Abstract
Endothelialization of artificial vascular grafts is a challenging process in cardiovascular tissue engineering. Functionalized biomaterials could be promising candidates to promote endothelialization in repair of cardiovascular injuries. The purpose of this study was to synthesize hyaluronic acid (HA) and heparin based hydrogels that could promote adhesion and spreading of endothelial progenitor cells (EPCs). We report that the addition of heparin into HA-based hydrogels provides an attractive surface for EPCs promoting spreading and the formation of an endothelial monolayer on the hydrogel surface. To increase EPC adhesion and spreading, we covalently immobilized CD34 antibody (Ab) on HA-heparin hydrogels using standard EDC/NHS amine coupling strategies. We found that EPC adhesion and spreading on CD34 Ab immobilized HA-heparin hydrogels was significantly higher than their nonmodified analogs. Once adhered, EPCs spread and formed an endothelial layer on both nonmodified and CD34 Ab modified HA-heparin hydrogels after 3 days of culture. We did not observe significant adhesion and spreading when heparin was not included in the control hydrogels. In addition to EPCs, we also used human umbilical cord vein endothelial cells (HUVECs), which adhered and spread on HA-heparin hydrogels. Macrophages exhibited significantly less adhesion compared to EPCs on the same hydrogels. This composite material could possibly be used to develop surface coatings for artificial cardiovascular implants, due to its specificity for EPC and endothelial cells on an otherwise non-thrombogenic surface.
Keywords: hyaluronic acid, heparin, endothelial progenitor cells, cardiovascular implants
1. Introduction
Cardiovascular injuries are a major focus of the current research in vascular tissue engineering as they account for the number one cause of death in the United States (de Mel et al., 2008; Center for Disease Control and Protection, 2011). Given the current demand in cardiovascular tissue engineering, studies have been performed to obtain substrates using new functional biomaterials compatible with blood contacting prostheses. Repair of vascular endothelium is a challenging process which is directly tied to the longterm performance of any therapy or implant (Schmidt et al., 2004). Thus, endothelialization studies, may be an attractive area of research in cardiovascular tissue engineering. Endothelial progenitor cells (EPCs) are predifferentiated stem cells, which originate from circulating blood (Schmidt et al., 2004). EPCs can potentially differentiate into endothelial cells and they may be a promising cell source for endothelialization (Kaushal et al., 2001; Melero-Martin et al., 2007). In addition, it has been reported that the formation of an endothelial lining could potentially be achieved by using EPCs for tissue engineered cardiovascular implants, making EPCs an attractive cell type for in vivo applications as it may be possible to recruit circulating EPCs to endothelialize the surface of biomaterials (He et al., 2003).
A commonly encountered complication following surgical treatment in vascular systems is thrombogenesis (Schopka et al., 2010). For this reason, biomaterials with non-thrombogenic features might improve the success rate when used in surface treatment of blood contacting devices in vivo. HA is a hydrophilic polysaccharide, which is present in a variety of native tissues (Ji et al., 2006; Peppas et al., 2006; Slaughter et al., 2009; Suri and Schmidt 2009; Fujie et al., 2010; Lei et al., 2011). Although HA is an abundant extracellular matrix (ECM) component in cardiovascular tissues, it is a non-adhesive (Hu et al., 2000; Leach et al., 2003) substrate, limiting its application for cell spreading. To increase the ability of HA to induce cell spreading, one can add cell-adhesive molecules into HA (Camci-Unal et al., 2010). Heparin is one possible candidate since it is a non-thrombogenic material and has the ability to interact with endothelial cells (Barzu et al., 1986; Patton et al., 1995) Due to its highly charged nature, heparin interacts with a variety of proteins via electrostatic interactions (Trindade et al., 2008). Furthermore, heparin binds to plasma proteins, such as, fibronectin, vitronectin, platelet derived growth factor 4 and histidine-rich glycoprotein in a non-specific manner (Cosmi et al. 1997). Heparin also has been shown to interact with a variety of cell types, such as, epithelial cells, smooth muscle cells, hepatocytes, melanoma cells, and CHO cells (Trindade et al., 2008). In addition, heparin is also known to bind to endothelial cells (Hiebert and Jaques 1976; Glimelius et al., 1978; Jaques 1982; Barzu et al., 1984; Barzu et al., 1986; Psuja et al., 1987; Patton et al., 1995). Molecular weight, charge density and relative affinity for antithrombin (AT) are the main factors in heparin binding to endothelial cells (Barzu et al., 1986; Chan et al., 2004). For example, high molecular weight heparins bind to endothelial cells with higher affinity. Higher charge density also enhances the degree of binding to endothelial cells (Barzu et al., 1986). Oversulphation of heparin has also been shown to affect its binding to the endothelium (Barzu et al., 1986). Thus, higher negative charge density increases the binding affinity for endothelial cells indicating the significance of electrostatic interactions. As mentioned above, heparin is a negatively charged polysaccharide that interacts with positively charged protein residues in the ECM via electrostatic forces. For example, it has been reported that fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) have affinities against heparin (Zhang et al., 2006; Zieris et al., 2010). This feature may aid in attracting endothelial cells on heparin containing materials, as endothelial cells possess receptors for these molecules (Tsou and Isik 2001; Casu and Naggi 2003; Murga et al., 2004; Zieris et al., 2010).
Rapid re-endothelialization is considered as a promising treatment for thrombosis and restenosis on artificial implants (Chen et al., 2010). For instance, titanium was coated with a thin layer of collagen/heparin to improve biocompatibility. On these metals substrates attachment and proliferation of EPCs was found to be significantly enhanced to generate a confluent layer of EPCs after a 3-day culture period. Albumin-heparin mixtures have also been used as coatings on artificial grafts. This hybrid coating was reported to enable endothelial cells to adhere on the graft surface.(Bos et al., 1998). It was stated that heparin enhanced endothelial cell adhesion by binding to proteins and also provided an anticoagulant effect.
In a previous work, we used CD34 Ab modified methacrylated hyaluronic acid (HAMA) and hyaluronic acid-gelatin methacrylate (GelMA) mixtures to capture EPCs (Camci-Unal et al., 2010). Addition of GelMA allowed EPCs to spread and elongate on the hydrogel surfaces due to GelMA containing cell-adhesive amino acid functionalities, which induce cell spreading following adhesion. However, GelMA interacts with a wide range of cell types greatly decreasing the specificity of the approach (Aubin et al., 2010; Nichol et al., 2010).
In this study, we hypothesized that the addition of heparin into HA hydrogels may support formation of an endothelial layer on the hydrogel surface. To test this hypothesis, we developed HA-heparin containing hydrogels that allowed for adhesion and spreading of EPCs to form an endothelial monolayer on the hydrogel surface. This approach could be useful in coating artificial implants for cardiovascular tissue engineering applications.
2. Materials and Methods
2.1. Materials
Methacrylic anhydride, glycidyl methacrylate, 3-(trimethoxysilyl) propyl methacrylate, N-hydroxysuccinimide, alginate sodium salt (average molecular weight 46 kDa), heparin sodium salt (average molecular weight 18 kDa), 3-(N-morpholino) propanesulfonic acid sodium salt were purchased from Sigma-Aldrich Inc. (St. Louis, MO). 2-Hydroxy-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone (Irgacure 2959) was supplied by Ciba Specialty Chemicals Inc. (Florham Park, NJ). Sodium hyaluronate (average molecular weight 53 kDa) was obtained from Lifecore Biomedical Inc. (Chaska, MN). N-(3-Dimethylaminopropyl)-N″-ethylcarbodiimide hydrochloride was purchased from Thermo Fisher Scientific Inc. (Rockford, IL). Antihuman anti-CD34 Ab and its FITC labeled analog were supplied by BioLegend (San Diego, CA). Calcein AM stain was obtained from Invitrogen Corp. (Carlsbad, CA). Pre-cleaned microscope glass slides were obtained from Fisher Scientific Inc. (Waltham, MA). Medium for EPCs, HUVECs and macrophages and their components were purchased from Lonza Walkersville Inc. (Walkersville, MD) and ATCC (Manassas, VA), respectively. All reagents were used as received without further purification.
2.2. Synthesis and characterization of methacrylated hydrogel precursors
To synthesize photocrosslinkable hydrogel precursors (Figure 1), HA (Burdick et al., 2005), alginate (Chou et al., 2009) and heparin (Nilasaroya et al., 2008) were methacrylated by standard chemical procedures. Briefly, to synthesize HAMA 1 g sodium hyaluronate was dissolved in 100 mL distilled water and combined with 1 mL of methacrylic anhydride at 4 °C under constant stirring. The pH of this solution was kept at 8.0 with the addition of 5 M NaOH during the course of the reaction. This reaction was carried out for 24 h and the resulting solution was dialyzed against distilled water for 3 days and lyophilized to a solid product. To methacrylate alginate, 1 g alginate sodium salt was dissolved in distilled water to obtain a homogeneous solution. Then 1 mL methacrylic anhydride was added under stirring at 4 °C. To keep the pH of the reaction vessel at 8.0 during the methacrylation reaction, 5 M NaOH was added. Dialysis was performed for 3 days and this was followed by lyophilization to obtain methacrylated alginate (AlgMA). 1 g of heparin sodium salt was dissolved in 10 mL PBS (pH 7.4) and 1 mL glycidyl methacrylate was added. The mixture was kept at room temperature under vigorous stirring for 14 days, then precipitated in acetone, dialyzed against distilled water for 3 days and finally freeze dried to obtain methacrylated heparin (HepMA). We used 1H NMR to confirm the incorporation of methacrylate functionalities in the final products.
Figure 1.
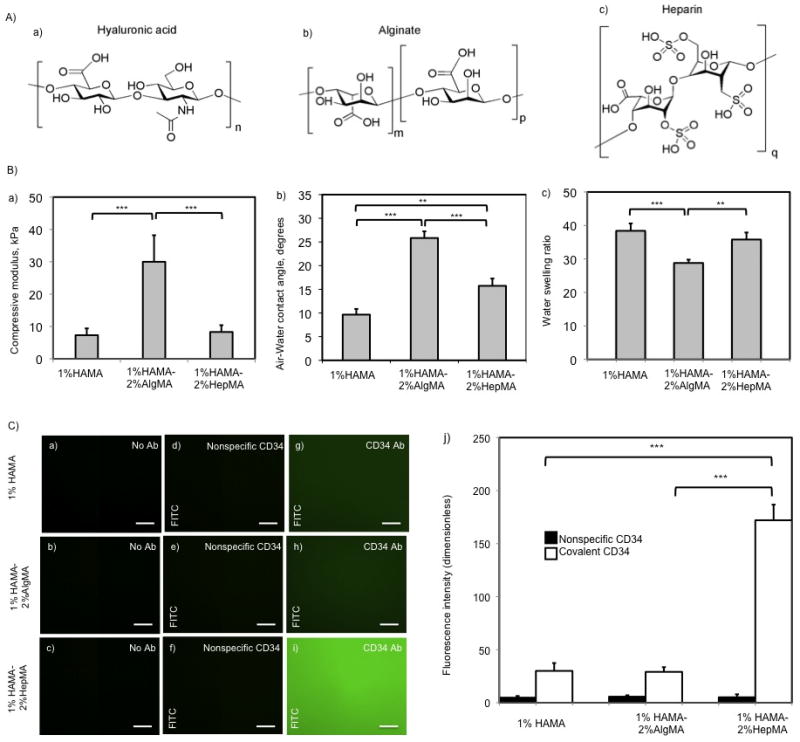
A) Molecules used to synthesize photocrosslinked hydrogels. a) Hyaluronic acid, b) Alginate, c) Heparin. B) Mechanical testing, contact angle measurement, and swelling ratio of 1%HAMA, 1%HAMA-2%HepMA, and 1%HAMA-2%AlgMA hydrogels. a) Compressive modulus of hydrogel substrates, b) Air-Water contact angles for hydrogels, c) Water swelling ratio for hydrogels used in the study (error bars: ±SD, **p < 0.01 and ***p < 0.001). C) Characterization of antibody conjugated HAMA-based hydrogels. (a,b,c) Fluorescent images of negative controls without antibody modification on (1% HAMA-2% AlgMA), 1% HA and (1% HAMA-2% HepMA) hydrogels respectively, (d,e,f) non-specific antibody adsorption on hydrogels without EDC/NHS activation, (g,h,i) fluorescent images for FITC-CD34 antibody immobilized 1% HA, (1% HAMA-2% AlgMA), and (1% HAMA-2% HepMA) hydrogel surfaces, respectively. Scale bars represent 100 μm. Error bars: ±SD, ***p < 0.001.
2.3. Photocrosslinked hydrogel formation
Photocrosslinkable hydrogels including 1% (w/v) HAMA, 1% (w/v) HAMA-2% (w/v) AlgMA and 1% (w/v) HAMA-2% (w/v) HepMA prepolymers were prepared in 0.5% (w/v) photoinitiator containing PBS. Once the solid components completely dissolved, we placed 20 μL of the prepolymer on a petri dish with 150 μm spacers. Then we covered the solution with a TMSPMA treated glass slide and placed a photomask under UV-light of 320–500 nm wavelength at an intensity of 2.5 mW/cm2 for 210 sec using the OmniCure Series 2000 (EXFO, Mississauga, Canada) to induce photocrosslinking. After this step unreacted polymers were washed away with PBS.
2.4. Physical properties of hydrogels
Mechanical properties for HA-based hydrogels were measured with an Instron 5542 mechanical tester (Norwood, MA). Equilibrium swollen hydrogel disks with 8 mm diameter and 1 mm thickness (n=4) were compressed at a rate of 0.2 mm per minute at room temperature. Compressive modulus was then calculated as the ratio of the stress to strain in the linear region of the curve.
To assess hydrophilicity of different hydrogel surfaces, static water contact angle measurements were carried out with a VCA 2500XE contact angle measurement system (Advanced Surface Technology, MA) using 10 μl deionized water droplet (n=3). The air-water contact angles were determined by using ImageJ software.
To measure swelling behavior of the gels, disks that were 8 mm in diameter and 1 mm in thickness (n=4) were immersed in 1 mL PBS for 24 h. Excess PBS was removed by gently blotting and the wet weight of the hydrogels was measured. The gel disks were then frozen and lyophilized to determine their dry weight. We calculated the equilibrium swelling ratio by dividing the wet weight with the corresponding dry weight of the hydrogels.
2.5. Covalent immobilization of CD34 Ab on HA-based hydrogels
We kept HA-based hydrogels in 0.5 M MES (pH 5.6) buffer for half an hour to prepare the surface for activation. Following aspiration of the buffer we added 0.2 M NHS and 0.1 M EDC on the hydrogels and incubated for an hour. Then we removed this solution and added PBS for 10 minutes to wash away unreacted functionalities. Afterwards, we placed 25 μg/mL FITC-labeled or unlabeled anti CD34 Ab on the surface of activated hydrogels and incubated at 4 °C overnight to facilitate covalent immobilization of the Abs. Subsequently we removed the Ab solution, washed the surface with PBS and incubated the hydrogels in PBS for 6 h to remove nonspecifically adsorbed Abs.
2.6. Characterization of CD34 Ab immobilized hydrogels
We used a Nikon inverted microscope (TE-2000-U) (Melville, NY) to measure the fluorescence from FITC-labeled CD34 Ab immobilized hydrogels. To quantify the fluorescence intensity from these images we utilized ImageJ software. Background fluorescence was obtained by imaging the nonmodified surfaces and these control values were subtracted from the fluorescence intensity of the CD34 Ab immobilized analogs. We performed these experiments in triplicate.
2.7. Cell culture experiments
Human EPCs were isolated from cord blood as published previously.(Melero-Martin, Khan et al. 2007) These cells were cultured in 20% fetal bovine serum (FBS) and 1% L-Glutamine supplemented EBM-2 media with all the components from the bullet kit except hydrocortisone. Human umbilical cord vein endothelial cells (HUVECs) were cultured in 2% FBS supplemented EBM-2 media with all the components from the bullet kit. Macrophage P388D1 cells were a kind gift from Dr. Blaine Pfeifer’s lab. Macrophages were cultured in 10% FBS supplemented RPMI-1640 media. 1% Pen-Strep was added to all culture media for the cell experiments. We changed the media every two to three days and harvested cells upon reaching confluence. We kept the cell cultures in a 5% CO2 supplemented incubator at 37 °C.
Once hydrogels were crosslinked under UV light, they were seeded with EPCs, HUVECs or macrophages and cultured in their corresponding media (as explained above) in a 37 °C incubator with 5% CO2 supplementation. Following 1 h incubation period, nonadherent cells were removed by changing the media of the cultured cells on the hydrogel surfaces. Media was then changed every day during the culture period.
To determine the effect of adsorbed serum proteins on adhesion and spreading of EPCs, cells were cultured on nonmodified 1%HAMA-2%HepMA hydrogels in endothelial media that was supplemented with 20%, 10% or 2% (v/v) fetal bovine serum (FBS). In addition, the effect of FGF and VEGF in endothelial media on adhesion and spreading of EPCs on nonmodified 1%HAMA-2%HepMA hydrogels were tested by excluding FGF and VEGF from the EPC media.
2.8. Cell adhesion
Nonmodified or CD34 Ab modified HA-based hydrogels were seeded with 2.8×103 cells/mm2. Samples were taken at different time points (1 h and 3 days). Nonadherent cells were washed away with PBS and samples were stained with Calcein AM to test cell viability on HA-based hydrogels. Calcein AM indicates esterase activity in metabolically active cells. Three different images from three replicate experiments were used in quantification of cell capture and spreading by ImageJ software.
2.9. Statistical analysis
Statistical analysis was processed by GraphPad Prism Inc (La Jolla, CA) by utilizing one-way, two-way ANOVA and Bonferroni post-hoc tests. All data were given as mean ± standard deviation (***p < 0.001, **p < 0.01, *p < 0.05).
3. Results
We fabricated and characterized HA-heparin based hydrogels (Figure 1) to increase adhesion and spreading of EPCs. We also examined the effect of immobilization of CD34 Ab on the HA-based hydrogels on increasing the EPC adhesion and spreading. Adhesion of EPCs was found to be affected by hydrogel type and Ab modification. Nonadhesive hydrogels made from 1% HAMA or 1% HAMA-2% AlgMA, did not show significant attachment or cell spreading following three days in culture (Figure 2). The 1% HAMA-2% HepMA combination with and without CD34 Ab modification provided significantly higher cell attachment compared to all other conditions and demonstrated formation of an endothelial monolayer on hydrogel surfaces following 3 days in culture (p<0.001). Here we will provide detailed results for hydrogel characterization, Ab immobilization and cell adhesion in the following sections.
Figure 2.
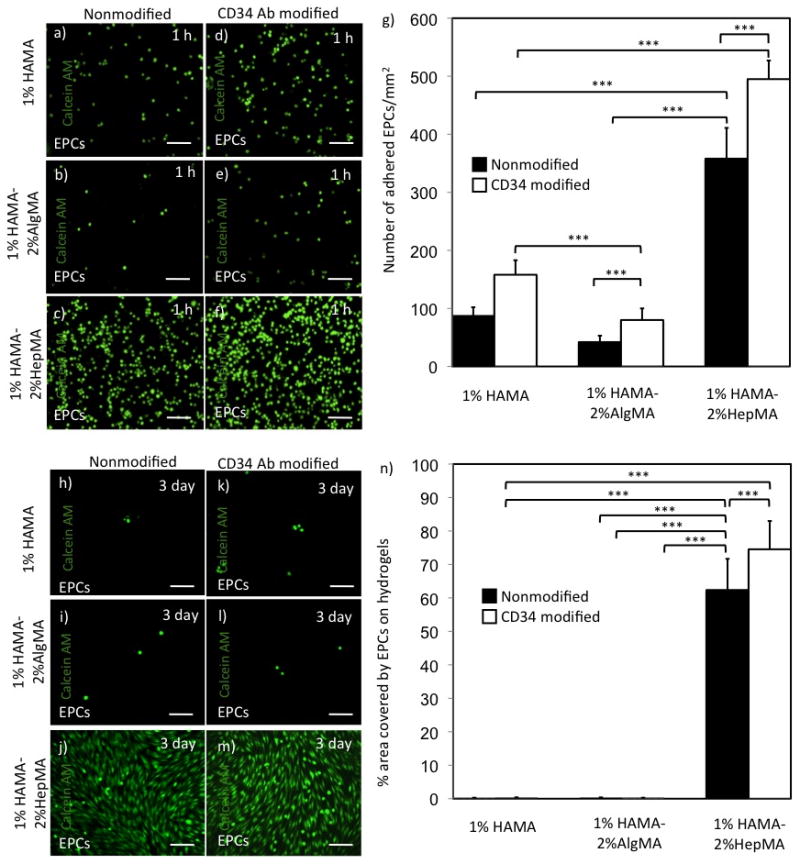
EPC adhesion and spreading on various hydrogel surfaces over time. Adhered EPCs on HA-based hydrogels at 1 h. (a,b,c) Number of adhered EPCs on nonmodified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels respectively, (d,e,f) EPC number on CD34 Ab modified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels, respectively. (g) Number of attached EPCs/mm2 at 1 h on HA-based hydrogels. EPC spreading and elongation on HA-based hydrogels at day 3. (h,i,j) EPCs on nonmodified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels respectively, (k,l,m) EPCs on CD34 Ab modified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels respectively, (n) % area covered by adhered EPCs at day 3 on HA-based hydrogels. Scale bars represent 100 μm. Error bars: ±SD, ***p < 0.001.
3.1. Physical properties of hydrogels
To characterize the physical properties of the synthesized gels, we determined compressive moduli, air-water contact angle and water swelling ratio. The compressive moduli for 1% HAMA and 1%HAMA-2%HepMA hydrogels were determined to be not statistically different (Figure 1.B). However, 1% HAMA-2% AlgMA hydrogels showed significantly higher compressive modulus (30.0±8.2 kPa) compared to the rest of the materials. Air-water contact angles were determined to be closer for 1% HAMA and 1%HAMA-2%HepMA hydrogels compared to their 1% HAMA-2% AlgMA analog, which was significantly higher, 25.8±1.4 degrees (p<0.001). Similarly, water swelling ratio for 1% HAMA and 1%HAMA-2%HepMA hydrogels were found to be close compared to 1% HAMA-2% AlgMA hydrogels (p>0.05). In this case, 1% HAMA-2% AlgMA hydrogels were swollen significantly less relative to the rest of the substrates, providing a swelling ratio of 28.8±1.0.
Swelling is an indication of the water content that reflects the hydrophilicity of the material. The greater the hydrophilicity of the material the smaller the air-water contact angle will be. In addition, higher water content makes the material less stiff. Our results are in good agreement with these facts. For instance, alginate containing surfaces afforded higher contact angles compared to the rest of the samples with higher stiffness and demonstrated less swelling.
3.2. Characterization of Ab immobilized hydrogels
We immobilized CD34 Abs on HA-based hydrogels to find out the effect of Ab addition on adhesion and spreading of EPCs. Following covalent CD34 Ab attachment, the relative amount of immobilized Ab on hydrogel surfaces was measured by fluorescent microscopy. The data was then processed by ImageJ for quantification (Figure 1.C). The fluorescence intensity of non-EDC/NHS activated nonmodified hydrogel surfaces was taken as the background value and subtracted from the corresponding materials. In addition to covalent CD34 Ab modification, nonspecific Ab adsorption was also studied. There was no statistical difference between the amount of nonspecific Ab adsorption on all three hydrogel surfaces. Furthermore, nonspecific Ab adsorption was quantified to be significantly less than that of covalently attached Abs for all three hydrogels. The amount of CD34 Ab immobilized on 1% HAMA and 1% HAMA-2% AlgMA surfaces was not significantly different (p>0.05). On the other hand, 1%HAMA-2%HepMA surface showed higher fluorescence intensity suggesting a substantial difference compared to other two surfaces (p<0.001).
3.3. Cell adhesion and spreading
Following synthesis and characterization, HA-based hydrogels were used for cell adhesion and spreading experiments. Cell adhesion was quantified 1 h after seeding the cells on these hydrogels (Figure 2.a–g). There were higher number of EPCs on CD34 Ab modified 1%HAMA-2%HepMA surfaces as compared to its nonmodified analog (495±32 and 358±53 EPCs/mm2, respectively, p<0.001). As expected, a higher number of EPCs were found on CD34 Ab modified 1% HAMA surfaces as compared to the nonmodified 1% HAMA hydrogels. Similarly, CD34 Ab immobilized 1% HAMA-2% AlgMA hydrogels captured higher number of EPCs in comparison to its nonmodified version.
To study the adhesion behavior of endothelial cells, we used HUVECs and performed similar experiments as done with EPCs. Higher number of HUVECs were counted on CD34 Ab modified 1%HAMA-2%HepMA hydrogels in comparison to its nonmodified counterparts (398±39 and 306±46 HUVECs/mm2, respectively, p<0.001). Similarly, there were more HUVECs adhered on CD34 modified 1% HAMA hydrogels as compared to its nonmodified analog (Figure 4.a–g). Also the other surfaces containing CD34 Ab modified 1% HAMA-2% AlgMA allowed for higher HUVEC adhesion as opposed to its nonmodified analog. We also measured cell adhesion for macrophages, which is taken as a model of a non-endothelial cell lineage. There was no significant difference in macrophage adhesion on the CD34 Ab modified and nonmodified 1%HAMA-2%HepMA surfaces (140±17 and 135±22 macrophages/mm2, respectively) (Figure 5.a–g). There was more macrophages, which adhered on CD34 Ab modified 1% HAMA surface compared to the nonmodified 1% HAMA hydrogels. Similarly, CD34 Ab immobilized 1% HAMA-2% AlgMA hydrogels captured higher number of macrophages compared to its nonmodified version.
Figure 4.
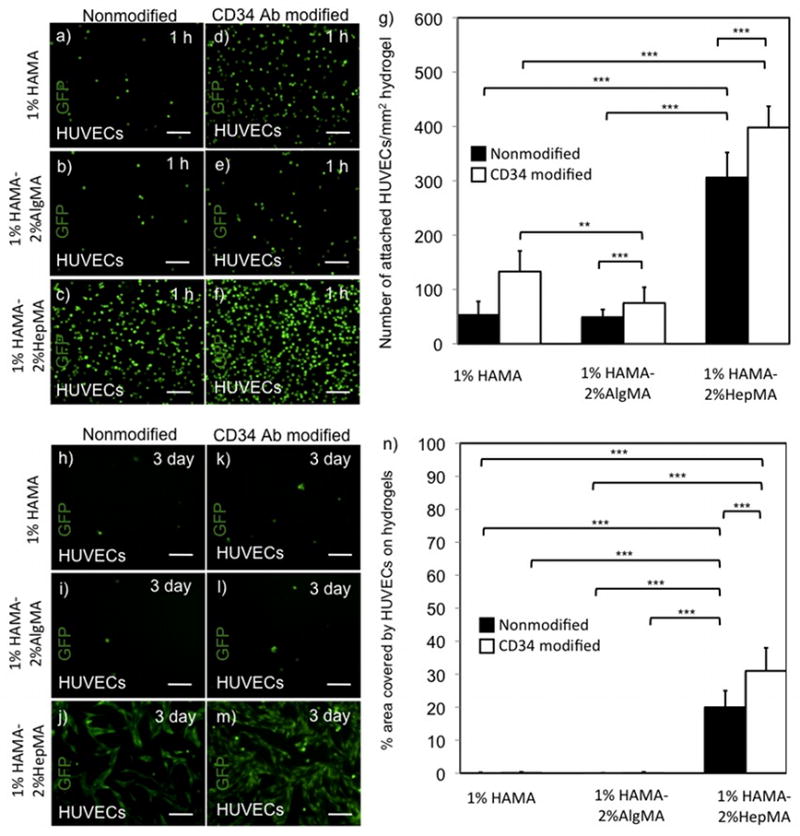
HUVEC attachment and spreading on hydrogel surfaces over time. Adhered HUVECs on HA-based hydrogels at 1 h. (a,b,c) HUVECs on nonmodified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels respectively, (d,e,f) HUVECs on CD34 Ab modified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels, respectively, (g) Number of attached HUVECs/mm2 at 1 h on HA-based hydrogels. HUVEC spreading and elongation on HA-based hydrogels at day 3. (h,i,j) HUVECs on nonmodified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels respectively, (k,l,m) HUVECs on CD34 Ab modified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels respectively, (n) % area covered by adhered HUVECs at day 3 on HA-based hydrogels. Scale bars represent 100 μm. Error bars: ±SD, **p < 0.01 and ***p < 0.001.
Figure 5.
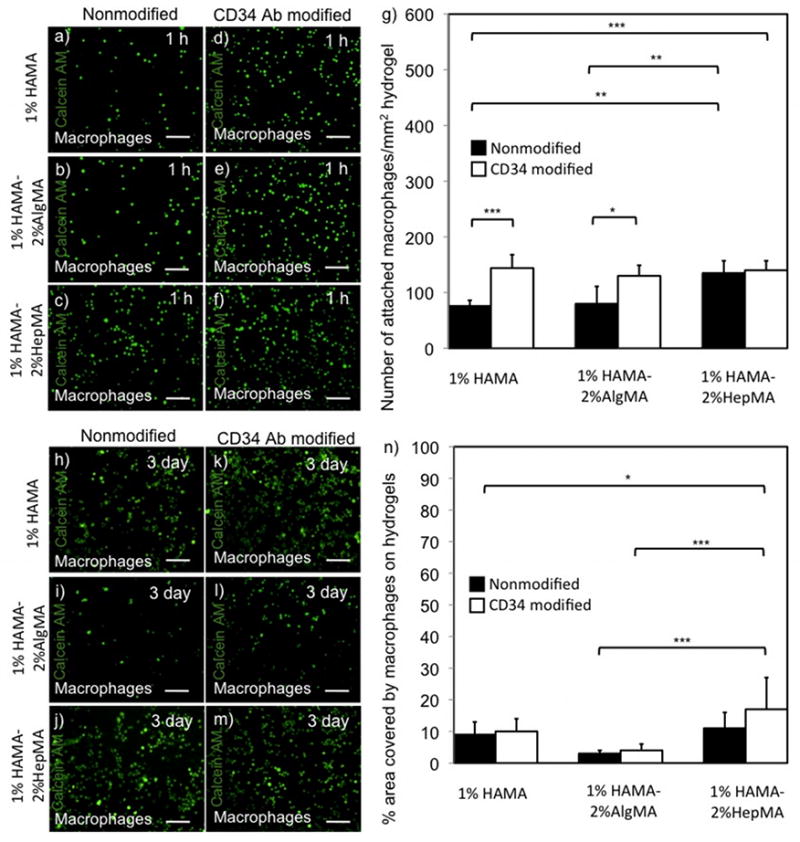
Macrophage adhesion and spreading on hydrogel surfaces over time. Adhered macrophages on HA-based hydrogels at 1 h. (a,b,c) macrophages on nonmodified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels respectively, (d,e,f) macrophages on CD34 Ab modified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels, respectively, (g) Number of attached macrophages/mm2 at 1 h on HA-based hydrogels. Macrophages on HA-based hydrogels at day 3. (h,i,j) macrophages on nonmodified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels respectively, (k,l,m) macrophages on CD34 Ab modified 1% HAMA+2% AlgMA, 1% HAMA and 1% HAMA+2% HepMA hydrogels respectively, (n) % area covered by adhered macrophages at day 3 on HA-based hydrogels. Scale bars represent 100 μm. Error bars: ±SD, *p < 0.05, **p < 0.01 and ***p < 0.001.
In addition to initial cell adhesion, we also quantified spreading at a later time point after 3 days of culture. To quantify spreading of cells on HA-based hydrogels we measured the percent area occupied by cells on these hydrogels. The percent surface area covered by EPCs on CD34 Ab modified 1%HAMA-2%HepMA hydrogels was higher than its nonmodified analog (75±9 and 62±9 percent, respectively) (Figure 2.h-n). Only a small area was occupied by EPCs on both CD34 Ab modified and nonmodified 1% HAMA surfaces. Similarly, both CD34 Ab immobilized and nonmodified 1% HAMA-2% AlgMA hydrogels provided significanty lower % area covered by EPCs. Spreading for the other endothelial cell line, HUVECs, on CD34 Ab modified and nonmodified 1%HAMA-2%HepMA hydrogels was significantly higher compared to all other hydrogels (31±7 and 20±5 percent, respectively) (Figure 4.h–n). The percent hydrogel area covered by macrophages on both CD34 Ab modified and non-modified 1%HAMA-2%HepMA hydrogels was lower as opposed to EPCs and HUVECs after 3 days of culture (17±10 and 11±5 percent, respectively) (Figure 5.h–n).
We also calculated cell shape factor as an indication of EPC spreading using the equation given below:
where A and P are the area and the perimeter length of the cell, respectively, and S is the unitless shape factor. We used ImageJ to calculate shape factor for EPCs on different hydrogel surfaces. A shape factor of 0 defines a straight line whereas 1 describes a perfect circle in our calculations. EPCs on nonmodified and CD34 Ab modified 1%HAMA-2%HepMA hydrogels yielded an average shape index of 0.14±0.03 and 0.13±0.02, respectively, indicating significant cell elongation (Figure 3). In contrast, 1%HAMA-2%AlgMA hydrogels resulted in shape factors of 0.92±0.04 and 0.93±0.02 on nonmodified and CD34 Ab modified surfaces, demonstrating no cell spreading. We obtained a shape factor of 0.93±0.01 and 0.93±0.03 for EPCs on nonmodified and Ab modified 1% HAMA hydrogels, which also demonstrates no elongation.
Figure 3.

Shape factor for EPCs on HA-based hydrogels after 3 days of culture. Scale bars represent 100 μm. Error bars: ±SD, ***p < 0.001.
4. Discussion
In this work we showed that incorporation of heparin into HA based hydrogels promotes adhesion and spreading of EPCs supporting formation of an endothelial layer on the hydrogel surface.
4.1. Physical properties of hydrogels
Stiffness of hydrogels has been reported to be a critical factor in cell adhesion and spreading.(Lee et al., 1998; Chen et al., 2008) We showed that 1% HAMA-2% AlgMA hydrogels had the highest stiffness (30.0±8.2 kPa) among the hydrogels used in this study (p<0.001); however, cell attachment to this substrate was found to be poor (Figure 1.B). On the other hand, excellent cell adhesion and spreading was found on 1%HAMA-2%HepMA hydrogels even though the stiffness was determined not to be significantly different than their 1% HAMA analogs. Therefore, it is possible that the adhesion of endothelial cells on 1%HAMA-2%HepMA surfaces is more strongly dominated in this system by factors other than stiffness. These factors can potentially be but not limited to protein adsorption (Hattori et al., 1985), surface charge (Hattori et al., 1985; Lee et al., 1998), water content (Hattori et al., 1985), functional groups (Maroudas 1975; Lee et al., 1998) and roughness (Lee et al., 1998), that may affect cell adhesion and growth on biomaterial surfaces.
Electrostatic charges and type of chemical functional groups are also important factors for cell adhesion and spreading in addition to the wettability characteristics of the surface.(Lee et al., 1998). Interactions between cells and biomaterial surfaces are complicated, hence, it is often unclear which factor plays a dominant role in cell adhesion and growth (Lee et al., 1998). Materials with moderate degree of hydrophilicity/hydrophobicity were reported to be more cell compatible compared to their extremely hydrophilic or hydrophobic analogs (Chen et al., 2005). Dispersive and electrostatic forces can also be used to explain cells adhesion events (Maroudas 1975). These forces can be effective depending on the location. For instance, negatively charged surfaces are repellant to cells in the long range, however, they can bind to polar functionalities on the cell membrane in the close range (Maroudas 1975). Another factor for cell attachment is serum proteins adsorbed on biomaterial surfaces promoting cell adhesion (Lee et al., 1998). Proteins contain polyelectrolytic charges bridging positive and negative charges on cell and hydrogel surfaces. For this reason, serum proteins might get adsorbed on negatively charged hydrogel surfaces enhancing the cell adhesion (Chen et al., 2010). Hydrogen-bond formation between polar functional groups on the cells and biomaterial surfaces has also been reported to enhance cell adhesion (Lee et al., 1998).
4.2. Covalent immobilization of CD34 Ab on HA-based hydrogels and characterization
We quantified the amount of CD34 Ab on hydrogel surfaces for both nonspecific adsorption and covalent immobilization cases using ImageJ by means of fluorescence intensity (Figure 1.C). The background value was taken as the fluorescence intensity from non-EDC/NHS activated hydrogel surfaces and was subtracted from corresponding materials. Background fluorescence was very low for all three materials in each case. No significant difference was measured for nonspecifically adsorbed FITC labeled CD34 Abs on any of the hydrogel surfaces. This result was expected since 150 μm thick hydrogels were incubated in PBS long enough to allow nonspecifically adsorbed Abs to diffuse out. For covalent modification with CD34 Abs, we did not observe a significant difference in the amount of Ab immobilized on 1% HAMA and 1% HAMA-2% AlgMA hydrogels. This could be due to having similar chemical structure and molecular weight for HA and alginate. However, the amount of covalently attached CD34 Ab on 1%HAMA-2%HepMA surfaces was significantly higher compared to the rest of the hydrogels. This could be occurring due to the strong anionic functional groups on heparin. In addition to covalent immobilization, there may be noncovalent interactions between ionic groups of heparin and CD34 Ab increasing the amount of immobilized Ab.
4.3. Cell adhesion and spreading
We quantified cell adhesion on HA-based hydrogels after 1 hour and spreading after 3 days of culture. All CD34 Ab immobilized hydrogel surfaces provided significant differences in initial EPC adhesion as expected as EPCs express CD34 antigens on their cell membranes (Yu et al., 2004; Aoki et al., 2005; de Mel et al., 2008). When heparin was not included in HA hydrogels, initially adhered cells detached from the surface and the remaining cells did not spread following a 3 day culture. This is likely to be a result of having a substrate, which is resistant for protein adsorption. Once 2% heparin methacrylate was incorporated into 1% HA, the number of adhered EPCs increased, then cell spreading was triggered possibly due to protein and growth factor adsorption on heparin hydrogels from the cells and culture media. This result was consistent for both Ab modified and nonmodified 1% HAMA-2% HepMA hydrogels. Moreover, it has been reported that FGF and VEGF have an affinity to bind to heparin (Zhang et al., 2006; Zieris et al., 2010). These growth factors are present in endothelial cell culture media possibly interacting with heparin containing materials inducing cell adhesion and spreading.
HUVECs were also used as another model for endothelialization of heparin included hydrogels. In this case, HUVECs provided similar cell adhesion and spreading results as with EPCs. In order to demonstrate the adhesion was specific for heparin we also used another polysaccharide, alginate, which is known as a nonadhesive substrate (Jeon et al., 2009). Although CD34 Ab immobilization made a significant difference in initial cell adhesion, no cell spreading was observed for alginate containing hydrogels as expected. Interestingly, macrophages also exhibited a small degree of adhesion on HA-based hydrogels which may have resulted from the presence of CD44 molecules on macrophages as CD44 is a well-known receptor for HA (Kennel et al., 1993). We observed higher macrophage adhesion on 1% HAMA hydogels in this study compared to our previous work (Camci-Unal et al., 2010). This could potentially be due to the combination of a number of factors, such as, higher cell density, higher passage number and different UV power and exposure.
4.4. Protein adsorption
We determined protein adsorption on HA-based hydrogels using 1 mg/mL fluorescein labeled BSA. HA and alginate are known to be resistant to adsorption of negatively charged proteins (Vanwachem et al., 1987; Rowley et al., 1999). Our data is in agreement with this observation. The amount of 1 mg/mL BSA adsorption on 1% HAMA and 1% HAMA-2% AlgMA surfaces were measured to be significantly lower than that of 1%HAMA-2%HepMA hydrogels (Supporting Figure S2). We did not observe a significant difference in protein adsorption on 1% HAMA and 1% HAMA-2% AlgMA hydrogels. This could be attributed to the abundance of hydroxyl functionalities in both structures. On the other hand, a high number of negatively charged sulfur groups in heparin structure produce interactions with positively charged protein functionalities (Trindade et al., 2008) resulting in accumulation of protein on 1%HAMA-2%HepMA surfaces. Furthermore, this is in agreement with other literature data that heparin is the most negatively charged glycosaminoglycan (GAG) because of the presence of negatively charged sulfo functionalities (REF Salek-Ardakani et al., 2000; Linhardt et al, 2002). Therefore, heparin interacts with positively charged ligands stronger than other GAGs. Due to this reason, we think that heparin containing hydrogels afforded higher degree of protein adsorption in our experiments.
4.5. Media conditions
We cultured EPCs on nonmodified 1%HAMA-2%HepMA hydrogels in media supplemented with 20%, 10% and 2% (v/v) FBS to observe the effect of serum protein adsorption by the hydrogels on cell adhesion and spreading. When the cultures were first prepared with the standard amount of FBS (20%) for EPCs, we observed that EPCs adhered and spread on these hydrogel surfaces. When the amount of FBS was decreased from 20% to 10% we observed a decrease in cell attachment and spreading (Supporting Figure S3). This could be related to adsorption of serum proteins onto heparin-incorporated hydrogels due to electrostatic interactions. Finally we further decreased the FBS concentration to 2% and did not observe any EPC spreading perhaps due to the protein adsorption effect mentioned above. Thus, we hypothesize that if the serum conditions are changed, different outcomes will be obtained in terms of endothelial cell attachment and spreading on HA-heparin hydrogels. This is in good agreement with previous reports that human endothelial cells did not adhere to polymer surfaces in serum-free condition implying the requirement of serum proteins for initial cell adhesion (Vanwachem et al., 1987).
Another important factor for endothelial cell adhesion is the interaction of growth factors, especially FGF and VEGF, with their corresponding receptors on endothelial cell surfaces. When FGF and VEGF were excluded from the EPC media we observed less cell attachment and almost no cell spreading on nonmodified 1%HAMA-2%HepMA hydrogels (Supporting Figure S4). In order to see the effect of preadsorbed FGF and VEGF on adhesion of EPCs we cultured them with FGF and VEGF excluded media on nonmodified 1%HAMA-2%HepMA surfaces. It has been reported previously (Tsou and Isik 2001; Murga et al., 2004) that FGF and VEGF receptors on endothelial cells bind to FGF and VEGF. In our case, FGF and VEGF from the culture media could be attracted by the heparin containing hydrogels, then corresponding receptors on endothelial cells could potentially bind to FGF and VEGF. When FGF and VEGF were preadsorbed on the heparin incorporated hydrogel surfaces, EPCs adhered and spread on these hydrogels even when FGF and VEGF excluded media was used. This could be due to FGF and VEGF, which possess well-known affinity for heparin, binding to heparin incorporated hydrogel surfaces then the FGF and VEGF receptors on EPCs bind to adhered FGF and VEGF growth factors facilitating the initial cell attachment.
In summary, we found that heparin addition to HA hydrogels provide an attractive environment for EPCs allowing formation of an endothelial monolayer in culture. This may possibly be occurring because of the strong ionic nature of heparin interacting with proteins and growth factors to promote cell adhesion and spreading. Due to the unique properties of both materials, this could be a useful approach to endothelialize artificial prostheses. In addition, HA (Mason et al., 2000) and heparin (Barzu et al., 1986; Patton et al., 1995) are non-thrombogenic substrates which could be an advantageous feature when developing coatings for blood contacting implants. It is obvious that biomaterials with non-thrombogenic features might improve the success rate when used in surface treatment of blood contacting devices in vivo.
5. Conclusions
In this study, we synthesized HA and heparin based hydrogels, which promoted adhesion and spreading of EPCs. We observed that the covalent CD34 Ab immobilization on HA-based hydrogels enhanced EPC adhesion and spreading. Although CD34 Ab modified hydrogel surfaces demonstrated significant improvements in cell adhesion and spreading for 1%HAMA-2%HepMA hydrogels, nonmodified 1%HAMA-2%HepMA surfaces also promoted formation of an endothelial monolayer by EPCs. This may be occurring due to the strong ionic nature of heparin producing electrostatic interactions with proteins and growth factors. Our data suggest that incorporation of heparin into HA based hydrogels supports adhesion and spreading of EPCs; in other cases where heparin was not included in the hydrogels no EPC spreading was observed. 1%HAMA-2%HepMA surfaces also allowed adhesion and spreading of HUVECs. We observed macrophage adhesion on 1%HAMA-2%HepMA hydrogels but this was significantly lower compared to that of EPCs. This strategy could help to treat cardiovascular injuries when recruiting EPCs from circulating blood to speed up re-endothelialization process. Therefore, this could potentially be used in endothelialization of artificial grafts and other related cardiovascular tissue engineering applications.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health (EB008392; DE019024; HL092836; HL099073; AR057837), the Institute for Soldier Nanotechnologies, and the US Army Corps of Engineers and National Science Foundation (DMR0847287). The authors acknowledge Dr Blaine Pfeifer for supplying them with the macrophages for this study.
Footnotes
Supporting information on the internet
The following supporting information may be found in the online version of this article.
Expression of CD34 from EPCs and macrophages by flow cytometry,
Protein adsorption on HA-based hydrogels,
Effect of serum concentration in media on EPC attachment on 1% HAMA-2% HepMA hydrogels,
Effect of FGF and VEGF in media on EPC attachment on 1% HAMA-2% HepMA hydrogels.
References
- Aoki J, Serruys PW, van Beusekom H, Ong ATL, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, Rowland S, Kutryk MJB. Endothelial progenitor cell capture by stents coated with antibody against CD34 - The HEALING-FIM (healthy endothelial accelerated lining inhibits neointimal growth-first in man) registry. JACC. 2005;45 (10):1574–1579. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Aubin H, Nichol JW, Hutson CB, Bae H, Sieminski AL, Cropek DM, Akhyari P, Khademhosseini A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010;31 (27):6941–6951. doi: 10.1016/j.biomaterials.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzu T, Molho P, Tobelem G, Petitou M, Caen JP. Binding of Heparin And Low-Molecular Weight Heparin Fragments to Human Vascular Endothelial-Cells in Culture. Nouv Rev Fr D Hematol. 1984;26 (4):243–247. [PubMed] [Google Scholar]
- Barzu T, Vanrijn J, Petitou M, Molho P, Tobelem G, Caen JP. Endothelial Binding-Sites for Heparin - Specificity and Role in Heparin Neutralization. Biochem J. 1986;238 (3):847–854. doi: 10.1042/bj2380847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos GW, Scharenborg NM, Poot AA, Engbers GHM, Terlingen JGA, Beugeling T, Van Aken WG, Feijen J. Adherence and proliferation of endothelial cells on surface-immobilized albumin-heparin conjugate. Tissue Eng. 1998;4 (3):267–279. doi: 10.1089/ten.1998.4.267. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Chung C, Jia XQ, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6 (1):386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camci-Unal G, Aubin H, Ahari AF, Bae H, Nichol JW, Khademhosseini A. Surface-modified hyaluronic acid hydrogels to capture endothelial progenitor cells. Soft Matter. 2010;6 (20):5120–5126. doi: 10.1039/c0sm00508h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casu B, Naggi A. Antiangiogenic heparin-derived heparan sulfate mimics. Pure Appl Chem. 2003;75 (2–3):157–166. [Google Scholar]
- Chan AKC, Paredes N, Thong B, Chindemi P, Paes B, Berry LR, Monagle P. Binding of heparin to plasma proteins and endothelial surfaces is inhibited by covalent linkage to antithrombin. Thromb Haemostasis. 2004;91 (5):1009–1018. doi: 10.1160/TH03-06-0365. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Protection. [accessed January 28, 2011];Heart Disease is the Number One Cause of Death. http://www.cdc.gov/features/heartmonth/
- Chen JL, Chen C, Chen ZY, Chen JY, Li QL, Huang N. Collagen/heparin coating on titanium surface improves the biocompatibility of titanium applied as a blood-contacting biomaterial. J Biomed Mater Res A. 2010;95A (2):341–349. doi: 10.1002/jbm.a.32847. [DOI] [PubMed] [Google Scholar]
- Chen RS, Chen YJ, Chen MH, Young TH. Cell-surface interactions of rat tooth germ cells on various biomaterials. J Biomed Mater Res A. 2008;84A (2):567–567. doi: 10.1002/jbm.a.31475. [DOI] [PubMed] [Google Scholar]
- Chen YM, Shiraishi N, Satokawa H, Kakugo A, Narita T, Gong JP, Osada Y, Yamamoto K, Ando J. Cultivation of endothelial cells on adhesive protein-free synthetic polymer gels. Biomaterials. 2005;26 (22):4588–4596. doi: 10.1016/j.biomaterials.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Chou AI, Akintoye SO, Nicoll SB. Photo-crosslinked alginate hydrogels support enhanced matrix accumulation by nucleus pulposus cells in vivo. Osteoarthr Cartilage. 2009;17 (10):1377–1384. doi: 10.1016/j.joca.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi B, Fredenburgh JC, Rischke J, Hirsh J, Young E, Weitz JI. Effect of nonspecific binding to plasma proteins on the antithrombin activities of unfractionated heparin, low-molecular-weight heparin, and dermatan sulfate. Circulation. 1997;95 (1):118–124. doi: 10.1161/01.cir.95.1.118. [DOI] [PubMed] [Google Scholar]
- de Mel A, Jell G, Stevens MM, Seifalian AM. Biofunctionalization of Biomaterials for Accelerated in Situ Endothelialization: A Review. Biomacromolecules. 2008;9 (11):2969–2979. doi: 10.1021/bm800681k. [DOI] [PubMed] [Google Scholar]
- Fujie T, Furutate S, Niwa D, Takeoka S. A nano-fibrous assembly of collagen-hyaluronic acid for controlling cell-adhesive properties. Soft Matter. 2010;6 (19):4672–4676. [Google Scholar]
- Glimelius B, Busch C, Hook M. Binding of Heparin on Surface of Cultured Human Endothelial Cells. Thromb Res. 1978;12 (5):773–782. doi: 10.1016/0049-3848(78)90271-2. [DOI] [PubMed] [Google Scholar]
- He HB, Shirota T, Yasui H, Matsuda T. Canine endothelial progenitor cell-lined hybrid vascular graft with nonthrombogenic potential. J Thorac Cardiovasc Surg. 2003;126 (2):455–464. doi: 10.1016/s0022-5223(02)73264-9. [DOI] [PubMed] [Google Scholar]
- Hiebert LM, Jaques LB. Observation of Heparin on Endothelium After Injection. Thromb Res. 1976;8 (2):195–204. doi: 10.1016/0049-3848(76)90262-0. [DOI] [PubMed] [Google Scholar]
- Hattori S, Andrade JD, Hibbs JB, Gregonis DE, King RN. Fibroblast Cell-Proliferation on Charged Hydroxyethyl Methacrylate Copolymers. J Colloid Inter Sci. 1985;104 (1):72–78. [Google Scholar]
- Hu M, Sabelman EE, Tsai C, Tan J, Hentz VR. Improvement of schwann cell attachment and proliferation on modified hyaluronic acid strands by polylysine. Tissue Eng. 2000;6 (6):585–593. doi: 10.1089/10763270050199532. [DOI] [PubMed] [Google Scholar]
- Jaques LB. Heparin - A Unique Misunderstood Drug. Trends Pharmacol Sci. 1982;3 (7):289–291. [Google Scholar]
- Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30 (14):2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Ji Y, Ghosh K, Shu XZ, Li BQ, Sokolov JC, Prestwich GD, Clark RAF, Rafailovich MH. Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials. 2006;27 (20):3782–3792. doi: 10.1016/j.biomaterials.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7 (9):1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel SJ, Lankford TK, Foote LJ, Shinpock SG, Stringer C. CD44 Expression on Murine Tissues. J Cell Sci. 1993;104:373–382. doi: 10.1242/jcs.104.2.373. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee JW, Khang G, Lee HB. Cell behavior on polymer surfaces with different functional groups. Plenum Press Div Plenum Publishing Corp; New York, NY, USA: 1998. [Google Scholar]
- Leach JB, Bivens KA, Patrick CW, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003;82 (5):578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- Lei YG, Gojgini S, Lam J, Segura T. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials. 2011;32 (1):39–47. doi: 10.1016/j.biomaterials.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas NG. Adhesion and Spreading of Cells on Charged Surfaces. J Theor Biol. 1975;49 (2):417–424. doi: 10.1016/0022-5193(75)90182-4. [DOI] [PubMed] [Google Scholar]
- Mason M, Vercruysse KP, Kirker KR, Frisch R, Marecak DM, Prestwich CD, Pitt WG. Attachment of hyaluronic acid to polypropylene, polystyrene, and polytetrafluoroethylene. Biomaterials. 2000;21 (1):31–36. doi: 10.1016/s0142-9612(99)00129-5. [DOI] [PubMed] [Google Scholar]
- Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109 (11):4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- Murga M, Yao L, Tosato G. Derivation of endothelial cells from CD34(−) umbilical cord blood. Stem Cells. 2004;22 (3):385–395. doi: 10.1634/stemcells.22-3-385. [DOI] [PubMed] [Google Scholar]
- Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31 (21):5536–44. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilasaroya A, Poole-Warren LA, Whitelock JM, Martens PJ. Structural and functional characterisation of poly(vinyl alcohol) and heparin hydrogels. Biomaterials. 2008;29 (35):4658–4664. doi: 10.1016/j.biomaterials.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Patton WA, Granzow CA, Getts LA, Thomas SC, Zotter LM, Gunzel KA, Lowekrentz LJ. Identification of a Heparin-Binding Protein Using Monoclonal-Antibodies that Block Heparin-Binding to Porcine Aortic Endothelial-Cells. Biochem J. 1995;311:461–469. doi: 10.1042/bj3110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18 (11):1345–1360. [Google Scholar]
- Psuja P, Drouet L, Zawilska K. Binding of Heparin to Human-Endothelial Cell Monolayer and Extracellular-Matrix in Culture. Thromb Res. 1987;47 (4):469–478. doi: 10.1016/0049-3848(87)90462-2. [DOI] [PubMed] [Google Scholar]
- Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20 (1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Breymann C, Weber A, Guenter CI, Neuenschwander S, Zund G, Turina M, Hoerstrup SP. Umbilical cord blood derived endothelial progenitor cells for tissue engineering of vascular grafts. Ann Thorac Surg. 2004;78 (6):2094–2098. doi: 10.1016/j.athoracsur.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Schopka S, Schmid T, Schmid C, Lehle K. Current Strategies in Cardiovascular Biomaterial Functionalization. Materials. 2010;3:638–655. [Google Scholar]
- Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in Regenerative Medicine. Adv Mater. 2009;21 (32–33):3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri S, Schmidt CE. Photopatterned collagen-hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomater. 2009;5 (7):2385–2397. doi: 10.1016/j.actbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Trindade ES, Oliver C, Jamur MC, Rocha HAO, Franco CRC, Boucas RI, Jarrouge TR, Pinhal MAS, Tersariol ILS, Gouvea TC, Dietrich CP, Nader HB. The binding of heparin to the extracellular matrix of endothelial cells up-regulates the synthesis of an antithrombotic heparan sulfate proteoglycan. J Cell Physiol. 2008;217 (2):328–337. doi: 10.1002/jcp.21504. [DOI] [PubMed] [Google Scholar]
- Tsou R, Isik FF. Integrin activation is required for VEGF and FGF receptor protein presence on human microvascular endothelial cells. Mol Cell Biochem. 2001;224 (1–2):81–89. doi: 10.1023/a:1011947301849. [DOI] [PubMed] [Google Scholar]
- Vanwachem PB, Hogt AH, Beugeling T, Feijen J, Bantjes A, Detmers JP, Vanaken WG. Adhesion of Cultured Human-Endothelial Cells onto Methacrylate Polymers with Varying Surface Wettability and Charge. Biomaterials. 1987;8 (5):323–328. doi: 10.1016/0142-9612(87)90001-9. [DOI] [PubMed] [Google Scholar]
- Yu Y, Flint AF, Mulliken JB, Wu JK, Bischoff J. Endothelial progenitor cells in infantile hemangioma. Blood. 2004;103 (4):1373–1375. doi: 10.1182/blood-2003-08-2859. [DOI] [PubMed] [Google Scholar]
- Zhang WQ, Swanson R, Xiong Y, Richard B, Olson ST. Antiangiogenic antithrombin blocks the heparan sulfate-dependent binding of proangiogenic growth factors to their endothelial cell receptors - Evidence for differential binding of antiangiogenic and anticoagulant forms of antithrombin to proangiogenic heparan sulfate domains. J Biol Chem. 2006;281 (49):37302–37310. doi: 10.1074/jbc.M604905200. [DOI] [PubMed] [Google Scholar]
- Zieris A, Prokoph S, Levental KR, Welzel PB, Grimmer M, Freudenberg U, Werner C. FGF-2 and VEGF functionalization of starPEG-heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials. 2010;31 (31):7985–7994. doi: 10.1016/j.biomaterials.2010.07.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


