Abstract
Cross-presentation is an important mechanism by which exogenous tumor antigens are presented to elicit immunity. Since neutrophil elastase (NE) and proteinase-3 (P3) expression is increased in myeloid leukemia, we investigated whether NE and P3 are cross-presented by dendritic cells (DC) and B-cells, and whether the NE and P3 source determines immune outcomes. We show that NE and P3 are elevated in leukemia patient serum and that levels correlate with remission status. We demonstrate cellular uptake of NE and P3 into lysosomes, ubiquitination and proteasome processing for cross-presentation. Using anti-PR1/HLA-A2 monoclonal antibody, we provide direct evidence that B-cells cross-present soluble and leukemia-associated NE and P3, while DCs cross-present only leukemia-associated NE and P3. Cross-presentation occurred at early time points but was not associated with DC or B-cell activation, suggesting that NE and P3 cross-presentation may favor tolerance. Furthermore, we show aberrant subcellular localization of NE and P3 in leukemia blasts to compartments that share common elements of the classical MHC class I antigen-presenting pathway, which may facilitate cross-presentation. Our data demonstrate distinct mechanisms for cross-presentation of soluble and cell-associated NE and P3, which may be valuable in understanding immunity to PR1 in leukemia.
Keywords: neutrophil elastase, proteinase 3, cross-presentation, leukemia, PR1, myeloid
Introduction
Neutrophil elastase (NE) and proteinase 3 (P3) are serine proteases normally stored in the azurophil granules of myeloid cells. They play a role in inflammation and anti-microbial defenses,1, 2 and are known leukemia-associated antigens (LAA) from which PR1, the human leukocyte antigen (HLA)-A2 restricted nonameric peptide, is derived.3, 4 PR1-specific cytotoxic T lymphocytes (CTLs) were shown to preferentially kill leukemia cells because of the high expression of NE and P3 by leukemia targets.4, 5 Clinical responses to interferon-α2b, hematopoietic stem cell transplantation (HSCT), and PR1-peptide vaccine have been correlated with circulating PR1-CTLs in patients with acute (AML) and chronic (CML) myelogenous leukemia.4, 6–8 Since NE and P3 are aberrantly expressed by leukemia and because the mechanisms involved in antigen presentation are critical for shaping immune outcomes, we sought to investigate the mechanisms involved in NE and P3 cross-presentation. Understanding these mechanisms is critical for understanding anti-leukemia immunity and for further development of PR1-targeting immunotherapies.
Cross-presentation is a primary mechanism whereby exogenous antigens are presented by antigen presenting cells (APCs)9 and is critical in eliciting antitumor immunity. Dendritic cells (DCs) were shown to be efficient cross-presenting APCs.10, 11 B-cells are also effective APCs, however, whether or not they can cross-present, the type of antigen that is favored for B-cell cross-presentation, and whether B-cell cross-presentation leads to immune priming (i.e. cross-priming) or tolerance (i.e. cross-tolerance) is not fully understood.12–14 Previous cross-presentation studies have used tumor whole cell lysates (WCLs) that elicit polyclonal T-cell responses or employed mouse models using the ovalbumin (ova)-derived peptide SIINFEKL.15, 16 These models have been valuable to our understanding of cross-presentation of tumor antigens, but have minimal direct clinical applicability. In this report, we studied two proteins that have demonstrated clinical applicability in leukemia.
Since azurophil-granule proteins were shown to be elevated in serum from leukemia patients,17 and since high-avidity PR1-CTLs are selectively deleted when exposed to P3 over-expressing CML,18 possibly due to cross-tolerance, we hypothesized that NE and P3 are cross-presented by APCs. Furthermore, we postulated that the source of antigen (soluble vs. leukemia cell-associated), the cell type that mediates PR1 cross-presentation, and the activation status of the APCs during PR1 cross-presentation determine the PR1 immune response.
In this report, we first show that NE and P3 are elevated in serum from leukemia patients, suggesting a source of soluble antigen, and show that their levels correlate with leukemia remission status. We provide evidence that NE and P3 are taken up by APCs, localize to lysosomes and are ubiquitinated, supporting the hypothesis that they are processed for cross-presentation. Using 8F4, the novel mouse monoclonal antibody (mAb) that recognizes the conformational epitope of PR1/HLA-A2,19 we show that PR1 is cross-presented by B-cells from soluble and leukemia-associated NE and P3, while myeloid DCs (mDCs) cross-present only leukemia-associated NE and P3. Cross-presentation occurred at early time points but did not coincide with activation of mDCs or B-cells. We also show that NE and P3 in leukemia blasts are mislocalized outside granules and are ubiquitinated, possibly facilitating their cross-presentation. Lastly, we demonstrate that in the presence of co-stimulation, NE and P3 cross-presentation leads to the proliferation of in vitro-expanded PR1-CTLs through proteasome dependent pathways. Overall, this study demonstrates that cross-presentation of soluble and cell-associated NE and P3 involves distinct mechanisms, which may modulate the anti-leukemia immune response to PR1.
Materials and methods
Patients, cells and cell lines
Patient and healthy donor (HD) samples were obtained after appropriate informed consent. HMy2.CIR (B lymphoblast), KG-1 (dendritic-like cells), U-937 (myelomonoblastic leukemia), HL-60 (acute promyelocytic leukemia) and T2 (B-cell/T-cell hybridoma) cell-lines were obtained from ATCC (Manassas, VA, USA). HLA-A2*0201-transduced HMy2.CIR, KG-1 and HL-60 cells were provided by Greg Lizee.20
B-cells were purified from HD peripheral blood (PB) mononuclear cells (PBMC) using CD19+ MicroBeads (Miltenyi, Auburn, CA, USA) and maintained in complete media (CM) supplemented with interleukin-4 (IL-4; 10 ng/mL; R&D Systems, Minneapolis, MN, USA) and CD40 ligand (L) (500 ng/mL) with enhancer (E) (1 µg/mL; Alexis Biochemicals, San Diego, CA, USA). Plasmacytoid DCs (pDCs) were purified using negative selection beads, and purity was confirmed using CD-303 (Miltenyi). mDC were generated by plastic adherence in tissue culture flasks and subsequent cultured in CM supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF; 1000 U/mL) and IL-4 (500 U/mL) (R&D) for 6 days. mDC were matured on day 6 with 50 ng/mL tumor necrosis factor-α (TNF-α) and 0.1 µM prostaglandin E2 (Sigma, St. Louis, MO, USA) for 2 days.
Hematopoietic stem cells (HSC) were stained with antibodies targeting CD34, CD38 and the lineage (lin) markers CD4, CD14, CD16 (all from Becton-Dickinson [BD], San Jose, CA, USA) and CD19 (eBioscience, San Diego, CA,USA). CD34+, CD38−, lin−, HSCs were sorted (Influx, BD) and stained for intracellular NE and P3.
Serum NE and P3 measurement
Serum samples were examined for NE using enzyme-linked immunosorbent assay (ELISA; Cell Sciences, Canton, MA, USA). P3 ELISA was performed after coating microtitre plates (Thermo-Scientific, Rochester, NY, USA) with purified goat anti-P3 polyclonal antibody (Santa Cruz, Santa Cruz, CA, USA). Plates were blocked and serum samples were added and incubated for 1 hour. Mouse anti-P3 mAb (NeoMarkers, Fremont, CA, USA) was added and following a wash step, conjugated HRP-labeled goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) was added. 3,3’, 5,5’-tetramethylbenzidine solution was used as the substrate and optical densities were read at 450 nm.
Cell pulsing for protein uptake and cross-presentation
To determine protein uptake, APCs were pulsed for 3 hours in serum-free medium containing 5 µg/mL NE or P3. Cells were then analyzed by flow cytometry. To determine cross-presentation, KG-1-A2 and HMy2.CIR-A2 cells were incubated with 5 µg/mL purified NE or P3 for 6 and 18 hours, respectively. B-cells and mDCs were cultured for 6 hours with 5 µg/mL NE or P3, or with irradiated cells at a ratio of 1:1 (APC: irradiated-cell).
Fluorescent confocal microscopy and flow cytometry analysis
Directly conjugated fluorescent antibodies targeting the following antigens were used: NE (Santa Cruz), P3 (NeoMarkers), lysosome-associated membrane protein-2 (LAMP-2; eBioscience), HLA-A2, HLA-DR, CD11c, CD19, CD86, CD14 (all from BD); CD83 (Biolegend, San Diego, CA, USA), and anti-PR1/HLA-A2 (8F4).19 We used Alexa-647/488 kits (Invitrogen) to conjugate anti-NE, P3 and 8F4. Aqua live/dead stain (Invitrogen) was used to assess viability. Confocal imaging was performed using Leica Microsystems SP2 SE confocal microscope (Illinois, USA) and analyzed using Leica LCS software (version 2.61). Flow cytometry was performed using the Cytomation CyAn flow cytometer (Dako, Carpinteria, CA, USA) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
APC activation studies
B-cells and mDCs were cultured for 72 hours and 1.5 hours, respectively, with NE or P3 (5µg/mL), or irradiated cells at 1:1 APC to irradiated-cell ratio. CD40L (500 ng/mL)/E (1 µg/mL) and TNF-α (50 ng/mL) were used as positive controls for B-cells and mDCs, respectively. Cells were stained for CD83 and HLA-DR an analyzed using flow cytometry.
Subcellular fractionation
Cytoplasmic, nuclear, membrane/membrane bound compartments (including granules, endosomes, lysosomes, Golgi complex, and endoplasmic reticulum), and skeletal fractions were generated using the ProteoExtract Subcellular Proteome Extraction kit (Calbiochem, San Diego, CA, USA).21 Anti-calpain (Calbiochem), LAMP-2 (Protein Tech Group, Chicago, IL, USA), nucleoporin-62 and GM-130 (both from BD) antibodies were used to confirm the subcellular fractions.
Immunoprecipitation (IP) and immunoblot (IB) analysis
WCLs were generated by suspending cell pellets in lysis buffer (10 mM/L HEPES [pH 7.9], 10 mM/L KCl, 0.1 mM/L EGTA, 0.1 mM/L EDTA, and 1 mM/L DTT) containing protease inhibitors and subsequent freeze-thaw cycles for 15 min. HMy2.CIR-A2 cells were pulsed for 3 hours with NE- or P3-containing supernatant harvested from NE (ELA2)- or P3 (PRTN3)-transfected HMy2.CIR-A2 cells. For IP reactions, anti-ubiquitin antibodies (Santa Cruz) were added to the pre-cleared WCLs and incubated overnight at 4°C. Subcellular fractions, IP products and WCLs were separated by electrophoresis on 10% SDS gels, transferred onto PVDF membranes and stained with anti-P3 (NeoMarkers) or anti-NE antibodies (Santa Cruz). Chemiluminescence was captured on Kodak film. Densitometric analysis was performed using Quantity One software (Bio-Rad, Hercules, CA, USA). The percentage of total protein (% ProteinTotal) was calculated using the formula: % ProteinTotal= Density Bandx/ Density BandTotal, where Density Bandx is the density of the NE or P3 band from a subcellular fraction and Density BandTotal is the sum of the densities of the NE or P3 bands in the 4 subcellular fraction from the same sample.
Expanding PR1-CTLs
HD HLA-A2+ PBMC were stimulated in vitro with PR1-peptide, as previously described.22 Briefly, T2 cells were incubated with 20 µg/mL of PR1 for 90 minutes at 37°C. Peptide-loaded T2 cells were irradiated and cultured with freshly isolated PBMC (1:1 ratio) in CM containing 10% human AB-serum. Re-stimulation with PR1-pulsed T2 cells was performed on days 7, 14, and 21, and the following day 20 IU/mL of recombinant human interleukin-2 (rhIL-2; R&D) was added. On day 25, T-cells were harvested and tested for peptide-specific proliferation.
Cell proliferation assay
PR1-CTLs were tested for cell proliferation using an ELISA assay to measure 5-bromodeoxyuridine (BrDU) incorporation (Roche, Indianapolis, IN, USA). PR1-CTLs were cultured with irradiated stimulator cells at a responder-to-stimulator ratio of 1:1 for 48 hours. HMy2.CIR cells were used as they lack endogenous NE and P3 and express the co-stimulatory molecules B7.1 (CD80) and B7.2 (CD86).23 Stimulator cells included HMy2.CIR-A2 cells, ELA2- or PRTN3-transfected HMy2.CIR-A2 cells and HMy2.CIR-A2 or HMy2.CIR (HLA-A2-negative) cells that were cultured with NE- or P3-containing supernatant from ELA2- or PRTN3-transfected HMy2.CIR cells. T2 cells were used alone or pulsed with PR1 peptide (10 µg/mL). To inhibit Golgi and proteasome processing, we added 1 µg/mL of brefeldin A (BFA; Sigma) or lactacystin (1–10 µg/mL; Calbiochem), respectively.12 Incorporation of BrDU was determined by measuring absorbance (A) at 370 nm (reference, 492 nm). The stimulation index (SI) was calculated using the formula: SI = (Aexperimental - Amedia)/(Acontrol - Amedia). Absorbance of PR1-CTLs from the unpulsed HMy2.CIR-A2 cells was used as the control (Acontrol).
Statistical Analysis
We used GraphPad Prism 5.0 software (La Jolla, CA, USA) to perform the statistical analyses and P-values <0.05 were used to establish significance.
Results
NE and P3 are elevated in leukemia patient serum
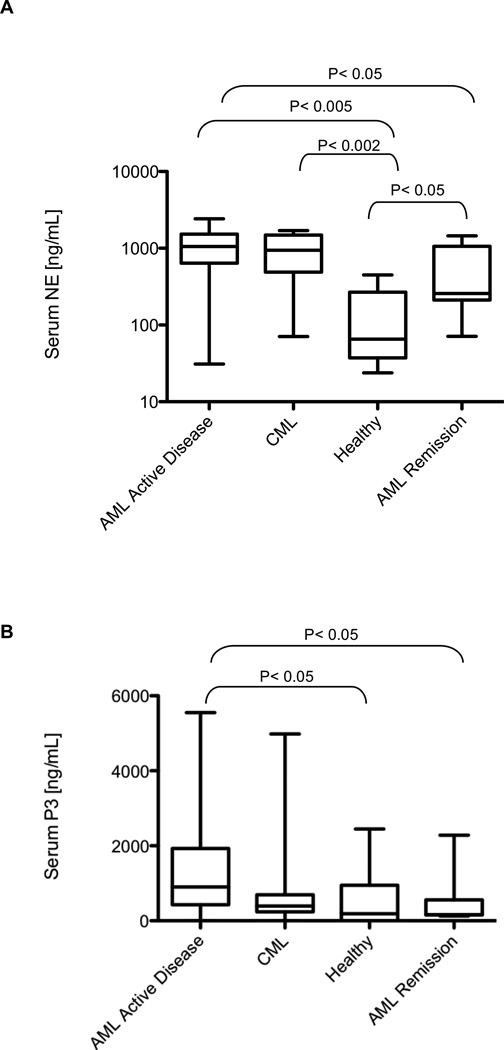
Since exogenous antigens are the protein source for cross-presentation, we used ELISA to examine whether NE and P3 were higher in leukemia patient serum (Fig. 1). NE was significantly higher in serum from patients with AML (median=1058 ng/mL; n=20) and CML (median= 941.7 ng/mL; n=15), compared with HD serum (median= 65.3 ng/mL; n=5) and AML patients in remission (median=257.9 ng/mL; n=17) (Fig. 1A). Similarly, P3 levels were also significantly higher in serum from AML patients (median= 902.1 ng/mL; n=22) compared with HD (median= 187.7 ng/mL; n=19) and patients in remission (median= 161.4 ng/mL; n=17) (Fig. 1B). Although CML patients had higher P3 levels (median=392.5 ng/mL; n=15) versus HD, it did not reach significance (P=0.46). Intracellular cytokine flow cytometry (CFC) assays showed poor PR1-CTL functions in patient with high NE and P3 levels (Supplementary Fig. 1 and Table 1). Furthermore, there was a correlation between serum NE and P3 levels and disease burden in CML, but not in AML (Supplementary Table 2).
Figure 1. NE and P3 are elevated in serum from patients with AML and CML.
Serum from HD and patients with AML and CML was analyzed using ELISA. (A) NE level was significantly higher in serum from AML (median= 1058 ng/mL; n = 20) and CML (median= 941.7 ng/mL; n = 15) patients, compared with serum from HD (median= 65.3 ng/mL; n = 5) and AML patients in remission (median= 257.9 ng/mL; n=17). (B) P3 level was also significantly higher in serum from AML patients (median= 902.1 ng/mL; n=22) compared with HD (median= 187.7 ng/mL; n=19) and patients in remission (median= 161.4 ng/mL; n=17).
Soluble NE and P3 are taken up by APCs
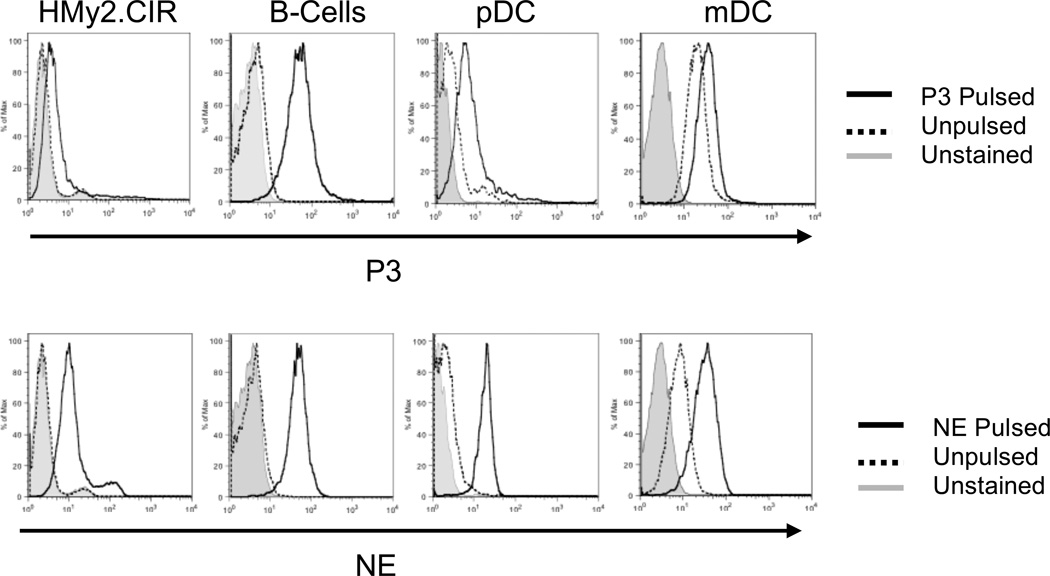
We next sought to determine if soluble NE and P3 could be taken up by B-cells and DCs. Although DCs are the principal cross-presenting cell type,11 we also examined B-cells since they are often spared in myeloid leukemia and were shown to cross-present.12, 14, 24 We used NE and P3 at 5 µg/mL, which approximates extracellular NE and P3 levels.25, 26 Using flow cytometry, we demonstrated that HD APCs and the NE- and P3-deficient B-cell line HMy2.CIR take up exogenous soluble NE and P3 (Fig. 2). Despite endogenous NE and P3 production,27, 28 mDCs took up exogenous NE and P3, thereby increasing their intracellular levels.
Figure 2. NE and P3 are taken up by APCs.
HMy2.CIR cells and healthy donor APCs, including B-cells, pDCs and mDCs, were pulsed with P3 (5 µg/mL) (top panel) or NE (5 µg/mL) (lower panel) for 3 hours, permeabilized and stained with FITC-conjugated antibodies to NE and P3, and then analyzed using flow cytometry. Unpulsed mDCs demonstrate baseline staining for NE and P3 as they endogenously express both proteins.
NE and P3 localize within lysosomes and are ubiquitinated
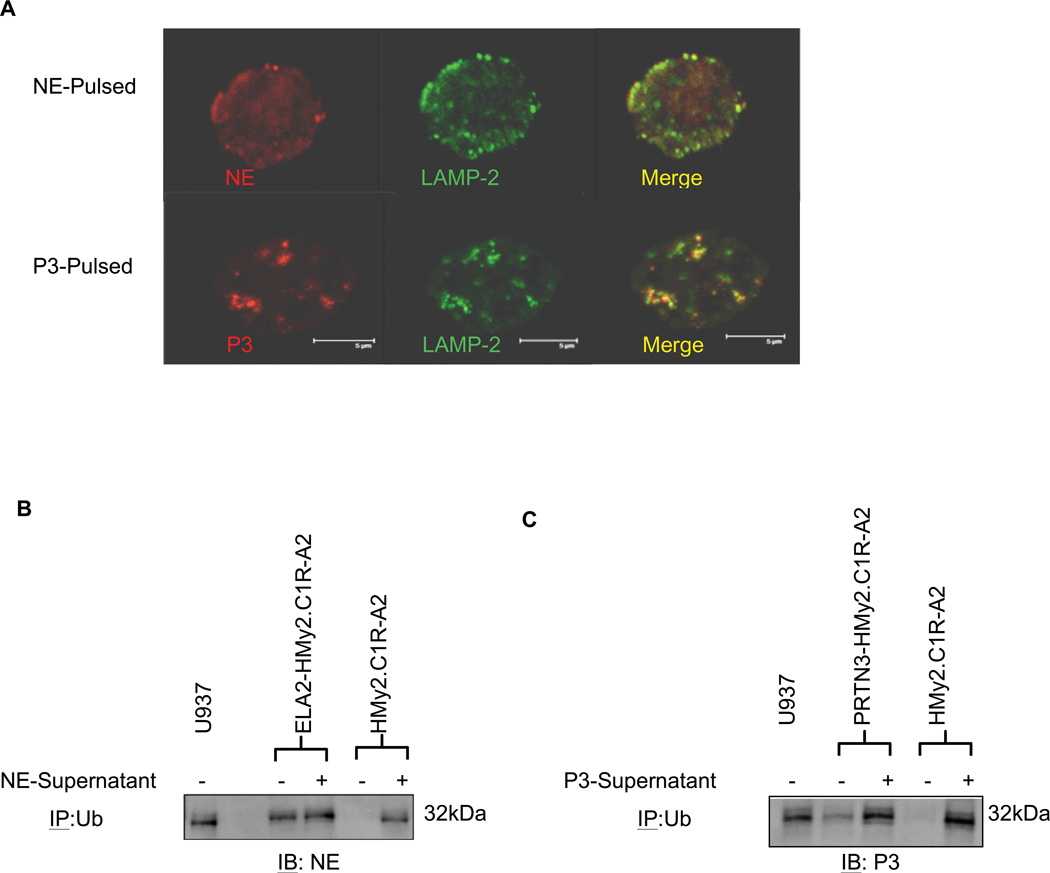
Since protein ubiquitination and degradation by proteasome were implicated in antigen presentation on major histocompatibility (MHC) class I,29–31 we examined the subcellular localization and ubiquitination of NE and P3 following uptake. Confocal microscopy showed that NE and P3 localized to LAMP-2-containing vesicles (i.e. lysosomes) following uptake where proteins may be degraded into small peptides (Fig. 3A). Furthermore, we used HMy2.CIR-A2 cells, which lack endogenous NE and P3, to demonstrate ubiquitination of exogenous NE and P3 following uptake. After 3-hour pulsing with NE- or P3-containing supernatant from ELA2- or PRTN3-transfected HMy2.CIR-A2 cells (Supplementary Fig. 2), IP demonstrated ubiquitination of NE and P3 following uptake (Fig. 3B and C). Exogenous NE and P3 was also ubiquitinated by the ELA2- and PRTN3-transfected HMy2.CIR-A2 cells following uptake, as evidenced by stronger NE and P3 intensities in the lanes from the pulsed/transfected cells. We also show that endogenous NE and P3 from the ELA2- or PRTN3-transfected HMy2.CIR-A2 cells are ubiquitinated (Fig. 3B and C). Since HMy2.CIR-A2 cells lack azurophil granules that normally store NE and P3, the localization of NE and P3 in the cytosolic compartment of ELA2- or PRTN3-transfected HMy2.CIR-A2 cells may have facilitated ubiquitination. This further suggests that MHC-I pathway is accessed by NE and P3, since cytosolic ubiquitination is important in MHC-I antigen processing.31 Taken together, these results suggest that exogenous and cytosolic NE and P3 may be preferentially processed by the MHC-I pathway.
Figure 3. NE and P3 localize to lysosomal compartments and are ubiquitinated following uptake.
(A) B-cells from healthy donors were enriched and pulsed with NE (5 µg/mL) or P3 (5 µg/mL) for 4 hours. Cells were permeabilized and stained with FITC-conjugated anti-LAMP2 antibody (green) and Alexa-647-conjugated anti-NE or anti-P3 antibodies (red). Cells were imaged using laser confocal microscopy, which shows localization of NE and P3 in lysosomal compartments following pulsing. Results are representative of 2 separate experiments from different healthy donors. (B) ELA2- and (C) PRTN3-transfected HMy2.CIR-A2, as well as wild-type HMy2.CIR-A2 cells (lacking NE and P3), were incubated for 3 hours with NE- or P3-containing supernatants that were harvested from the ELA2- and PRTN3-transfected HMy2.CIR-A2 cells, respectively. Cell lysates were immunoprecipitated with anti-ubiquitin and subsequently analyzed for NE (B) or P3 (C) using immunoblotting. The leukemia cell line U-937 was used as a positive control for NE and P3 ubiquitination. Ub indicates ubiquitin; IP, immunoprecipitation; and IB, immunoblots.
NE and P3 source affects cross-presentation by mDCs and B-cells
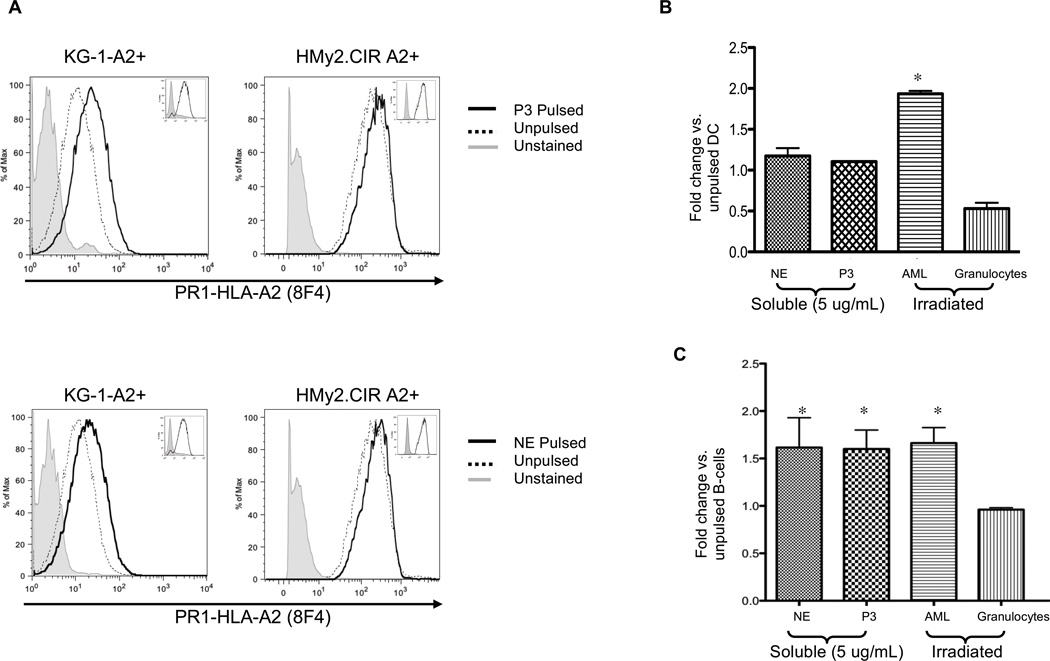
Having demonstrated uptake and ubiquitination of NE and P3, we next investigated whether they are cross-presented in normal and leukemia APCs, the latter includes KG-1-A2 and HMy2.CIR-A2.23, 32 Since HMy2.CIR-A2 cells lack endogenous NE, P3 and surface PR1/HLA-A2, they are an ideal cell type for studying NE and P3 cross-presentation. KG-1-A2 and HMy2.CIR-A2 cell lines were cultured for 6 and 18 hours, respectively, with 5 µg/mL NE or P3. These time points were selected as they provided maximal cross-presentation and >90% cell viability. Additionally, cross-presentation by DC subsets was shown to occur at early time points.33 After surface staining with 8F4,19 we detected increased PR1/HLA-A2 expression on the KG-1-A2 and HMy2.CIR-A2 (Fig. 4A). Since 8F4 binds PR1/HLA-A2, in which the HLA-A2 molecule makes up a large portion of the conformational epitope,19, 34 8F4 antibody bound HLA-A2 on unpulsed cells. However, greater staining in pulsed cells indicates PR1 cross-presentation.
Figure 4. Leukemia, mDCs and B-cells cross-present soluble and cell-associated NE and P3.
(A) A2-transfected KG-1 (KG-1-A2+) and -HMy2.CIR (HMy2.CIR A2+) leukemia cell lines were pulsed with NE or P3 (5 µg/mL) for 6 and 18 hours, respectively. Cells were stained with Alexa-647 conjugated 8F4 antibody and analyzed by flow cytometry. Results show increased PR1 presentation following cell pulsing. Insets show no change in HLA-A2 expression following NE or P3 pulsing. Results are representative of 3 separate experiments. (B) HLA-A2 positive normal mDCs or (C) B-cells were cultured with soluble NE (5 µg/mL), P3 (5 µg/mL), or irradiated leukemia (n=4) or granulocytes (n=3) at a 1:1 APC to irradiated-cell ratio for 6 hours. Cells were stained with Alexa-647-conjugated 8F4 antibody. CD33, CD34, CD38, HLA-A2, aqua live/dead stain and light scatter were used to exclude irradiated cells. CD19+ and CD11c+/CD14− were used to gate on B-cells and mDCs, respectively. (A–C) Since 8F4 binds PR1/HLA-A2, in which the HLA-A2 molecule makes up a large portion of the conformational epitope,19, 34 8F4 antibody bound HLA-A2 on unpulsed cells. However, greater staining in pulsed cells indicates PR1 cross-presentation. Results represent duplicate wells from 3 representative experiments. All data points are expressed as mean fold-change ± SD in the MFI of 8F4 staining compared with unpulsed cells. ANOVA followed by Tukey tests were performed using Prism 5.0 software (*P<0.05).
Since DCs take up apoptotic and necrotic neutrophils,35 and because neutrophils and myeloid leukemia cells express NE and P3 and have a high cell turnover, we investigated whether cell-associated NE and P3 are a source for cross-presentation. Primary leukemia and cell lines (HL-60 and U-937), as well as granulocytes, were irradiated and then co-cultured with normal APCs for 6 hours. Our results showed that cross-presentation by normal mDCs was significantly higher (P<0.05) when the NE and P3 source was irradiated leukemia (Fig. 4B). In contrast, B-cells cross-presented soluble and leukemia-associated NE and P3, but like mDCs, failed to cross-present NE and P3 from irradiated granulocytes (Fig. 4C). Results were similar irrespective of the HLA-A2 status of the irradiated cells, indicating that PR1 is cross-presented from NE and P3, and not transferred from the surface of the HLA-A2+ irradiated cells onto the APCs (data not shown).
NE and P3 are cross-presented but do not activate mDC or B-cells
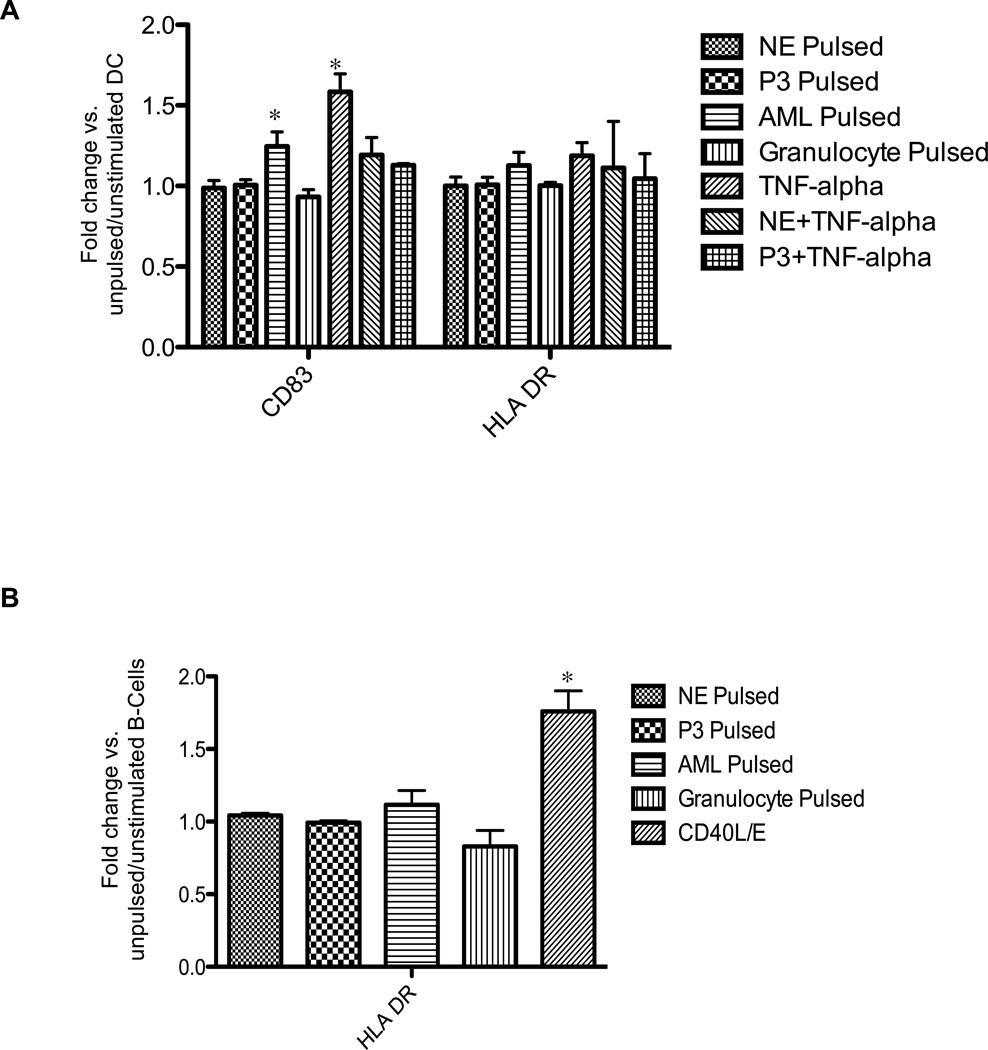
Because up-regulation of co-stimulatory markers on APCs is necessary for eliciting immune responses, we investigated the effects of NE and P3 source on APC surface expression of CD83 and HLA-DR. Immature mDCs and B-cells were grown in CM in the presence of 5 µg/mL of soluble NE or P3, irradiated leukemia or normal granulocytes. Flow cytometry showed that at early time points, coinciding with cross-presentation (90 minutes to 6 hours), mDCs (CD11c+CD14−) significantly increased expression of CD83 after pulsing with irradiated leukemia cells, but not after pulsing with soluble NE or P3, or granulocytes (Fig. 5A). Furthermore, soluble NE and P3 diminished TNF-α induced maturation. In addition, there was no increase in mDC activation as measured by HLA-DR. Similar down-regulation of mDC activation was previously reported following DC pulsing with apoptotic and necrotic neutrophils.35 With respect to normal B-cells, none of the NE or P3 sources activated normal B-cells as measured by surface staining for HLA-DR (Fig. 5B). We chose the 72-hour time point because B-cell activation by CD40L/E (positive control) was not evident at earlier time points and furthermore, there was no B-cell activation by any of the NE or P3 sources at earlier time points (data not shown).
Figure 5. Effects of NE and P3 on activation of normal mDCs and B-cells.
(A) Normal immature mDCs (CD11c+/CD14−) were cultured up to 6 hours with soluble NE or P3 (5 µg/mL), irradiated leukemia or granulocytes at 1:1 DC to irradiated-cell ratio, or with TNF-α (10 ng/mL) as a positive control. Cells were stained for CD83 or HLA-DR. Results represent 5 experiments performed in duplicate. (B) Normal CD19+ B-cells were enriched from healthy donor blood and cultured for 72 hours with NE or P3 (5 µg/mL), irradiated leukemia or granulocytes at 1:1 B-cell to irradiated-cell ratio, or with CD40L (500 ng/mL)/E (1 µg/mL) as a positive control. Cells were then stained for HLA-DR. Results represent 3 experiments performed in duplicate. All data points represent mean fold change ± SD in MFI of CD83 or HLA-DR compared with unpulsed cells. ANOVA followed by Tukey tests were performed using Prism 5.0 software (*P<0.05).
NE and P3 in AML share common elements of the MHC-I antigen-presenting pathway
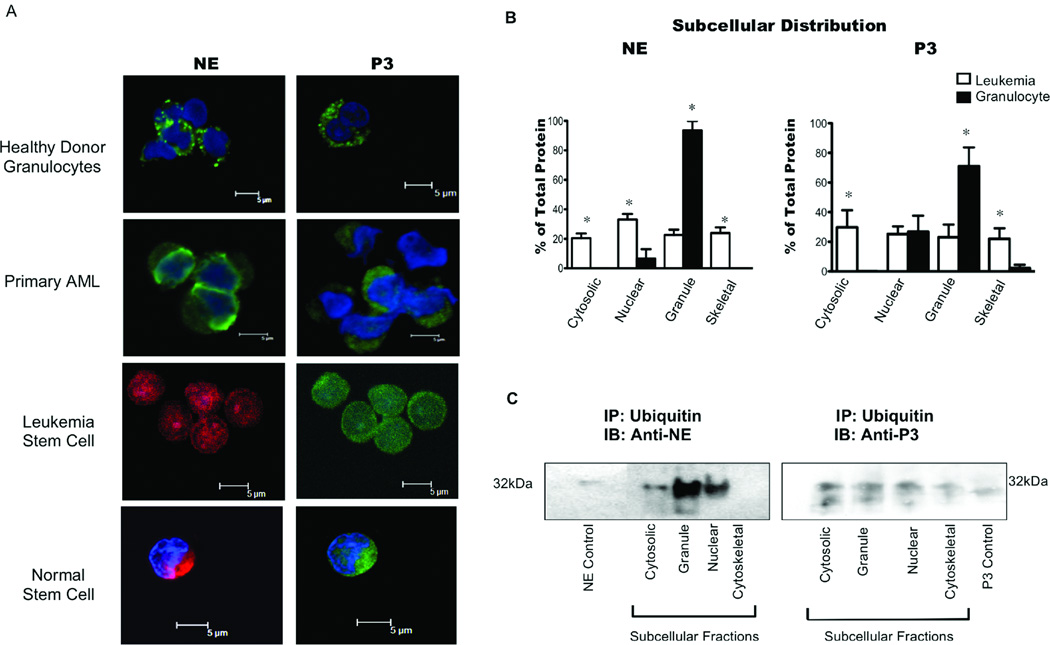
Cytosolic localization and ubiquitination of antigens facilitate their presentation on MHC-I.9, 31 We demonstrated ubiquitination of cytosolic NE and P3 in U-937 cells and the ELA2- and PRTN3-transfected HMy2.CIR cells (Fig. 3B and C) and previously reported higher levels of PR1 on leukemia cells compared with granulocytes.19 Thus, we investigated whether common elements of the MHC-I pathway are accessible to NE and P3 in primary leukemia by virtue of their cytosolic localization and ubiquitination. To identify the location of NE and P3 in granulocytes and leukemia blasts, we performed confocal microscopy and found that NE and P3 were present diffusely in leukemia blasts indicating localization outside the granules, in contrast with granulocytes where NE and P3 were within granules (Fig. 6A). Furthermore, we detected extragranular NE and P3 in stem cells from HD and leukemia patients (Fig. 6A).
Figure 6. Aberrant localization and ubiquitination of NE and P3 in myeloid leukemia.
(A) Confocal microscopy showed NE and P3 confined to granules in normal granulocytes as shown by distinct foci of fluorescence, and outside granules in AML blasts, as well as leukemic and normal stem cells. NE and P3 (Alexa-488) appear green in primary AML and healthy donor (HD) granulocytes. Because of their low frequencies, normal stem cells were FACS sorted from 1 HD bone marrow and pooled with sorted stem cells from 5 GM–CSF mobilized HD apheresis samples; sorted stem cells were also pooled from 7 different AML samples. All stem cells were simultaneously co-stained with anti-NE Alexa-647 (red) and anti-P3 Alexa-488 (green). DAPI blue was used to stain the nuclei. (B) Distribution of intracellular NE and P3 as a percentage of total NE and P3. IB of subcellular fractions from leukemia and granulocytes were analyzed by densitometry. Bars represent mean percent NE or P3 distribution ± SD from leukemia (n=20) and normal granulocyte (n = 4) samples. Two-tailed unpaired T-tests were used to compare the same fractions in healthy granulocytes and leukemia (*P<0.05). (C) Representative IB of NE and P3 following ubiquitin immunoprecipitation of representative AML patient sample. Subcellular fractions were immunoprecipitated with anti-ubiquitin antibodies, and resolved on a 10% polyacrylamide gel under reducing conditions. IB analysis for NE and P3 was subsequently carried out. NE (0.5 µg) and P3 (0.5 µg) were used as positive controls for IB.
To confirm extragranular localization of NE and P3, we performed IB on subcellular fractions from normal granulocytes and leukemia blasts. Quantitative assessment showed NE and P3 in all 4 fractions in the leukemia samples, in contrast to normal granulocytes where they were primarily located in the granule-containing membrane fraction and nucleus (Fig. 6B). IBs showed a significantly higher distribution of NE in cytosolic (20% vs. <1%), nuclear (33% vs. 6%) and skeletal (24% vs. <1%) fractions in leukemia (P<0.05) (Fig. 6B). The distribution of P3 was also higher in cytosolic (30% vs. <1%) and skeletal fractions (22% vs. 2%) in leukemia (P<0.05) (Fig. 6B). As expected, the distribution of NE (94% vs. 23%) and P3 (71% vs. 23%) were higher in the granule fractions in granulocytes (P<0.05). Table 1 shows the leukemia subtypes represented. We detected P3 in the nucleus of granulocytes, similar to what was previously reported for NE (Fig. 6B).36, 37 Furthermore, IB analysis of primary AML cells showed ubiquitinated NE and P3 in the cytosolic and granule fractions (Fig. 6C), supporting our hypothesis that in leukemia, aberrant localization of NE and P3 outside granules and ubiquitination may facilitate uptake and cross-presentation by APCs.
Table 1.
Myeloid leukemia samples used in determining subcellular localization of NE and P3.
| Sample | Leukemia type |
Source | Cell Count* (K/uL) |
%Blasts* | Cytogenetic/Molecular Abnormalities | Phenotype |
|---|---|---|---|---|---|---|
| Evaluated for NE | ||||||
| Pt #1 | FAB-M1 | LP | 42 | 89 | Diploid 46XY; FLT3+ | CD13/33/38/117+; MPO+ |
| Pt #2 | FAB-M1 | LP | 86 | 86 | Diploid 46XX; FLT3+ | CD13/33/117+; HLA-DR+ |
| Pt #3 | FAB-M1 | LP | 38 | 92 | Hyperdiploid 47XY; +4; FLT3+;NPM1+ | CD13/33/34/38/117+; MPO+ |
| Pt #4 | FAB-M5 | LP | 114 | 89 | Pseudodiploid 46XY; t(6;11) | CD13/14/33/64+; HLA-DR+; MPO+ |
| Pt #5 | FAB-M5 | LP | 58 | 87 | Hyperdiploid 47XY; t (9;11); +8 | CD33/117+; HLA-DR+ |
| Pt #6 | FAB-M5 | LP | 103 | 83 | Pseudodiploid 46XX; t (9;11); Ras+; FLT3+ | CD13/14/15/33/38/64+; MPO+ |
| Pt #7 | FAB-M1 | PB | 55 | 90 | Hyperdiploid 47XX; +13 | CD13/15/33/34/38/117+; HLA-DR+; MPO+ |
| Pt #8 | FAB-M1 | PB | 157 | 92 | Diploid 46XX; FLT3+ | CD13/33/34/38/64/117+; HLA-DR+; MPO+ |
| Pt #9 | FAB-M5 | PB | 34 | 98 | Hypodiploid 43–46XY; del 5q; del 7q; del17p; del12p | CD13/33+; HLA-DR+ |
| Pt #10 | N/D | LP | 48 | 68 | Pseudodiploid, 46XX, t(1;16) | CD13/33/34/117+; HLA-DR+ |
| U-937 | Monocytic | N/A | N/A | N/A | N/A | N/A |
| HL-60 | Promyelocytic | N/A | N/A | N/A | N/A | N/A |
| Evaluated for P3 | ||||||
| Pt #11 | FAB-M1 | LP | 38 | 97 | Diploid 46XX; FLT3+ | CD7/13/33/34/38/117+; HLA-DR+; MPO+ |
| Pt #12 | FAB-M5 | LP | 90 | 57 | Diploid 46XX | CD13/14/15/33/38/45/64+; MPO+ |
| Pt #13 | CML-CP | PB | 25 | 30 | Diploid 46XX; t (9;22) | N/D |
| Evaluated for Both Proteins | ||||||
| Pt #14 | FAB-M1 | LP | 373 | 97 | Diploid 46XY; FLT3+; b2a2 BCR-ABL | CD7/13/33/34/38/117+; HLA-DR+; MPO+ |
| Pt #15 | FAB-M2 | LP | 65 | 43 | N/D | CD13/33/64/38+ |
| Pt #16 | FAB-M7 | PB | 7 | 76 | Hyperdiploid 49XY; del (5); del (7) | CD34/41/117+, HLA-DR+ |
| Pt #17 | 2nd AML | LP | 40 | 73 | Pseudodiploid 46XX; del (5); +12; Ras+ | CD13/33/34/117+; HLA-DR+ |
| Pt #18 | CML-AP | LP | 305 | 13 | Diploid 46XY; t(9;22); b3a2 b2a2 BCR-ABL | CD7/13/33/34/38/117+; HLA-DR+; MPO+ |
FAB indicates French-American-British classification; LP, leukapheresis; PB, peripheral blood; del, deletion; CML-AP, accelerated phase CML; CML-CP, chronic phase CML; MPO, myeloperoxidase, N/D, not determined; and N/A, not applicable.
In leukapheresis samples, cell count and blast percent was determined prior to sample apheresis.
NE and P3 are cross-presented through a proteasome-dependent pathway
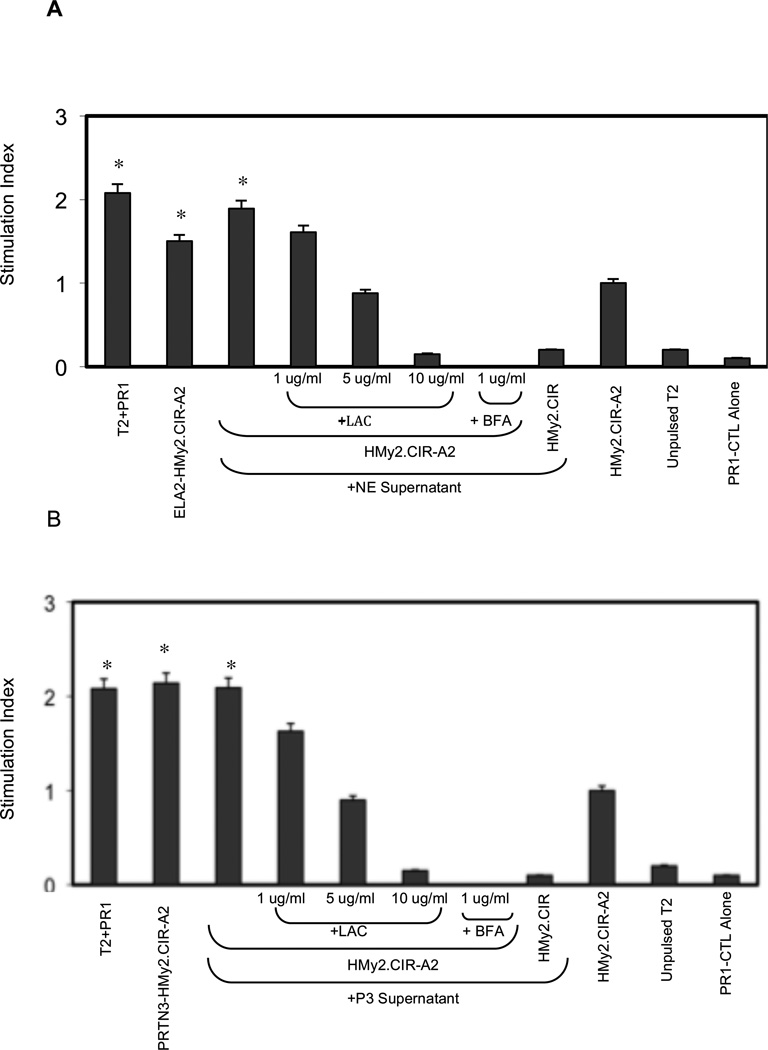
To determine whether the proteasome and Golgi are involved in PR1 cross-presentation by HMy2.CIR-A2 cells,12, 31, 38 which constitutively express the co-stimulatory molecules CD80 and CD86,23 we used lactacystin and BFA to inhibit proteasome and antegrade Golgi transporter, respectively. HMy2.CIR-A2 cells that were transfected with ELA2 or PRTN3 (Supplementary Fig. 2), or pulsed with NE or P3 containing supernatant caused increased proliferation of PR1-CTLs compared with NE- or P3-pulsed HMy2.CIR-A2 cells to which lactacystin or BFA were added (Figs. 7A, B). PR1-specific proliferation elicited by cross-presentation of exogenously pulsed cells was similar to the proliferation induced by ELA2 and PRTN3 transfected HMy2.CIR-A2 and PR1-pulsed T2 cells. These data suggest that NE and P3 are cross-presented via mechanisms involving proteasome and Golgi transport, since both lactacystin and BFA treatment of HMy2.CIR-A2 inhibited PR1-CTL proliferation.
Figure 7. Cross-presentation of PR1 by HMy2.CIR-A2 cells induces PR1-CTL proliferation through proteasome- and Golgi-dependent mechanisms.
Proliferation of PR1-CTL, measured by 48-hour BrDU uptake, was preferentially induced by HMy2.CIR-A2 cells transfected with (A) ELA2 (ELA2-HMy2.CIR-A2) and (B) PRTN3 (PRTN3-HMy2.CIR-A2) and by non-transfected HMy2.CIR-A2 cells that were cultured with (A) NE- or (B) P3-containing supernatant from ELA2-HMy2.CIR-A2 or PRTN3-HMy2.CIR-A2 cells, respectively. Cross-presentation of NE and P3 were proteasome- and Golgi-dependent, since it was abrogated by pretreatment of HMy2.CIR-A2 cells with lactacystin (LAC) and brefeldin A (BFA). Negative controls included unpulsed HMy2.CIR-A2, NE- or P3-pulsed HMy2.CIR cells (lacking HLA-A2), and unpulsed T2 cells. PR1-pulsed T2 cells were used as a positive control. Data represent the mean proliferation index ± SD of PR1-CTL in the experimental groups vs. PR1-CTL proliferation in control unpulsed HMy2.CIR-A2 group from 2 representative experiments (*P< 0.01).
Discussion
Since PR1-targeted therapies for myeloid leukemia have shown efficacy,4–7 we investigated factors that influence immunity to PR1. We show that in myeloid leukemia patients, serum and leukemia-associated NE and P3 may be an antigen source for cross-presentation. B cells and mDC cross-presented NE and P3 at early time points through mechanisms involving endosomes and proteasomal processing. mDC cross-presented NE and P3 only from irradiated leukemia, while B cells cross-presented from both cellular and non-cellular sources. Irrespective of antigen source, NE and P3 cross-presentation had no effect on activation status of either APC. We also provide evidence that in myeloid leukemia blasts, NE and P3 are ubiquitinated in extra-granular compartments, which can provide direct access to MHC-I antigen presenting pathways and may facilitate cross-presentation of leukemia-associated NE and P3. Finally as a clinical observation, we show that elevated serum NE or P3 in leukemia patients, which may be cross-presented, are correlated with dysfunctional PR1-CTLs.
Our data highlight the role of antigen source, co-stimulation and type of APC in determining immunity to cross-presented antigens. Immunity following antigen cross-presentation has been shown to be dependent on the viability of the cells providing the antigens. Spisek et al15 showed that antigen cross-presentation from apoptotic leukemia blasts induced leukemia immunity while Matheoud et al10 demonstrated immunity following DC cross-presentation of antigens from live cells. Other studies have correlated cross-presentation of apoptotic cells with tolerance and cross-presentation of necrotic cells with immune priming.39, 40 These different outcomes were attributed to the cytokines produced by the apoptotic cells and differential expression and activation of cell receptors and signaling pathways in the apoptotic and effector cells.15, 40, 41 Our data clarifies some of these discrepancies as we detected differences in the ability of mDC and B-cells to cross-present based on the antigen source and also demonstrated variable effects of antigen source on APC activation.
We show that cell-associated NE and P3 are more efficiently cross-presented by DCs. We believe that this is because NE and P3 are ubiquitinated and located in leukemia cytoplasm (Figs. 3b and 6), where they have access to MHC-I processing machinery42 and can undergo proteasome dependent cross-presentation, supporting our original hypothesis that PR1 cross-presentation may be facilitated by NE and P3 mislocalization in primary leukemia. The actual protein sources of cell-associated NE and P3 used by APC for cross-presentation of PR1 may be leukemia-associated ubiquitinated NE and P3 or NE and P3 peptides that are cleaved by the leukemia that may be favored for further antigen processing by proteasome.31, 43 Additionally, better antigen processing by DCs may be due to increased antigen uptake of chaperone-rich cell (i.e. leukemia) lysates, which in addition to facilitating antigen entry into the DC also prolong the protein half-life, two processes that are known to facilitate cross-presentation.44–46
Whether B-cells are able to cross-present antigen remains controversial. Keller et al13 showed that B-cells failed to present exogenous antigen on MHC-I, while others have shown that cross-presentation by B-cells elicits immune responses.47, 48 These conclusions about cross-presentation by B-cells were complicated by use of exogenous factors such as gene gun vaccination or CpG-DNA47, 48 that contribute to cell activation by stimulation of pattern recognition receptors. In addition, the studies were conducted in vivo49 where it may be difficult to discern cross-presentation from other cellular and molecular interactions that determine immune outcomes. In contrast, we used in vitro methods to directly detect PR1 cross-presentation by B-cells using anti-PR1/HLA-A2. Thus, we were able to delineate the relationship of peptide/HLA-A2 presentation vs. co-stimulatory molecule expression, a difficult differentiation to make using functional assays as the sole measurement of cross-presentation. Our results support the observation that B-cells can cross-present (Figs. 4 and 7). Because B-cells are not derived from malignant progenitor cells in myeloid leukemia, they are often present in normal numbers,24 and therefore may play a significant role in NE and P3 cross-presentation in leukemia. However, since exposure of B-cells to NE and P3 led to PR1 cross-presentation in the absence of B-cell activation (Figs. 4 and 5), our data suggest that B-cells may not play a role in the induction of PR1 immunity.
While B-cells and DC showed no significant evidence of activation with cross-presentation of NE or P3, PR1-CTLs were induced to proliferate by NE and P3 cross-presentation by HMy2.CIR-A2 cells, which constitutively express the co-stimulatory molecules CD80 and CD86.23 These results support a previous report that showed in vitro PR1-CTL expansion using NE- and P3-loaded monocytes.50 However, in that study the monocytes/DCs that were used to expand PR1-CTLs were cultured with GM-CSF, which up-regulates endogenous NE and P351 and therefore confounds the distinction between endogenous NE/P3 presentation and cross-presentation. Because we measured PR1/HLA-A2 surface expression on cells that lack endogenous NE and P3 (i.e. HMy2.CIR-A2 and normal B cell), our results provide direct evidence that PR1 is cross-presented from NE and P3 and in the setting of co-stimulation, can induce a PR1-specific immune response. Taken as a whole, our results suggest that eliciting in vitro immunity to PR1 may be enhanced by using leukemia cells as the source of antigen, which can be taken up by both DC and B cells, and ensuring sustained expression of co-stimulatory molecules by APCs. Such an approach could be utilized to enhance PR1 immunity in stem cell grafts prior to their administration to patients, and may be applicable to eliciting immunity to other LAAs. Moreover, since pulsing DCs directly with peptides or leukemia lysates was shown to generate immunity against leukemia in DC vaccine trials,15, 44, 52 our results expand on these prior reports by highlighting that immunity to such antigens could be made even more potent by ensuring appropriate sustained co-stimulation to accompany the cross-presentation of LAA by APCs.
Furthermore, the proliferation of PR1-CTL by P3-pulsed HMy2.CIR-A2 cells (Fig. 7) also suggests that anti-leukemia immunity might be elicited in vivo with interventions to increase and sustain PR1/HLA-A2 presentation coupled with treatments that maintain co-stimulation by APCs. This is supported by a previous study showing that in patients with Wegener's granulomatosis (WG), co-incubation of patient DC with P3 resulted in proliferation of autologous P3-specific T cells, which are thought to mediate the autoimmune vasculitis in WG.1 However, because PR1-CTL are deleted from the repertoire of leukemia patients,18 which confounds the direct effects of NE and P3 cross-presentation on PR1 immunity, further in vivo validation of PR1 cross-tolerance mechanisms using animal models is required.
Since we detected increased P3 and NE levels in leukemia patient serum and because soluble P3 and NE can lead to PR1 cross-presentation by APCs without cell activation, our data suggest that increased P3 and NE in myeloid leukemia may favor tolerance. Consistent with this hypothesis, we observed a decreased functional response by PR1-CTL in AML patients with elevated serum P3 or NE, compared with PR1-CTL from AML patients with low serum P3 or NE levels (Supplementary Fig. 1 and Table 1). While soluble P3 and NE correlated with disease burden in CML, which may lead to more tolerizing conditions (Supplementary Table 2), the lack of correlation of soluble P3 or NE with disease burden in AML suggests that there are additional mechanisms for facilitating tolerance conditions. Aberrant subcellular localization of P3 and NE in both AML and CML suggests a role for protein localization and cell turnover differences53, 54 in determining immune outcomes.
In summary, we provide direct evidence for cross-presentation of two clinically significant human leukemia-associated self antigens, NE and P3, and show that antigen mislocalization in leukemia critically affects antigen cross-presentation and possibly tumor susceptibility to immune attack. Our observations may be relevant to other tumors since many tumor antigens are derived from mislocalized self antigens. Further studies are under way to investigate this possibility in non-hematologic malignant cells.
Supplementary Material
Intracellular cytokine flow cytometry (CFC) analysis of 5 representative AML patient samples showed functional PR1-CTL responses in patients with low serum NE and P3 levels (Pt #1 and #2). AML patient samples were incubated with unpulsed T2 cells (top panel) or PR1-pulsed T2 cells (bottom panel). Live, lineage negative, CD8+ cells were analyzed for INF-γ and TNF-α production using intracellular CFC. Percent of CD8+ cells responding to PR1-T2 or unpulsed T2 cells is listed in each quadrant. A description of the patients is shown in supplementary Table S1.
HMy2.CIR-A2 cells were transfected using pCMS-GFP vector containing PRTN3 or ELA2 coding sequences. (A) Fluorescence microscopy demonstrating successful expression of GFP by HMy2.CIR-A2 cells following transfection with PRTN3- or ELA2-containing plasmid. (B) IB analysis showing expression of P3 (left panel) or NE (right panel) by bone marrow and peripheral blood cells, as well as PRTN3- or ELA2-transfected HMy2.CIR-A2 cells. HL-60 and HMy2.CIR-A2 were used as positive and negative controls, respectively. (C) IB analysis showing P3 and NE in supernatant from PRTN3- and ELA2-HMy2.CIR-A2 cells, respectively, but not in supernatant from un-transfected cells. (D) P3 and NE from PRTN3- and ELA2-transfected HMy2.CIR-A2 cells are glycosylated, similar to endogenous proteins from myeloid cells, as shown by the decrease in the molecular weight following digestion with N-Glycosidase F (PGnase F).
Acknowledgements
The authors thank Tomaz Zal and Anna Zal for their assistance in performing confocal microscopy.
Sources of Support:
This work was supported by grants from the National Institutes of Health (CA81247, CA49639, and CA100632 to J.J.M.), Leukemia and Lymphoma Society (SCOR 7262 to J.J.M.) and the American Society of Clinical Oncology Young Investigator Award (to G.A.)
G.A. and P.A. received partial salary support from the MD Anderson Barbara Rattay Advanced Scholars Program.
E.A.M. was supported by NIH grant 4R00CA133244-03
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Csernok E, Ai M, Gross WL, et al. Wegener autoantigen induces maturation of dendritic cells and licenses them for Th1 priming via the protease-activated receptor-2 pathway. Blood. 2006;107(11):4440–4448. doi: 10.1182/blood-2005-05-1875. [DOI] [PubMed] [Google Scholar]
- 2.Geraghty P, Rogan MP, Greene CM, et al. Neutrophil elastase up-regulates cathepsin B and matrix metalloprotease-2 expression. J Immunol. 2007;178(9):5871–5878. doi: 10.4049/jimmunol.178.9.5871. [DOI] [PubMed] [Google Scholar]
- 3.Molldrem J, Dermime S, Parker K, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88(7):2450–2457. [PubMed] [Google Scholar]
- 4.Molldrem JJ, Lee PP, Wang C, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6(9):1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 5.Yong AS, Rezvani K, Savani BN, et al. High PR3 or ELA2 expression by CD34+ cells in advanced-phase chronic myeloid leukemia is associated with improved outcome following allogeneic stem cell transplantation and may improve PR1 peptide-driven graft-versus-leukemia effects. Blood. 2007;110(2):770–775. doi: 10.1182/blood-2007-02-071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezvani K, Grube M, Brenchley JM, et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood. 2003;102(8):2892–2900. doi: 10.1182/blood-2003-01-0150. [DOI] [PubMed] [Google Scholar]
- 7.Rezvani K, Price DA, Brenchley JM, et al. Transfer of PR1-specific T-cell clones from donor to recipient by stem cell transplantation and association with GvL activity. Cytotherapy. 2007;9(3):245–251. doi: 10.1080/14653240701218524. [DOI] [PubMed] [Google Scholar]
- 8.Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111(1):236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cresswell P, Ackerman AL, Giodini A, et al. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 10.Matheoud D, Perie L, Hoeffel G, et al. Cross-presentation by dendritic cells from live cells induces protective immune responses in vivo. Blood. 2010;115(22):4412–4420. doi: 10.1182/blood-2009-11-255935. [DOI] [PubMed] [Google Scholar]
- 11.Robson NC, Hoves S, Maraskovsky E, et al. Presentation of tumour antigens by dendritic cells and challenges faced. Curr Opin Immunol. 2010;22(1):137–144. doi: 10.1016/j.coi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Robson NC, Donachie AM, Mowat AM. Simultaneous presentation and cross-presentation of immune-stimulating complex-associated cognate antigen by antigen-specific B cells. Eur J Immunol. 2008;38(5):1238–1246. doi: 10.1002/eji.200737758. [DOI] [PubMed] [Google Scholar]
- 13.Keller SA, von Allmen CE, Hinton HJ, et al. Follicular and marginal zone B cells fail to cross-present MHC class I-restricted epitopes derived from viral particles. J Immunol. 2009;182(10):6261–6266. doi: 10.4049/jimmunol.0804035. [DOI] [PubMed] [Google Scholar]
- 14.Perchellet A, Brabb T, Goverman JM. Crosspresentation by nonhematopoietic and direct presentation by hematopoietic cells induce central tolerance to myelin basic protein. Proc Natl Acad Sci U S A. 2008;105(37):14040–14045. doi: 10.1073/pnas.0804970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spisek R, Chevallier P, Morineau N, et al. Induction of leukemia-specific cytotoxic response by cross-presentation of late-apoptotic leukemic blasts by autologous dendritic cells of nonleukemic origin. Cancer Res. 2002;62(10):2861–2868. [PubMed] [Google Scholar]
- 16.Burgdorf S, Scholz C, Kautz A, et al. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9(5):558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 17.Olsson I, Olofsson T, Ohlsson K, et al. Serum and plasma myeloperoxidase, elastase and lactoferrin content in acute myeloid leukaemia. Scand J Haematol. 1979;22(5):397–406. doi: 10.1111/j.1600-0609.1979.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 18.Molldrem JJ, Lee PP, Kant S, et al. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest. 2003;111(5):639–647. doi: 10.1172/JCI16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sergeeva A, Alatrash G, He H, et al. An anti-PR1/HLA-A2 T cell receptor-like antibody mediates complement dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood. doi: 10.1182/blood-2010-07-299248. Epub 2011 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Cruz TG, Liu S, Khalili JS, et al. Natural splice variant of MHC class I cytoplasmic tail enhances dendritic cell-induced CD8 T-cell responses and boosts anti-tumor immunity. PLoS One. 2011;6(8):e22939. doi: 10.1371/journal.pone.0022939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabio G, Arthur JS, Kuma Y, et al. p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005;24(6):1134–1145. doi: 10.1038/sj.emboj.7600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molldrem JJ, Lee PP, Wang C, et al. A PR1-human leukocyte antigen-A2 tetramer can be used to isolate low-frequency cytotoxic T lymphocytes from healthy donors that selectively lyse chronic myelogenous leukemia. Cancer Res. 1999;59(11):2675–2681. [PubMed] [Google Scholar]
- 23.Kang X, Robbins PF, Fitzgerald EB, et al. Induction of melanoma reactive T cells by stimulator cells expressing melanoma epitope-major histocompatibility complex class I fusion proteins. Cancer Res. 1997;57(2):202–205. [PubMed] [Google Scholar]
- 24.Haferlach T, Winkemann M, Nickenig C, et al. Which compartments are involved in Philadelphia-chromosome positive chronic myeloid leukaemia? An answer at the single cell level by combining May-Grunwald-Giemsa staining and fluorescence in situ hybridization techniques. Br J Haematol. 1997;97(1):99–106. doi: 10.1046/j.1365-2141.1997.9662656.x. [DOI] [PubMed] [Google Scholar]
- 25.Henshaw TJ, Malone CC, Gabay JE, et al. Elevations of neutrophil proteinase 3 in serum of patients with Wegener's granulomatosis and polyarteritis nodosa. Arthritis Rheum. 1994;37(1):104–112. doi: 10.1002/art.1780370116. [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson S, Falk R, Yang JJ, et al. Increased expression of the secretory leukocyte proteinase inhibitor in Wegener's granulomatosis. Clin Exp Immunol. 2003;131(1):190–196. doi: 10.1046/j.1365-2249.2003.02024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dollery CM, Owen CA, Sukhova GK, et al. Neutrophil elastase in human atherosclerotic plaques: production by macrophages. Circulation. 2003;107(22):2829–2836. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- 28.Just J, Moog-Lutz C, Houzel-Charavel A, et al. Proteinase 3 mRNA expression is induced in monocytes but not in neutrophils of patients with cystic fibrosis. FEBS Lett. 1999;457(3):437–440. doi: 10.1016/s0014-5793(99)01098-4. [DOI] [PubMed] [Google Scholar]
- 29.Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat Immunol. 2004;5(7):670–677. doi: 10.1038/ni1089. [DOI] [PubMed] [Google Scholar]
- 30.Delamarre L, Pack M, Chang H, et al. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307(5715):1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 31.Rock KL, Gramm C, Rothstein L, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78(5):761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 32.Ackerman AL, Cresswell P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J Immunol. 2003;170(8):4178–4188. doi: 10.4049/jimmunol.170.8.4178. [DOI] [PubMed] [Google Scholar]
- 33.Di Pucchio T, Chatterjee B, Smed-Sorensen A, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9(5):551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porgador A, Yewdell JW, Deng Y, et al. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6(6):715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 35.Clayton AR, Prue RL, Harper L, et al. Dendritic cell uptake of human apoptotic and necrotic neutrophils inhibits CD40, CD80, and CD86 expression and reduces allogeneic T cell responses: relevance to systemic vasculitis. Arthritis Rheum. 2003;48(8):2362–2374. doi: 10.1002/art.11130. [DOI] [PubMed] [Google Scholar]
- 36.Kollner I, Sodeik B, Schreek S, et al. Mutations in neutrophil elastase causing congenital neutropenia lead to cytoplasmic protein accumulation and induction of the unfolded protein response. Blood. 2006;108(2):493–500. doi: 10.1182/blood-2005-11-4689. [DOI] [PubMed] [Google Scholar]
- 37.Papayannopoulos V, Metzler KD, Hakkim A, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chefalo PJ, Harding CV. Processing of exogenous antigens for presentation by class I MHC molecules involves post-Golgi peptide exchange influenced by peptide-MHC complex stability and acidic pH. J Immunol. 2001;167(3):1274–1282. doi: 10.4049/jimmunol.167.3.1274. [DOI] [PubMed] [Google Scholar]
- 39.Sauter B, Albert ML, Francisco L, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffith TS, Kazama H, VanOosten RL, et al. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178(5):2679–2687. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- 41.Chen W, Frank ME, Jin W, et al. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14(6):715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 42.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267(5195):243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 43.Bansal P, Mukherjee P, Basu SK, et al. MHC class I-restricted presentation of maleylated protein binding to scavenger receptors. J Immunol. 1999;162(8):4430–4437. [PubMed] [Google Scholar]
- 44.Zeng Y, Graner MW, Thompson S, et al. Induction of BCR-ABL-specific immunity following vaccination with chaperone-rich cell lysates derived from BCR-ABL+ tumor cells. Blood. 2005;105(5):2016–2022. doi: 10.1182/blood-2004-05-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 46.Donohue KB, Grant JM, Tewalt EF, et al. Cross-priming utilizes antigen not available to the direct presentation pathway. Immunology. 2006;119(1):63–73. doi: 10.1111/j.1365-2567.2006.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heit A, Huster KM, Schmitz F, et al. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol. 2004;172(3):1501–1507. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- 48.Hon H, Oran A, Brocker T, et al. B lymphocytes participate in cross-presentation of antigen following gene gun vaccination. J Immunol. 2005;174(9):5233–5242. doi: 10.4049/jimmunol.174.9.5233. [DOI] [PubMed] [Google Scholar]
- 49.Lazdina U, Alheim M, Nystrom J, et al. Priming of cytotoxic T cell responses to exogenous hepatitis B virus core antigen is B cell dependent. J Gen Virol. 2003;84(Pt 1):139–146. doi: 10.1099/vir.0.18678-0. [DOI] [PubMed] [Google Scholar]
- 50.Fujiwara H, El Ouriaghli F, Grube M, et al. Identification and in vitro expansion of CD4+ and CD8+ T cells specific for human neutrophil elastase. Blood. 2004;103(8):3076–3083. doi: 10.1182/blood-2003-07-2424. [DOI] [PubMed] [Google Scholar]
- 51.Sturrock A, Franklin KF, Hoidal JR. Human proteinase-3 expression is regulated by PU.1 in conjunction with a cytidine-rich element. J Biol Chem. 1996;271(50):32392–32402. doi: 10.1074/jbc.271.50.32392. [DOI] [PubMed] [Google Scholar]
- 52.Lee JJ, Kook H, Park MS, et al. Immunotherapy using autologous monocyte-derived dendritic cells pulsed with leukemic cell lysates for acute myeloid leukemia relapse after autologous peripheral blood stem cell transplantation. J Clin Apher. 2004;19(2):66–70. doi: 10.1002/jca.10080. [DOI] [PubMed] [Google Scholar]
- 53.Strife A, Lambek C, Wisniewski D, et al. Discordant maturation as the primary biological defect in chronic myelogenous leukemia. Cancer Res. 1988;48(4):1035–1041. [PubMed] [Google Scholar]
- 54.Faderl S, Harris D, Van Q, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces antiapoptotic and proapoptotic signals in acute myeloid leukemia. Blood. 2003;102(2):630–637. doi: 10.1182/blood-2002-06-1890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intracellular cytokine flow cytometry (CFC) analysis of 5 representative AML patient samples showed functional PR1-CTL responses in patients with low serum NE and P3 levels (Pt #1 and #2). AML patient samples were incubated with unpulsed T2 cells (top panel) or PR1-pulsed T2 cells (bottom panel). Live, lineage negative, CD8+ cells were analyzed for INF-γ and TNF-α production using intracellular CFC. Percent of CD8+ cells responding to PR1-T2 or unpulsed T2 cells is listed in each quadrant. A description of the patients is shown in supplementary Table S1.
HMy2.CIR-A2 cells were transfected using pCMS-GFP vector containing PRTN3 or ELA2 coding sequences. (A) Fluorescence microscopy demonstrating successful expression of GFP by HMy2.CIR-A2 cells following transfection with PRTN3- or ELA2-containing plasmid. (B) IB analysis showing expression of P3 (left panel) or NE (right panel) by bone marrow and peripheral blood cells, as well as PRTN3- or ELA2-transfected HMy2.CIR-A2 cells. HL-60 and HMy2.CIR-A2 were used as positive and negative controls, respectively. (C) IB analysis showing P3 and NE in supernatant from PRTN3- and ELA2-HMy2.CIR-A2 cells, respectively, but not in supernatant from un-transfected cells. (D) P3 and NE from PRTN3- and ELA2-transfected HMy2.CIR-A2 cells are glycosylated, similar to endogenous proteins from myeloid cells, as shown by the decrease in the molecular weight following digestion with N-Glycosidase F (PGnase F).