies, which is likely due to different genetic penetrance. HIV-1 transgenic mice (Tg26) originally reported by Kopp et al developed renal disease that is less severe with a later onset 32. HIV-Tg mice used in the current studies were derived from a Tg26 mouse with severe kidney disease. These mice developed proteinuria at age of 4 weeks and mild to moderate renal insufficiency at age of 8–10 weeks. The severity of disease in HIV-Tg mice more closely mimics human HIVAN. It is also interesting that RARα agonist and roflumilast improve kidney disease without affecting the expression of HIV genes. These findings suggest that these two drugs protect kidney cells against injury likely through affecting the host response of the kidney cells to the HIV gene expression.
Even though our data suggest that this new therapy regime is effective in a murine model of HIVAN, previous studies have shown that RA is effective in animal models of other non-HIVAN kidney diseases 11. We believe this new therapeutic combination could be applied to the treatment of other kidney glomerular diseases caused by podocyte injury. Our unpublished data suggests that Am580 may also improve kidney disease in a murine model of diabetic nephropathy.
In summary, the combination of a PDE4 inhibitor with a RARα agonist provides renal protection in HIV-Tg mice by activating the cAMP/PKA/CREB pathway. These new findings provide a scientific basis to design a therapeutic regimen that could be used for patients with glomerular diseases including HIVAN.
Methods
1. Animal studies
It is known that HIV-Tg mice from different colonies have variable severity of kidney phenotype. In this rederived colony, about 80% mice develop more than 1+ proteinuria at the age of 4 weeks based on urine dipstick and mild-moderate renal insufficiency at age of 8–10 weeks. These mice also developed cataract, skin papillomas, and mild edema by age of 8–10 weeks. For the current studies, we pre-screened the HIV-Tg mice by using urine dipstick and mice with 1+ to 2+ of proteinuria at the age of 4 weeks were selected for the studies (10 mice were selected, 3 mice had 1+ and 7 mice had 2+ proteinuria by urine dipstick) and the age-matched littermates were used as the control. Since both male and female HIV-Tg mice develop kidney disease similarly we decided to use 5 male and 5 female in each group (n=10). Mice were fed with the vehicle (1.3% polyethylene glycol 400; 4% methylcellulose solution), roflumilast (5mg/kg/day; purchased from LGM Pharma, Boca Raton, Florida), Am580 (0.3mg/kg/day; supplied by Dr. K Shudo at Research Foundation ITSUU Laboratory, Molecular and Functional Bioscience, Japan), or both Am580 and roflumilast for a total of 5 weeks. Roflumilast was dissolved in the vehicle and given as daily gavage and Am580 was mixed in the animal chow. Unrestricted food and water were provided throughout the duration of the experiment. Body weight was recorded every week. Urine samples were collected weekly for determination of albuminruia and creatinine ratio. After 5 weeks of treatment, mice were euthanized at 9 weeks of age for blood, urine, and tissue collection. Blood urea nitrogen, a marker of glomerular function, was measured. Kidneys were collected and kidney weight was recorded. A section of the kidney was fixed in formalin. Glomeruli were isolated and total RNA extracted from the glomeruli for determination of gene expression by real-time PCR. Western blot was performed for phosphorylation of CREB. All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee at the Mount Sinai School of Medicine.
2. Measurement of BUN, urine protein, and creatinine
Blood urea nitrogen (BUN) was measured using a commercially available assay (Bioassay Systems). Urine albumin was quantified by ELISA (Bethyl Laboratory Inc., Houston, TX, USA). Urine creatinine was measured in the same samples using the QuantiChrom™ Creatinine Assay Kit (DICT-500, BioAssay Systems) following manufacturer’s protocol. Urine albumin excretion is expressed as the ratio of albumin to creatinine.
3. Quantitative Histopathology
Mice were perfused with PBS containing 4% paraformaldehyde and kidneys were further fixed in 4% paraformaldehyde for 2 hours. Kidney tissue was embedded into paraffin by American Histolabs, Inc. (Gaithersberg, MD). Kidney histology was examined after periodic acid-Schiff (PAS) staining. Glomerulosclerosis was scored as described previously by Dr. D’Agati 33. Briefly, each specimen received a score for three parameters: percentage of collapsing glomerular sclerosis, percentage of tubular cysts or casts, and podocyte hypertrophy. The percentage of collapsing glomerulosclerosis was obtained by identifying the total number of glomeruli with collapse and segmental or global sclerosis and dividing this number by the total number of glomeruli seen. The percentage of tubular cysts or casts score was obtained by the number of tubules with either microcystic dilatation or filled with casts divided by the total number of tubular cross sections in a representative area. Finally, the degree of podocyte hypertrophy was scored as 0 (absent), 1+ (podocyte hypertrophy observed in less than 25% of all glomeruli), 2+ (podocyte hypertrophy observed in between 25 – 50% of all glomeruli), and 3+ (podocyte hypertrophy in greater than 50% of all glomeruli). The podocyte hypertrophy is evaluated based on the morphology under light microscopy as described previously 33.
4. Electronic microscopy
Mice were perfused with PBS and then immediately fixed in glutaraldehyde for electron microscopy (EM) study performed at the histopathology core facility of the Mount Sinai School of Medicine.
5. Isolation of glomeruli from mice for western blot and real-time PCR
Glomeruli were isolated as described 34. Briefly, animals were perfused with 60ml of Hank’s Buffered Salt Solution (HBSS) containing 2.5 mg/ml iron oxide and 1% bovine serum albumin. After perfusion, kidneys were removed, decapsulated, minced into 1-mm3 pieces, and digested in HBSS containing 1mg/ml collagenase A and 100U/ml deoxyribonuclease I. Digested tissue was passed through a 100 micron cell strainer and collected by centrifugation. The pellet was resuspended in 2 ml of HBSS and glomeruli were collected using a magnet. The purity of glomerular was verified under microscopy and by western blot analysis for podocyte specific markers including synaptopodin, nephrin, and WT-1.
6. Real-time PCR
Total RNA was isolated from glomeruli using TRIzol (Invitrogen). Extracted RNA samples were reverse transcribed to cDNA using SuperScript™ III First-Strand Synthesis System for RT-PCR. Real-time PCR was performed on cDNA samples using Quantitect SYBR Green PCR Kit (Qiagen) in a Roche Lightcycler (Roche). Primers used for synaptopodin, nephrin, WT-1, cyclin E, and Ki67 were the same as described 16. Data were normalized to housekeeping genes (GAPDH) and presented as fold increase compared to cDNA from WT animals using the 2−ΔΔCT method.
7. Western blot
Glomeruli are lysed with a buffer containing 1% NP40, a protease inhibitor cocktail and tyrosine and serine-threonine phosphorylation inhibitors. After determination of protein concentration, glomerular lysates were subjected to Western blot analysis using the following specific antibodies: anti-phospho CREB antibody from Cell Signaling Technology, Inc. (Danvers, MA) and anti-total CREB from Millipore (Billerica, MA). Anti-cylcin E antibody was from Santa Cruz and anti-GAPDH antibody was from Sigma. Densitometric analysis of western blot results was quantified using ImageJ.
8. Immunofluorescence
Paraffin-embedded sections were de-paraffinized prior to incubation with primary antibodies for 1 h at RT. Fluorescent-labeled 2nd antibodies with different wavelengths were used for co-localization study. Sections were examined by epi-fluorescent microscopy. Antibodies used for immunostaining are anti-nephrin antibody (a gift from Dr. Larry Holzeman), anti-synaptopodin (Fitzgerald Industries International, Acton, MA), anti-WT1 and anti-nestin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and anti-phosphor-CREB (Cell Signaling Technology, Inc.), and anti-Ki67 (Vector Laboratories, Inc., Burlingame, CA).
9. Statistical Analysis
Data were expressed as mean ± standard deviation (X±SD). The unpaired T-test was used to analyze data between two groups. ANOVA was used for multiple group analysis and the comparison between the groups was further analyzed using Bonferroni correction. The renal scoring data was analyzed by using non-parametric Wilcoxon Signed Rank Test. The percentage of mice in each group did or did not develop renal disease was summarized in a contingency table and analyzed by chi square test. Statistical significance will be considered when p<0.05.
Acknowledgement
JCH is supported by NIH 1R01DK078897, 1R01DK088541, P01-DK-56492, and VA Merit Award; PYC is supported by NIH 5K08DK082760.
Footnotes
Competing interest statement: The authors declare that they have no competing financial interests.
References
- 1.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 57(A8):e1–e526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt CM, Klotman PE. HIV-associated nephropathy in the era of antiretroviral therapy. Am J Med. 2007;120:488–492. doi: 10.1016/j.amjmed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 3.Lucas GM, Eustace JA, Sozio S, et al. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. AIDS. 2004;18:541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 4.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 5.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 6.Barisoni L, Kriz W, Mundel P, et al. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 7.Barisoni L, Bruggeman LA, Mundel P, et al. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 2000;58:173–181. doi: 10.1046/j.1523-1755.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- 8.Sunamoto M, Husain M, He JC, et al. Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int. 2003;64:1695–1701. doi: 10.1046/j.1523-1755.2003.00283.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Q, Tao J, Zhu Q, et al. Rapid induction of cAMP/PKA pathway during retinoic acid-induced acute promyelocytic leukemia cell differentiation. Leukemia. 2004;18:285–292. doi: 10.1038/sj.leu.2403226. [DOI] [PubMed] [Google Scholar]
- 10.Zhong J, Zuo Y, Ma J, et al. Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int. 2005;68:1048–1060. doi: 10.1111/j.1523-1755.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 11.Xu Q, Lucio-Cazana J, Kitamura M, et al. Retinoids in nephrology: promises and pitfalls. Kidney Int. 2004;66:2119–2131. doi: 10.1111/j.1523-1755.2004.66002.x. [DOI] [PubMed] [Google Scholar]
- 12.He JC, Lu TC, Fleet M, et al. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol. 2007;18:93–102. doi: 10.1681/ASN.2006070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughan MR, Pippin JW, Griffin SV, et al. ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int. 2005;68:133–144. doi: 10.1111/j.1523-1755.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu TC, He JC, Wang ZH, et al. HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem. 2008;283:8173–8182. doi: 10.1074/jbc.M708920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrella E, Gianni M, Cecconi V, et al. Phosphodiesterase IV inhibition by piclamilast potentiates the cytodifferentiating action of retinoids in myeloid leukemia cells. Cross-talk between the cAMP and the retinoic acid signaling pathways. J Biol Chem. 2004;279:42026–42040. doi: 10.1074/jbc.M406530200. [DOI] [PubMed] [Google Scholar]
- 16.Ratnam KK, Feng X, Chuang PY, et al. Role of the retinoic acid receptor-alpha in HIV-associated nephropathy. Kidney Int. 79:624–634. doi: 10.1038/ki.2010.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu TC, Wang Z, Feng X, et al. Retinoic acid utilizes CREB and USF1 in a transcriptional feed-forward loop in order to stimulate MKP1 expression in human immunodeficiency virus-infected podocytes. Mol Cell Biol. 2008;28:5785–5794. doi: 10.1128/MCB.00245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng X, Lu TC, Chuang PY, et al. Reduction of Stat3 Activity Attenuates HIV-Induced Kidney Injury. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2008080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorner PS, Ho M, Eremina V, et al. Podocytes contribute to the formation of glomerular crescents. J Am Soc Nephrol. 2008;19:495–502. doi: 10.1681/ASN.2006101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans TR, Kaye SB. Retinoids: present role and future potential. Br J Cancer. 1999;80:1–8. doi: 10.1038/sj.bjc.6690312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronemeyer H, Miturski R. Molecular mechanisms of retinoid action. Cell Mol Biol Lett. 2001;6:3–52. [PubMed] [Google Scholar]
- 22.Benkoussa M, Brand C, Delmotte MH, et al. Retinoic acid receptors inhibit AP1 activation by regulating extracellular signal-regulated kinase and CBP recruitment to an AP1-responsive promoter. Mol Cell Biol. 2002;22:4522–4534. doi: 10.1128/MCB.22.13.4522-4534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Na SY, Kang BY, Chung SW, et al. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J Biol Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- 24.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 25.Radominska-Pandya A, Chen G, Czernik PJ, et al. Direct interaction of all-trans-retinoic acid with protein kinase C (PKC). Implications for PKC signaling and cancer therapy. J Biol Chem. 2000;275:22324–22330. doi: 10.1074/jbc.M907722199. [DOI] [PubMed] [Google Scholar]
- 26.Hong HY, Varvayanis S, Yen A. Retinoic acid causes MEK-dependent RAF phosphorylation through RARalpha plus RXR activation in HL-60 cells. Differentiation. 2001;68:55–66. doi: 10.1046/j.1432-0436.2001.068001055.x. [DOI] [PubMed] [Google Scholar]
- 27.Canon E, Cosgaya JM, Scsucova S, et al. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol Biol Cell. 2004;15:5583–5592. doi: 10.1091/mbc.E04-05-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 29.Bek M, Nusing R, Kowark P, et al. Characterization of prostanoid receptors in podocytes. J Am Soc Nephrol. 1999;10:2084–2093. doi: 10.1681/ASN.V10102084. [DOI] [PubMed] [Google Scholar]
- 30.Endlich N, Endlich K. cAMP pathway in podocytes. Microsc Res Tech. 2002;57:228–231. doi: 10.1002/jemt.10079. [DOI] [PubMed] [Google Scholar]
- 31.Lu TC, He JC, Klotman P. Animal models of HIV-associated nephropathy. Curr Opin Nephrol Hypertens. 2006;15:233–237. doi: 10.1097/01.mnh.0000222688.69217.8e. [DOI] [PubMed] [Google Scholar]
- 32.Kopp JB, Klotman ME, Adler SH, et al. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci U S A. 1992;89:1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Agati V. Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 34.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]