Abstract
Patients surviving severe brain injury may regain consciousness without recovering their ability to understand, move and communicate. Recently, electrophysiological and neuroimaging approaches, employing simple sensory stimulations or verbal commands, have proven useful in detecting higher order processing and, in some cases, in establishing some degree of communication in brain-injured subjects with severe impairment of motor function. To complement these approaches, it would be useful to develop methods to detect recovery of consciousness in ways that do not depend on the integrity of sensory pathways or on the subject's ability to comprehend or carry out instructions. As suggested by theoretical and experimental work, a key requirement for consciousness is that multiple, specialized cortical areas can engage in rapid causal interactions (effective connectivity). Here, we employ transcranial magnetic stimulation together with high-density electroencephalography to evaluate effective connectivity at the bedside of severely brain injured, non-communicating subjects. In patients in a vegetative state, who were open-eyed, behaviourally awake but unresponsive, transcranial magnetic stimulation triggered a simple, local response indicating a breakdown of effective connectivity, similar to the one previously observed in unconscious sleeping or anaesthetized subjects. In contrast, in minimally conscious patients, who showed fluctuating signs of non-reflexive behaviour, transcranial magnetic stimulation invariably triggered complex activations that sequentially involved distant cortical areas ipsi- and contralateral to the site of stimulation, similar to activations we recorded in locked-in, conscious patients. Longitudinal measurements performed in patients who gradually recovered consciousness revealed that this clear-cut change in effective connectivity could occur at an early stage, before reliable communication was established with the subject and before the spontaneous electroencephalogram showed significant modifications. Measurements of effective connectivity by means of transcranial magnetic stimulation combined with electroencephalography can be performed at the bedside while by-passing subcortical afferent and efferent pathways, and without requiring active participation of subjects or language comprehension; hence, they offer an effective way to detect and track recovery of consciousness in brain-injured patients who are unable to exchange information with the external environment.
Keywords: coma, TMS, EEG, minimally conscious state, cerebral cortex
Introduction
The level of consciousness of patients who survive severe brain injury is assessed clinically based on their ability to interact with the environment and communicate. Patients who, after careful testing, remain unresponsive to the environment even though their eyes may be open, are considered unconscious or in a vegetative state (Jennett and Plum, 1972; Royal College of Physicians, 1994). The appearance of non-reflexive behaviours, such as visual tracking or responding to simple commands, is considered a sufficient clinical criterion for a minimally conscious state (Giacino et al., 2002, 2004), while functional communication marks the unambiguous emergence of consciousness (emergence from minimally conscious state). As a result of concurrent motor impairments, however, it may happen that brain-injured patients recover consciousness but are unable to signal it behaviourally (Giacino et al., 2009; Schnakers et al., 2009). For this reason, electrophysiological (Kotchoubey et al., 2005; Fellinger et al., 2011) and neuroimaging protocols (Owen et al., 2006; Monti et al., 2010a) have been developed to probe for signs of awareness even in patients who are completely unable to move. In these protocols, subjects are instructed with verbal commands to enter and sustain specific mental states (such as imagining to play tennis) while their brain activity is recorded; in this way, a patient can signal that she/he is aware by producing specific neural responses and may, in exceptional cases, establish a basic form of communication (Monti et al., 2010a). These mental imagery tasks require high-order cognitive abilities and can be very demanding for many brain-injured subjects; thus, patients in a minimally conscious state (Monti et al., 2010a), as well as locked-in patients (Bardin et al., 2011), may fail the test resulting in a significant rate of false negatives. Event-related EEG potentials elicited by simpler sensory (auditory) stimulations, such as P3b, N400, P3a and mismatch negativity, involve lower cognitive resources compared to mental imagery tasks; for example, while the late P3b requires that subjects intentionally pay attention to a target stimulus, the mismatch negativity is an early, automatic reaction to a deviant stimulus that is generated at the pre-attentive level. Although these components can be absent in patients who show behavioural signs of consciousness (Kotchoubey et al., 2005; Fischer et al., 2010; Holler et al., 2011), detecting them in non-responsive subjects is clearly informative. In these particular cases, similarly to functional MRI active paradigms, late event-related EEG potential components, such as P3b, can reveal the recovery of residual cognitive function in patients that are otherwise unresponsive (Schnakers et al., 2008; Faugeras et al., 2011). More generally, event-related EEG potentials allow assessment of the integrity of sensory processing at different hierarchical levels (Kotchoubey et al., 2005; Boly et al., 2011); as such, event-related EEG potentials recorded in the early stages of coma are a good predictor of outcome (Fischer et al., 2004; Daltrozzo et al., 2007; Wijnen et al., 2007; Luaute et al., 2010; Duncan et al., 2011; Faugeras et al., 2011).
In parallel to event-related EEG potential recordings and active paradigms, it would be useful to develop more sensitive methods to detect recovery of consciousness that do not depend on the integrity of sensory and motor pathways nor on the subject's ability to comprehend or carry out instructions. Theoretical considerations (Tononi, 2004; Laureys, 2005; Dehaene et al., 2006; Tononi and Koch, 2008; Seth et al., 2008) as well as experimental data (Del Cul et al., 2007; Alkire et al., 2008) suggest that a basic requirement for consciousness is that multiple, specialized areas of the thalamocortical system can engage in rapid causal interactions (effective connectivity). One way to gauge effective connectivity among thalamocortical modules involves perturbing directly a subset of cortical neurons with transcranial magnetic stimulation (TMS) and recording the reaction of the rest of the brain with millisecond resolution by means of EEG (Ilmoniemi et al., 1997; Litvak et al., 2007; Morishima et al., 2009; Akaishi et al., 2010; Casali et al., 2010). When consciousness is present, the thalamocortical system should respond to TMS with a complex pattern of activation, involving different cortical areas at different times; conversely, it should react with a simple response that remains localized to the stimulated area if consciousness is reduced (Alkire et al., 2008; Massimini et al., 2009a). In a recent series of experiments these hypotheses were tested during wakefulness, deep sleep and anaesthesia. In healthy awake subjects, TMS induced a sustained EEG response involving the sequential activation of different brain areas and affecting much of the cortex (Massimini et al., 2005). In contrast, after loss of consciousness induced by general anaesthesia (Ferrarelli et al., 2010), TMS pulses invariably produced a simple response that remained localized to the site of stimulation, indicating a breakdown of effective interactions among thalamocortical modules. A similar breakdown of effective connectivity was observed during slow wave sleep early in the night (Massimini et al., 2005), when subjects report little or no conscious content upon awakening (Hobson et al., 2000). Importantly, during rapid eye movement sleep, when subjects are unresponsive to sensory stimuli and virtually paralysed but report vivid dreams upon awakening, the cortical response to TMS recovered its complexity and became similar to that observed during wakefulness (Massimini et al., 2010).
In the present work we employed TMS/EEG to measure effective connectivity at the bedside of 17 patients who evolved from coma into different clinical states (vegetative state, minimally conscious state, emergence from minimally conscious state and locked-in syndrome). We predicted that measuring effective connectivity should reliably discriminate between patients in a vegetative state and patients in a minimally conscious state with a stable clinical diagnosis (between-subject comparisons) and that a clear-cut resumption of causal interactions should be detectable in the brains of patients who gradually regain consciousness and functional communication (within-subject comparisons).
Materials and methods
Patients
The study was approved by the Ethics Committee of the Medicine Faculty of the University of Liège. Written informed consents were obtained by the patient's legal surrogates and consents were obtained directly from the patients when they recovered the ability to communicate.
We performed a first set of TMS/EEG experiments (single session) in a group of 12 patients (Group I: five females; mean age ± SD: 50.3 ± 26.21; for more details see Supplementary Table 1). These patients were behaviourally evaluated by means of the Coma Recovery Scale-Revised (CRS-R; Giacino et al., 2004). Evaluation sessions took place four times a week (evaluation week), every other day. These repeated evaluations were carried out in order to avoid diagnostic errors due to fluctuations in responsiveness and to obtain a stable clinical diagnosis. Five patients of Group I (Patients 1–5), showed only reflexive behaviour and were diagnosed as vegetative state during the four behavioural evaluations. Five patients (Patients 6–10) satisfied the CRS-R criteria for minimally conscious state in at least three evaluations, including the one performed on the day of the TMS/EEG session (reported in Supplementary Table 1). The two remaining patients (Patients 11 and 12) could communicate reliably and were diagnosed with locked-in syndrome. The vegetative and minimally conscious state subgroups did not differ systematically in aetiology and time from injury (Supplementary Table 1); in particular, Group I included three chronic patients, one vegetative state (Patient 5: 172 days from injury), one minimally conscious state (Patient 8: 1334 days from injury) and one locked-in syndrome (Patient 12: 1399 days from injury).
A second group of five patients (Group II: three females; mean age ± SD: 51.2 ± 23.05; for more details see Supplementary Table 2) were recruited from intensive care; these patients underwent longitudinal TMS/EEG measurements (Sessions 1–3) as they awakened from coma and progressed towards different clinical states. As assessed by the CRS-R, three of these patients (Patients 13–15) recovered consciousness evolving from a vegetative state, through a minimally conscious state to emergence from minimally conscious state, while two patients (Patients 16 and 17) remained in a vegetative state. Session 1 was performed in all cases, at least 48 h after withdrawal of sedation, when patients exited from coma and entered the vegetative state. In the three subjects (Patients 13–15) who recovered, Session 2 was performed on the day after they transitioned from vegetative to minimally conscious state (however, Patient 15 temporarily slipped back into a vegetative state); in the two patients who did not recover (Patients 16 and 17), Session 2 was performed after 1 month. Session 3 was performed only in the three patients who recovered, after they regained functional communication and emerged from a minimally conscious state (emergence from minimally conscious state).
In summary, we first performed experiments in Group I patients in order to test whether TMS/EEG measures of effective connectivity are able to discriminate between unconscious (vegetative state) and conscious (minimally conscious state, locked-in syndrome) patients with a clear, stable clinical diagnosis (across-subjects comparisons). Then, we recruited Group II patients in order to test, by means of repeated measures, whether changes in effective connectivity are also detectable over time in the brains of individual patients who recover consciousness (within-subjects comparisons).
Transcranial magnetic stimulation targeting and stimulation parameters
A single TMS/EEG session consisted of up to five TMS/EEG measurements that differed either for the site or the intensity of stimulation.
Cortical targets were identified on CT scans acquired with a Siemens Senatom Sensation 16. The TMS stimulator consisted of a Focal Bipulse figure-of-eight coil (mean/outer winding diameter ∼50/70 mm, biphasic pulse shape, pulse length ∼280 µs, focal area of the stimulation 0.68 cm2) driven by a Mobile Stimulator Unit (eXimia TMS Stimulator, Nexstim Ltd.). We controlled TMS parameters by means of a Navigated Brain Stimulation system (Nexstim Ltd.) that employed a 3D infrared tracking position sensor unit to locate the relative positions of the coil and subject's head within the reference space of individual CT scan. Navigated brain stimulation also calculated, online, the distribution and the intensity (expressed in V/m) of the intracranial electric field induced by TMS. The location of the maximum electric field induced by TMS on the cortical surface (hot spot) was always kept on the convexity of the targeted gyrus with the induced current perpendicular to its main axis. At least 300 trials were collected for each stimulation site. Stimulation was delivered with an interstimulus interval jittering randomly between 2000 and 2300 ms (0.4–0.5 Hz), at an intensity ranging from 140 V/m up to 200 V/m on the cortical surface; TMS pulses within this range are largely above the threshold (50 V/m) for an EEG response (Komssi et al., 2007; Rosanova et al., 2009; Casali et al., 2010). The CT-guided intracranial electric field estimation was a crucial step during the experimental procedure; due to shifts of intracranial volumes in brain-injured patients, it is difficult to assess whether TMS is on target and effective based on extra-cranial landmarks alone and this may result in false-negatives (absence of EEG response due to missed target or sub-threshold stimulation).
The reproducibility of the stimulation coordinates, within and across sessions (Group II), was guaranteed by a software aiming device that indicated, in real-time, any deviation from the designated target >3 mm. As shown by previous works (Casali et al., 2010; Casarotto et al., 2010), this device ensures high test–retest reproducibility in longitudinal TMS/EEG measurements.
By means of the navigated brain stimulation, TMS was targeted to four cortical sites: the left and right medial third of the superior parietal gyrus and the left and right medial third of the superior frontal gyrus. These cortical targets were selected for the following reasons: (i) they are easily accessible and far from major head or facial muscles whose unwanted activation may affect EEG recordings; (ii) the posterior parietal cortex as well as its interactions with more frontal areas, is thought to be particularly relevant for consciousness (Laureys et al., 2004); and (iii) previous TMS/EEG studies have been successfully performed in these areas during wakefulness (Rosanova et al., 2009), sleep (Massimini et al., 2005, 2007) and anaesthesia (Ferrarelli et al., 2010). In practice, all four cortical sites were not always accessible in all subjects due to skull breaches or external drain derivations. In these cases, TMS/EEG measurements were restricted to two or three cortical sites. In all cases, we avoided stimulating over focal cortical lesions that were clearly visible in CT scans, since the EEG response of these areas may be absent or unreliable.
Electroencephalogram recordings
Both TMS/EEG measurements and spontaneous EEG recordings were performed using a TMS-compatible 60-channel amplifier (Nexstim Ltd). This device prevents amplifier saturation and reduces, or abolishes, the magnetic artefacts induced by the coil's discharge (Virtanen et al., 1999). To further optimize TMS compatibility, the impedance at all electrodes was kept <5 kΩ. EEG signals were referenced to an additional electrode on the forehead, filtered (0.1–500 Hz) and sampled at 1450 Hz. Two extra sensors were used to record the electrooculogram. In the present study, most recordings were free from TMS-induced magnetic or electric artefacts and in all cases the EEG response was artefact-free starting from ∼10 ms after stimulation.
Besides the magnetic artefact, other factors may confound the interpretation of TMS-evoked potentials, if not adequately controlled for. For example, TMS may directly stimulate or activate trigeminal sensory afferents and head muscles evoking somatosensory potentials or muscle potentials, respectively. Moreover, the ‘click’ associated with the coil's discharge propagates through air and bone possibly inducing auditory evoked potentials. In the present experiments, as in previous studies (Massimini et al., 2005; Ferrarelli et al., 2008; Rosanova et al., 2009), we have applied the following procedures in order to eliminate, or control for, these confounding factors. (i) Trigeminal stimulation and muscle artefacts were minimized by placing the coil on a scalp area close to the midline, far away from facial or temporal muscles and nerve endings; (ii) to prevent contamination of TMS-evoked EEG potentials by the auditory response to the coil's ‘click’, subjects wore earphones through which a noise masking, reproducing the time-varying frequency components of the TMS ‘click’, was played throughout each TMS/EEG session. Additionally, in two patients, sham stimulation was performed as in previous works (Massimini et al., 2005; Rosanova et al., 2009) and demonstrated the absence of auditory evoked potentials. Noise masking was also effective in preventing TMS from causing blinks or eye muscle reactions; and (iii) bone conduction of the TMS-associated ‘click’ was minimized by placing a thin foam layer between the coil and the EEG cap. These procedures ensure genuine EEG responses to direct cortical stimulation with TMS. These responses reveal patterns of excitability and connectivity that are specific for the site (Kahkonen et al., 2005a; Rosanova et al., 2009; Casali et al., 2010), the angle (Bonato et al., 2006; Casarotto et al., 2010) and the intensity of stimulation (Komssi et al., 2004b; Kahkonen et al., 2005b; Rosanova et al., 2009). During off-line data processing, all trials that contained spontaneous blinks, eye movement, or muscle artefacts were rejected using an automatic algorithm (Casali et al., 2010). After artefact rejection, 72 good TMS/EEG measurements were further analysed and were included in the present study.
General experimental procedures
During each TMS/EEG session patients were lying on their beds, awake and with their eyes open. If signs of drowsiness appeared, recordings were momentarily interrupted and subjects were stimulated using the CRS-R arousal facilitation protocols. Throughout every recording session the stability of stimulation coordinates was continuously monitored. If the virtual aiming device was signalling a displacement >4 mm, the session was interrupted and the coil was repositioned. At the end of the experiment, the stimulation coordinates were recorded and the electrodes positions were digitized.
Data analysis and statistics
Data analysis was performed using Matlab R2006a (The MathWorks). First, TMS/EEG trials containing noise, muscle activity or eye movements were automatically detected and rejected (Casali et al., 2010). Then, EEG data were average referenced; down-sampled to half of the original sampling rate (725 Hz), band pass filtered (1–80 Hz) and baseline corrected over 300 ms prestimulus. After trials rejection, each TMS-evoked response was obtained by averaging 150–250 artefact-free trials. In order to obtain the overall amount of electrical activity induced by TMS, we calculated the global mean field power (GMFP) (Lehmann and Skrandies, 1980) from the multichannel average signals as follows:
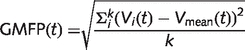 |
where k is the number of channels, Vi is the voltage measured with channel i, and Vmean is the mean of the measured voltages across channels (average reference).
Effective connectivity is defined as the effect of the activation of a subset of neurons on other neuronal groups (Friston, 2002). TMS/EEG allows activating directly a subset of cortical neurons and recording the immediate effects of this initial activation in the rest of the brain. Thus, detecting significant TMS-evoked cortical activations (primary neuronal currents) far away from the site of the initial perturbation provides a coarse, but straightforward, indication of effective connectivity (Paus, 2005). In order to detect significant TMS-evoked cortical activations we proceeded as follows. First, we detected primary currents by performing source modelling. Statistical Parametric Mapping software (SPM, freely available at http://www.fil.ion.bpmf.ac.uk/spm) was used to compute cortex, skull and scalp meshes (3004, 2000 and 2000 vertices, respectively) and to co-register these meshes with EEG sensors positions by rigid rotations and translations of anatomical landmarks (nasion, left tragus and right tragus). Conductive head volume was modelled according to the three-spheres BERG method (Berg and Scherg, 1994) as implemented in the Brainstorm software package (http://neuroimage.usc.edu/brainstorm). Finally, the inverse solution was computed on the average of all artefact-free TMS/EEG trials using the minimum norm estimate with smoothness prior, a method that has the advantage of requiring no a priori assumption about the nature of the source distribution (Hamalainen and Ilmoniemi, 1994) and of providing stable solutions also in the presence of noise (Silva et al., 2004). Though the minimum norm estimate tends to result in a blurred picture of cortical activation, the location of the maximum estimated current has been shown to reflect the location of the generator of neural activity with good accuracy (<20 mm) (Babiloni et al., 2000; Hauk, 2004; Komssi et al., 2004a).
As in previous TMS/EEG works performed during sleep (Massimini et al., 2005, 2007, 2010), we considered only the cortical activations that corresponded to significant GMFP values (see Supplementary Fig. 1 for a graphical example). To assess the threshold for significance (Supplementary Fig. 1), a bootstrap method (Delorme and Makeig, 2004; Lv et al., 2007; McCubbin et al., 2008), which does not assume normal distribution of the observations, was applied by shuffling the time samples of GMFP prestimulus activity (from −300 to −50 ms) at the single-trial level and by calculating 500 surrogated prestimulus GMFP time-series. From each random realization, the maximum value across all latencies was selected to obtain a maximum distribution (control for type I error) and significance level was set at P < 0.01. At each significant latency of the post-stimulus GMFP, the location of maximum neuronal current (10 most active sources) was detected on the cortical surface. Plotting and counting the sources involved by maximum neuronal currents across all significant time points in the first 300 ms post-stimulus resulted in the cortical maps and in the values reported in Figs 1–3 and Supplementary Figs 1–3 and 5.
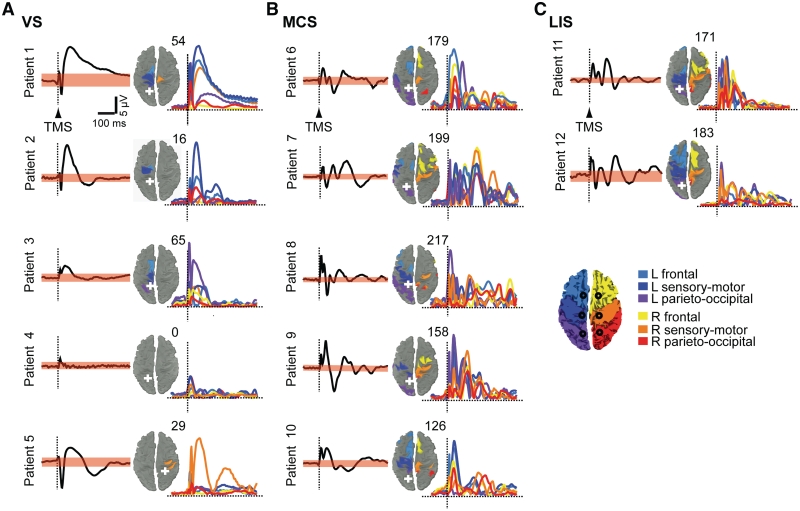
Figure 1.
TMS-evoked cortical responses in Group I patients. A group of five vegetative state (VS, A), five minimally conscious state (MCS, B), and two patients with locked-in syndrome (LIS, C) underwent one TMS/EEG session after 7 days of repeated evaluations by means of the CRS-R. For each patient, the averaged TMS-evoked potentials recorded at one electrode under the stimulator (black trace) and the respective significance threshold (upper and lower boundaries of the pink bands; bootstrap statistics, P < 0.01) are shown. The sources involved by maximum cortical currents (10 most active sources) during the significant post-stimulus period of the global mean field power are plotted on the cortical surface and colour-coded according to their location in six anatomical macro-areas as indicated in the legend; the number of detected sources is indicated at the top right of each map. The time-series (colored traces) represent TMS-evoked cortical currents recorded from an array of six sources (black circles on the cortical map in the legend) located ∼2 cm lateral to the midline, one for each macro-area (Supplementary Fig. 1). The white crosses mark the sites of stimulation. For all patients, the responses to the left parietal cortex stimulation are shown, except for one patient (Patient 5) in whom a significant response could only be detected in the right hemisphere (Supplementary Fig. 2). EEG positivity is upward. L = left; R = right.
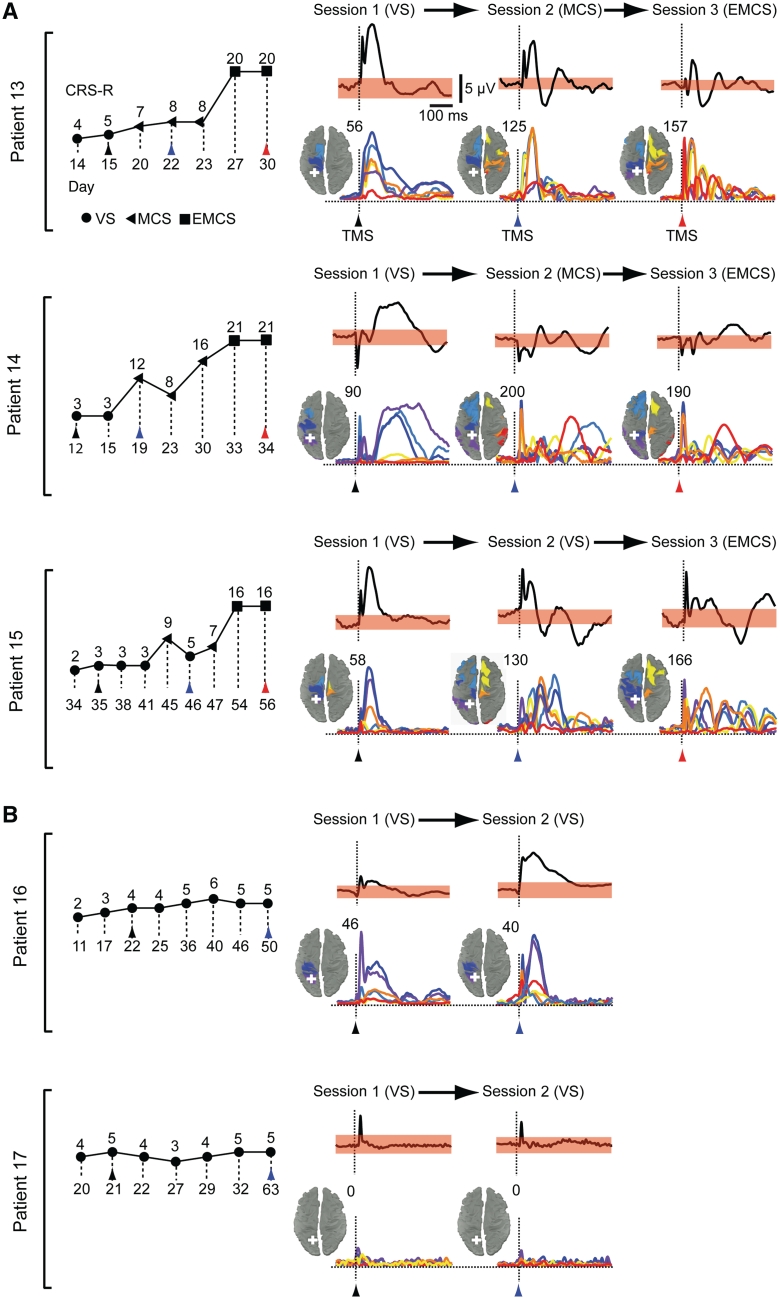
Figure 2.
Clinical evaluation and TMS-evoked cortical responses in Group II patients. CRS-R total scores are plotted for the patients who were studied longitudinally (Group II) and eventually emerged from a minimally conscious state (EMCS, A) or remained in a vegetative state (VS, B); the first assessment (Session 1) was carried out 48 h after withdrawal of sedation, as patients exited from coma. The symbols indicate the associated clinical diagnosis (filled circles = vegetative state; filled triangles = minimally conscious state; filled squares = emergence from minimally conscious state). Coloured arrow tips mark the days when TMS/EEG recordings were performed and the time of TMS delivery (black = Session 1; blue = Session 2; red = Session 3). For every patient and measurement, averaged potentials triggered by TMS (vertical dashed lines) of parietal cortex and recorded from the electrode under the stimulator are shown. The corresponding spread and the time-course of the cortical currents evoked by TMS is measured. The sources involved by maximum neuronal currents during the significant post-stimulus period are plotted on the cortical surface and colour-coded according to their location in six anatomical macro-areas (Fig. 1); the number of detected sources is indicated at the top right of each map. The time-series represent TMS-evoked cortical currents recorded from an array of six sources (see their locations in Fig. 1) located ∼2 cm lateral to the midline, one for each macro-area. The white crosses mark the sites of stimulation; in each patient, the left parietal cortex was stimulated when patients entered a vegetative state from coma (Session 1), soon after transition to a minimally conscious state or at least 30 days of permanence in a vegetative state (Session 2) and after emergence from a minimally conscious state (Session 3), when subjects recovered functional communication. See Supplementary Fig. 3 for the remaining cortical sites targeted in patients from Group II. EEG positivity is upward.
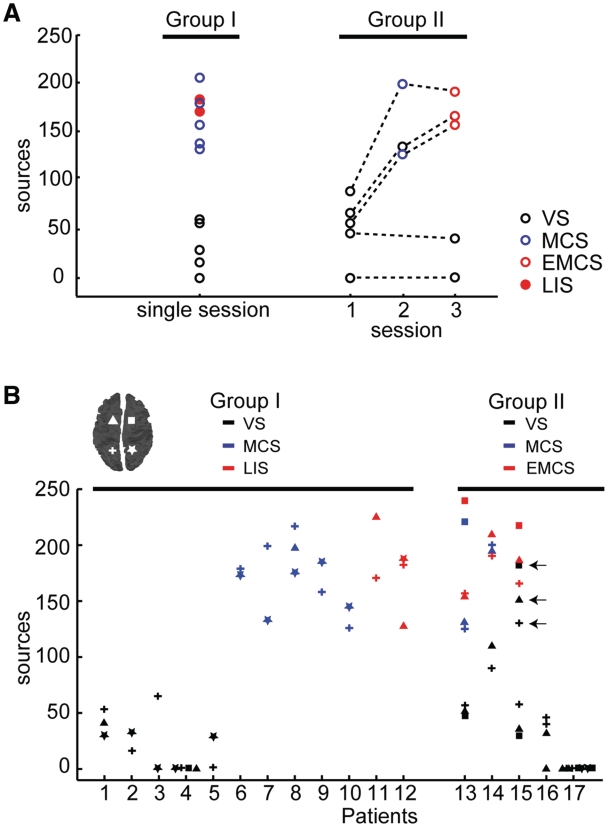
Figure 3.
Effective connectivity for all patients and TMS/EEG measurements. (A) For each patient and TMS/EEG measurement (same measurements as Figs 1 and 2), the number of sources involved by TMS-evoked currents are plotted. The circles indicate the clinical diagnosis at the time of recording [open black circles for vegetative state (VS); open blue circles for minimally conscious state (MCS); open red circles for emergence from minimally conscious state (EMCS) and filled red circles for locked-in syndrome (LIS)]. (B) The number of cortical sources involved by maximum cortical currents detected in all TMS/EEG measurements (n = 72) is plotted for all patients (Group I on the left and Group II on the right). Each value refers to one cortical target and is marked according to both the site of stimulation (the correspondence between symbols and stimulation sites is graphically reported on the cortical map in the left upper corner) and the CRS-R diagnosis at the time of recording (black for vegetative state; blue for minimally conscious state; red for locked-in syndrome in Group I and emergence from minimally conscious state in Group II). In all cases, effective connectivity is higher in patients who showed some level of consciousness (minimally conscious state, emergence from minimally conscious state and locked-in syndrome) compared to patients in a vegetative state. An exception is represented by the three measurements (left parietal, left frontal, right frontal) performed in Patient 15 during Session 2 (open black circles indicated by arrows). This patient was diagnosed as being in a minimally conscious state the day before the measurement, slipped back into a behavioural vegetative state on the day of Session 2 and within days, was reassessed clinically as being in a minimally conscious state and then emerged from minimally conscious state (during Session 3). Effective connectivity was null in the two anoxic subjects (Patient 4 from Group I and Patient 17 from Group II).
According to this procedure, the number of detected sources is small if TMS triggers neuronal activations (maximum currents) that remain confined to the stimulated area during the entire post-stimulus period. On the contrary, the number of detected sources is large if TMS triggers maximum cortical currents that involve different cortical areas at different times. In order to describe the time course of TMS-evoked cortical activations in different areas, the currents from a grid of six cortical sources (Fig. 1 and Supplementary Figs 1 and 2) were extracted and auto-scaled to the maximum value of each session. Sources and time series of cortical currents were colour-coded according to their anatomical location in six arbitrary macro-areas.
Spontaneous electroencephalogram recordings and analysis
The spontaneous EEG was recorded immediately before each TMS/EEG session. Similarly to TMS/EEG recordings, EEG recordings were obtained while subjects were behaviourally awake and with their eyes open; if signs of drowsiness appeared, the recordings were momentarily interrupted and patients were stimulated according to the CRS-R arousal facilitation protocol. Hence, all recordings were presumably carried out during a state of high activation of brainstem arousal systems. Continuous EEG acquisitions were split into 5-s epochs. Based on an automatic procedure (Casali et al., 2010), epochs displaying electrooculogram deflections exceeding 70 μV (indicating ocular activity) and/or absolute power of EEG channel F8 in the fast beta range (>25 Hz) exceeding 0.9 μV2/Hz (indicating activity of fronto-temporal muscles) (van de Velde et al., 1998) were rejected. After epochs rejection the average overall duration of retained epochs was 133.67 s (min: 125 s; max: 170 s). In order to be consistent across subjects, we restricted the analysis to the first 2 min of artefact-free EEG recorded in each patient. Following EEG filtering (1–40 Hz) and channels rejection, Fast Fourier Transform (FFT) was performed and each frequency bin was normalized to total power. The obtained power spectral densities were then subdivided into standard EEG frequency bands (δ, 1–4 Hz; θ, 4–8 Hz; α, 8–12 Hz; and β, 12–25 Hz). At the group level, differences between conditions (vegetative state, minimally conscious state and locked-in syndrome-emergence from minimally conscious state) were tested by performing separately for each frequency band a one-way ANOVA, followed by post hoc Bonferroni corrected t-test. In the five subjects who underwent longitudinal recording sessions (Group II) and in the two patients recorded both during behavioural sleep and wakefulness, changes in the EEG spectrum were assessed statistically at the individual level by means of a two tailed paired t-test.
Results
Using a TMS-compatible 60-channel EEG amplifier we recorded TMS-evoked EEG potentials in 17 patients. In each subject, we performed different stimulation/recording sessions during which we targeted the parietal lobe (superior parietal gyrus) and the frontal lobe (superior frontal gyrus) bilaterally. In total, 72 stimulations were successfully performed and analysed. During all stimulation/recording sessions patients were lying on their beds, awake and with their eyes open.
Measuring cortical effective connectivity allows single subject discrimination between vegetative patients and patients who show some level of consciousness
Building on previous measurements performed in awake, sleeping (Massimini et al., 2005, 2010) and anaesthetized subjects (Ferrarelli et al., 2010), we first tested the ability of TMS/EEG to discriminate between consciousness and unconsciousness in brain-injured patients. A group of 12 patients (Group I) underwent a TMS/EEG session after 1 week of repeated behavioural evaluations by means of the CRS-R (Giacino et al., 2004). Five subjects from this group showed only reflexive behaviour, remained unresponsive to the environment during the whole observation period and were diagnosed in a vegetative state (Supplementary Table 1). As shown in Fig. 1A and Supplementary Fig. 2A, TMS evoked a slow, positive-negative EEG response in all patients in a vegetative state except for one anoxic patient (Patient 4) in whom no response could be elicited even when TMS was delivered at high intensity (200 V/m) in both hemispheres (Supplementary Fig. 2A and 4). The coloured maps show, for each subject, the cortical sources that were involved by TMS-evoked maximum neuronal currents during the significant intervals of the post-stimulus period (0–300 ms) (see ‘Materials and methods’ section and Supplementary Fig. 1 for details about the statistical procedure). At the right side of each map the number of detected sources is reported together with the time series of neuronal currents recorded from six selected cortical areas (Supplementary Fig. 1). In all patients in a vegetative state, TMS elicited maximum cortical currents that remained localized during the entire significant post-stimulus period, involving a small number of sources around the stimulated area. The stereotypical, local positive-negative wave triggered by TMS in patients in a vegetative state closely resembled the one previously observed during deep sleep (Massimini et al., 2005) and anaesthesia (Ferrarelli et al., 2010), when subjects, if awakened, report little or no conscious experience. Thus, TMS/EEG measurements revealed a substantial impairment of inter-areal causal interactions in the brain of patients who were open-eyed, behaviourally awake but presumably unconscious.
Five subjects of Group I satisfied the CRS-R criteria for a minimally conscious state during the observation period (Supplementary Table 1). These patients showed fluctuating signs of non-reflexive reactions to external stimuli (such as visual pursuit or responses to simple commands) but were unable to communicate reliably with the examiners. In these cases, TMS invariably triggered a complex EEG response associated with a rapidly changing pattern of cortical activation, where maximum neuronal currents shifted over time from the stimulated site to a large number of distant sources (Fig. 1B and Supplementary Fig. 2B). This pattern contrasted starkly with the local, simple wave recorded in patients in a vegetative state and was, instead, comparable to the one obtained in two subjects with locked-in syndrome (Fig. 1C and Supplementary Fig. 2C). Subjects with locked-in syndrome, though being largely paralysed at the time of recording, could signal that they were fully aware through vertical eye movements. In Fig. 3 the number of sources involved by the propagation of TMS-evoked maximum currents (effective connectivity) is reported for all TMS/EEG sessions (Fig. 3A), all sites of stimulation (Fig. 3B) and all patients; clear-cut differences in cortical effective connectivity discriminate between individual patients in a vegetative state and patients in a minimally conscious state with a stable clinical diagnosis (Group I in Fig. 3A and B).
Cortical effective connectivity recovers in the brain of patients who recover their ability to communicate
If effective connectivity among thalamocortical modules is a key neurophysiological mechanism for some level of consciousness to emerge, then it should clearly recover in the brain of an individual patient before he recovers his ability to communicate reliably. To test this hypothesis we performed longitudinal TMS/EEG measurements in a group of five patients (Group II) who were recruited from the intensive care as soon as they awakened from coma. As assessed by means of the CRS-R (Supplementary Table 2), three of these patients (Fig. 2A and Supplementary Fig. 3A) recovered consciousness and functional communication, evolving from a vegetative state through minimally conscious state to emergence from minimally conscious state, whereas two patients (Fig. 2B and Supplementary Fig. 3B) remained in a vegetative state. In all cases the first TMS/EEG session (Session 1) was performed at least 48 h after withdrawal of sedation, when patients opened their eyes and were diagnosed as in a vegetative state. At this time, similar to the patients in a vegetative state in Group I, TMS evoked a simple wave and a local pattern of activation or no response at all (Patient 17, anoxic) (Fig. 2B, Supplementary Fig. 3B and 4). Following Session 1, two additional TMS/EEG sessions were performed in the three patients who eventually recovered consciousness: Session 2 was recorded as soon as they satisfied the CRS-R criteria for minimally conscious state and Session 3 when they recovered functional communication and emerged from the minimally conscious state. In these patients, TMS triggered a complex pattern of activation that sequentially involved a large set of cortical areas already during Session 2; this response was substantially different from the simple, local activation of Session 1 and was instead comparable to the one obtained in Session 3, when subjects had recovered their ability to communicate (Figs 2A and 3 and Supplementary Fig. 3A). In the two patients who did not show any clinical improvement beyond vegetative state, a second TMS/EEG measurement (Session 2) was performed >1 month after Session 1 and showed either a local, simple wave of activation (Patient 16) or no response (Patient 17, anoxic), although subjects were awake and open-eyed when their brains were stimulated (Figs 2B, 3, Supplementary Fig. 3B and 4). The results of Group II experiments are also reported in Fig. 3 and indicate that the breakdown of effective connectivity observed in patients in a vegetative state can be reversible and that a substantial improvement in the brain's ability to sustain internal communication occurs at an early stage during recovery of consciousness, before reliable communication can be established with the patient.
The recovery of cortical effective connectivity is not contingent on overt changes in the background electroencephalogram spectrum
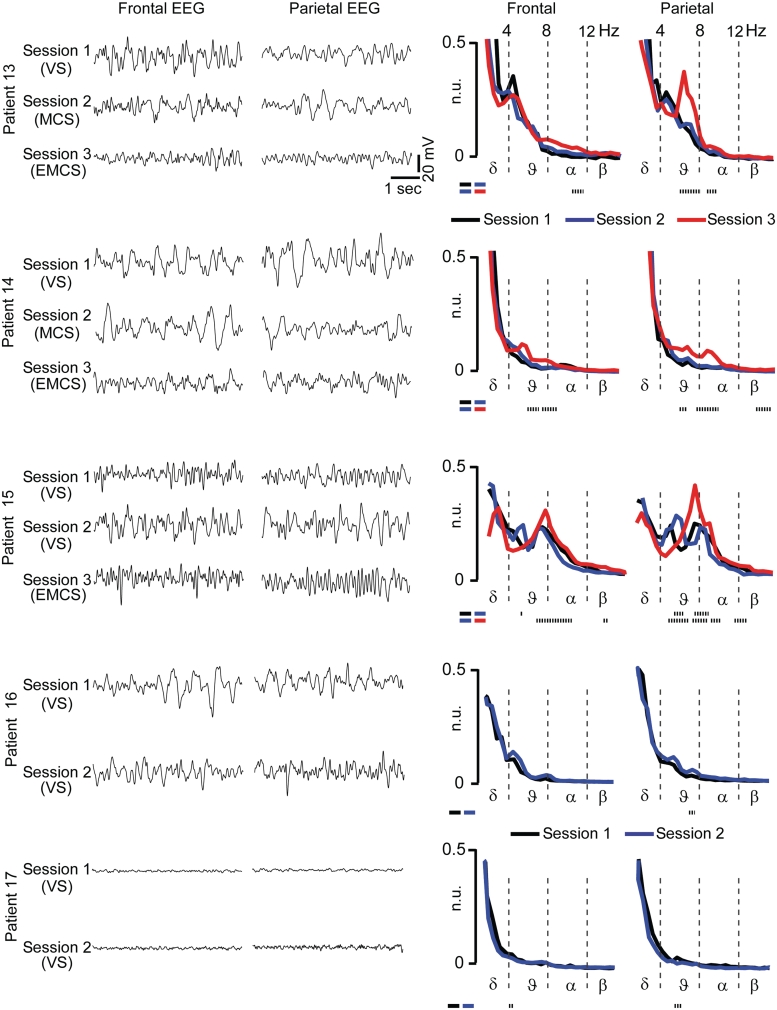
Patients in a vegetative state showed a local response to TMS whether they were behaviourally ‘awake’ (eyes open) or ‘asleep’ (eyes closed) (Supplementary Fig. 5), suggesting a dissociation between the mechanisms of cortical integration and behavioural arousal. Therefore, we assessed whether changes in effective connectivity could also be dissociated from changes in electrophysiological arousal (EEG activation). Spectral analysis of spontaneous EEG (see ‘Materials and methods’ section) showed, at the group-level, a significant increase of high-frequency oscillations (α- and β-bands) in locked-in syndrome-emergence from minimally conscious state compared with the minimally conscious state (one-way ANOVA testing group differences, followed by post hoc Bonferroni corrected t-test) (Supplementary Table 3). In contrast, in spite of a clear-cut change in the electrical response to TMS, no systematic changes of the background EEG could be detected between vegetative state and minimally conscious state both at single subject (Fig. 4) and at the group level (Supplementary Table 3), consistent with previous reports (Kotchoubey et al., 2005). These results suggest that the transition from vegetative to minimally conscious state involves a substantial improvement of effective connectivity that is not necessarily associated with an obvious change in the level of activation of the ongoing EEG.
Figure 4.
EEG spectra show evident changes from minimally conscious state (MCS) to emergence from minimally conscious state (EMCS) but not from vegetative state (VS) to minimally conscious state. Spontaneous EEG traces (5 s) and EEG spectra (calculated on 2 min; average of 5 s epochs) are shown for the five subjects who underwent longitudinal recording sessions (Group II); in these patients, changes in the EEG spectrum were assessed statistically by means of a two-tailed paired t-test. The dotted lines at the bottom of each plot indicate the frequency bins that show statistically significant differences of power (t-test, P < 0.01). EEG positivity is upward. n.u. = normalised units.
Discussion
In this work we employed TMS/EEG to measure cortical effective connectivity at the bedside of patients emerging from coma after severe brain injury. The specific aim of the present study was to develop a novel approach to detect and track the neural correlates of recovery of consciousness in non-communicating patients. This approach can complement event-related EEG potential protocols and functional MRI active paradigms because it does not rely on a subject's ability to process sensory stimuli, to understand and follow instructions or communicate; instead, it aims at gauging directly the ability of distributed thalamocortical modules to interact among each other on a millisecond time-scale, a condition that is considered critical for consciousness to emerge (Tononi, 2004; Laureys, 2005; Tononi and Koch, 2008; Alkire et al., 2008; Seth et al., 2008). Practically, such an approach can be important because the capacity of brain-injured patients to interact with the external environment may be impeded by lesions of sensory/motor pathways and cortices, by difficulties in language comprehension (Majerus et al., 2009) and may fluctuate significantly over time (Monti et al., 2010b). It could prove especially useful in patients at the lower boundary of consciousness, by providing an objective biomarker that could be used to monitor and guide their rehabilitation and treatment (Schiff, 2010; Shah and Schiff, 2010).
As in previous studies (Massimini et al., 2005, 2010; Ferrarelli et al., 2010), in order to probe the ability of distributed thalamocortical modules to interact, we stimulated a subset of cortical neurons with TMS and performed EEG source modelling to detect, on a millisecond time-scale, the chain of effects triggered in the rest of the brain by this initial perturbation. Compared to methods based on the observation of resting brain activity, this perturb-and-measure approach (Paus, 2005) readily dissociates functional connectivity (temporal correlations) from effective connectivity (causal interactions), which is defined as the ability of a subset of neurons to causally affect the activity of other groups of neurons (Friston, 2002; Lee et al., 2003). Recent studies have shown that by employing TMS/EEG and source modelling it is possible to detect patterns of effective connectivity that are generally predicted by main anatomical pathways (Ilmoniemi et al., 1997; Litvak et al., 2007; Morishima et al., 2009; Casali et al., 2010). On the other hand, since TMS tends to activate a large set of cortical axons in a way that is difficult to control fully (Wagner et al., 2007), this technique is more likely to provide a coarse rather than a fine-grained estimation of effective connectivity. In the present context, a broader estimation of effective connectivity may constitute an advantage, since theoretical works (Tononi, 2004; Tononi and Koch, 2008), experimental data (Maandag et al., 2007; Alkire et al., 2008; Shulman et al., 2009) and clinical evidence (Markowitsch and Kessler, 2000; Mataro et al., 2001; Schiff, 2010) suggest that consciousness depends not so much on some specific circuits, but rather on the capacity of distributed regions of the brain to interact through divergent cortico–cortical and cortico–thalamo–cortical connections. Indeed, as demonstrated by previous experiments, TMS/EEG measures of effective connectivity can distinguish readily between conditions in which consciousness is present (alert wakefulness, dreaming) (Massimini et al., 2005, 2010) and conditions in which consciousness is reduced, or lost (sleep and anaesthesia) (Massimini et al., 2005; Ferrarelli et al., 2010).
Figure 3 summarizes the results obtained after applying TMS in all 17 patients and shows that it is possible to discriminate reliably between a vegetative and minimally conscious state, at the single-subject level. Crucially, this discrimination was achieved in a way that is completely independent on the patient's ability to exchange information with the surrounding environment. The fact that TMS/EEG detected a clear-cut difference between vegetative state and minimally conscious state (unconsciousness versus low-level of consciousness) but not between minimally conscious state, emergence from minimally conscious state and locked-in syndrome (lower versus higher levels of consciousness) suggests that the availability of effective interactions among thalamocortical modules may be a critical mechanism that correlates closely with the presence/absence of a minimal level of consciousness. This aspect is particularly relevant if one considers that the most challenging task at the bedside is distinguishing between patients in a vegetative state and non-communicating patients in a minimally conscious state (Majerus et al., 2005). As an example, in the present work, TMS/EEG detected the resumption of rapid, effective intracortical interactions in the brain of a patient (Patient 15) who (during Session 2) had temporarily slipped back into a clinically vegetative state, possibly due to transient fluctuations in her ability to interact with the environment; this patient was reassessed clinically as minimally conscious state and then emerged from minimally conscious state.
Clearly, validating an objective marker of consciousness that can be applied to patients that are unable to interact with the external environment is challenging by definition, since, in these cases, there is no behavioural reference to assess the presence of consciousness. In an attempt to overcome this circularity, we have previously tested TMS/EEG measures in states in which consciousness is unambiguously present [alert wakefulness (Massimini et al., 2005), dreaming (Massimini et al., 2010)] or unambiguously reduced [early slow wave sleep (Massimini et al., 2005), general anaesthesia (Ferrarelli et al., 2010)]. Here, we demonstrate that TMS/EEG measures are reliable when they are applied to brain-injured patients with a stable clinical diagnosis (Group I) and that they are sensitive in detecting a clear-cut resurgence of cortical effective connectivity in the brains of individual patients who gradually recover consciousness and functional communication (Group II). In future works, the same approach should be further tested, in a back-and-forth process, both in definite and in ambiguous clinical conditions, such as the one of Patient 15. It will be equally important to directly compare the ability of TMS/EEG to discriminate between vegetative and minimally conscious states at the individual level with the diagnostic capacity of other neurophysiological methods, such as peripherally evoked potentials (Kotchoubey et al., 2005; Bekinschtein et al., 2009; Fischer et al., 2010; Boly et al., 2011) and long-term EEG recordings (Landsness et al., 2011). The lack of a direct comparison with other techniques represents a clear limitation of the present study and is due to logistical and time constraints (in each patient, we stimulated from two to four cortical sites) in the intensive care unit. For now, we can only compare the present results to the current literature and, in particular, to a number of works in which the mismatch negativity was evaluated systematically in patients in a vegetative state and patients in a minimally conscious state. Altogether, this body of literature suggests that, while the mismatch negativity may differ significantly between vegetative state and minimally conscious state at the group level, it does not discriminate reliably between these two conditions at the individual patient's level; in fact, this late component may be undetectable in a large proportion (up to 60%) of patients who are behaviourally in a minimally conscious state (Kotchoubey et al., 2005; Fischer et al., 2010; Holler et al., 2011). Since in the present study we found consistent TMS/EEG results across sites of stimulation, in future work it will be feasible to directly compare the EEG response to TMS of a single cortical area with a battery of sensory evoked potentials (N20, mismatch negativity and P3b) recorded in the same patient, on the same day. These joint measurements will be crucial to precisely quantify the relative diagnostic power of complementary neurophysiological techniques that may enter the routine evaluation of severely brain-injured patients. To this regard, the present experiments show that, like peripheral evoked potentials, TMS-evoked potentials can be recorded at the patient's bedside, in the intensive care unit. A technical disadvantage of TMS/EEG is that it requires a more complex set-up, which includes a TMS main unit, a TMS-compatible EEG amplifier and, ideally, a navigation system in order to precisely target TMS on the cerebral cortex. Navigating TMS based on prior anatomical knowledge (CT or MRI scan) may be especially important in the assessment of brain-injured patients for two reasons. First, because it allows avoiding obvious cortical lesions and stimulating the cortical surface at supra-threshold intensity (see ‘Materials and methods’ section and Casali et al., 2010) and second (and most important) because it ensures high test-retest reproducibility when TMS-evoked potentials are performed longitudinally (Lioumis et al., 2009; Casarotto et al., 2010). Hardware solutions aside, developing TMS/EEG towards routine clinical applications may require the implementation of a standard, fast data analysis procedure to calculate the spatial-temporal complexity of the cortical response to TMS.
Besides their potential diagnostic value, TMS/EEG measurements may provide novel insights on the physiopathology of disorders of consciousness as well as a valuable marker to guide rehabilitation and treatment (Giacino et al., 2006; Shah and Schiff, 2010). In patients in a vegetative state, who were aroused but unaware, TMS failed to trigger complex, long-range activations pointing to a dissociation between arousal and the mechanisms of thalamocortical integration. In patients in a vegetative state caused by anoxia (Patients 4 and 17) no significant EEG responses could be elicited, even when TMS was delivered at high intensity at multiple stimulation sites (Fig. 3 and Supplementary Fig. 4), consistent with an extensive necrosis of the cerebral cortex (Kinney and Samuels, 1994). In non-anoxic patients in a vegetative state TMS elicited, at both frontal and parietal sites, a strong response that remained local (Figs 1, 2, Supplementary Fig. 2 and 3) corroborating the notion that the brain of these patients may retain islands of cortex (including associative areas) that are responsive, but reciprocally disconnected (Schiff et al., 2002; Laureys et al., 2004). According to post-mortem (Adams et al., 2000) and in vivo (Fernandez-Espejo et al., 2011) neuropathological studies, this disconnection is primarily structural and may be largely due to widespread injury of cortico–cortical fibres but also to thalamic damage, leading to a substantial impairment of cortico–thalamo–cortical circuits. Notably, the present results indicate that, in addition to the anatomical damage, functional disturbances in thalamocortical networks may play a significant role. Indeed, in non-anoxic patients in a vegetative state TMS triggered a slow wave similar to the one recorded during sleep (Massimini et al., 2005, 2007) and anaesthesia (Ferrarelli et al., 2010) suggesting that, besides structural lesions and disconnections, functional alterations such as disfacilitation (Englot et al., 2010), network bistability (Massimini et al., 2009b) and altered excitation–inhibition balance (Schiff, 2010), may contribute to the overall impairment of effective connectivity. These alterations were possibly reversed in the patients of Group II in whom repeated TMS/EEG measurements revealed a resumption of fast, long-range interactions, which paralleled recovery of consciousness; further measurements should be performed, longitudinally, at the bedside of patients who recuperate spontaneously and in patients who undergo pharmacological treatment (Brefel-Courbon et al., 2007), or protocols of neuromodulation (Schiff et al., 2007), in order to gain better insight on the mechanisms of recovery of consciousness after brain injury.
Funding
EU-grant-224328-PredictAD; PUR 2009 (University of Milan); Prin 2008 (Italian Government); Belgian National Funds for Scientific Research (FNRS); James S. McDonnell Foundation; Mind Science Foundation; European Commission (Mindbridge, DISCOS, DECODER & COST); Concerted Research Action (ARC 06/11-340); Public Utility Foundation “Université Européenne du Travail” and “Fondazione Europea di Ricerca Biomedica”.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank Simone Sarasso, Didier Ledoux, Andrea Pigorini and Martino Napolitani for their help and comments.
Glossary
Abbreviations
- CRS-R
Coma Recovery Scale-Revised
- TMS
transcranial magnetic stimulation
References
- Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123:1327–38. doi: 10.1093/brain/123.7.1327. [DOI] [PubMed] [Google Scholar]
- Akaishi R, Morishima Y, Rajeswaren VP, Aoki S, Sakai K. Stimulation of the frontal eye field reveals persistent effective connectivity after controlled behavior. J Neurosci. 2010;30:4295–305. doi: 10.1523/JNEUROSCI.6198-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni F, Babiloni C, Locche L, Cincotti F, Rossini PM, Carducci F. High-resolution electro-encephalogram: source estimates of Laplacian-transformed somatosensory-evoked potentials using a realistic subject head model constructed from magnetic resonance images. Med Biol Eng Comput. 2000;38:512–9. doi: 10.1007/BF02345746. [DOI] [PubMed] [Google Scholar]
- Bardin JC, Fins JJ, Katz DI, Hersh J, Heier LA, Tabelow K, et al. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain. 2011;134:769–82. doi: 10.1093/brain/awr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L, Naccache L. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci USA. 2009;106:1672–7. doi: 10.1073/pnas.0809667106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P, Scherg M. A fast method for forward computation of multiple-shell spherical head models. Electroencephalogr Clin Neurophysiol. 1994;90:58–64. doi: 10.1016/0013-4694(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, et al. Preserved feedforward but impaired top-down processes in the vegetative state. Science. 2011;332:858–62. doi: 10.1126/science.1202043. [DOI] [PubMed] [Google Scholar]
- Bonato C, Miniussi C, Rossini PM. Transcranial magnetic stimulation and cortical evoked potentials: a TMS/EEG co-registration study. Clin Neurophysiol. 2006;117:1699–707. doi: 10.1016/j.clinph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Brefel-Courbon C, Payoux P, Ory F, Sommet A, Slaoui T, Raboyeau G, et al. Clinical and imaging evidence of zolpidem effect in hypoxic encephalopathy. Ann Neurol. 2007;62:102–5. doi: 10.1002/ana.21110. [DOI] [PubMed] [Google Scholar]
- Casali AG, Casarotto S, Rosanova M, Mariotti M, Massimini M. General indices to characterize the electrical response of the cerebral cortex to TMS. Neuroimage. 2010;49:1459–68. doi: 10.1016/j.neuroimage.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Romero Lauro LJ, Bellina V, Casali AG, Rosanova M, Pigorini A, et al. EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS One. 2010;5:e10281. doi: 10.1371/journal.pone.0010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daltrozzo J, Wioland N, Mutschler V, Kotchoubey B. Predicting coma and other low responsive patients outcome using event-related brain potentials: a meta-analysis. Clin Neurophysiol. 2007;118:606–14. doi: 10.1016/j.clinph.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci. 2006;10:204–11. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Del Cul A, Baillet S, Dehaene S. Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol. 2007;5:e260. doi: 10.1371/journal.pbio.0050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Duncan CC, Summers AC, Perla EJ, Coburn KL, Mirsky AF. Evaluation of traumatic brain injury: brain potentials in diagnosis, function, and prognosis. Int J Psychophysiol. 2011;82:24–40. doi: 10.1016/j.ijpsycho.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Yang L, Hamid H, Danielson N, Bai X, Marfeo A, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–77. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein TA, Galanaud D, Puybasset L, et al. Probing consciousness with event-related potentials in the vegetative state. Neurology. 2011;77:264–8. doi: 10.1212/WNL.0b013e3182217ee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellinger R, Klimesch W, Schnakers C, Perrin F, Freunberger R, Gruber W, et al. Cognitive processes in disorders of consciousness as revealed by EEG time-frequency analyses. Clin Neurophysiol. 2011;122:2177–84. doi: 10.1016/j.clinph.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espejo D, Bekinschtein T, Monti MM, Pickard JD, Junque C, Coleman MR, et al. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage. 2011;54:103–12. doi: 10.1016/j.neuroimage.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci USA. 2010;107:2681–6. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Luaute J, Adeleine P, Morlet D. Predictive value of sensory and cognitive evoked potentials for awakening from coma. Neurology. 2004;63:669–73. doi: 10.1212/01.wnl.0000134670.10384.e2. [DOI] [PubMed] [Google Scholar]
- Fischer C, Luaute J, Morlet D. Event-related potentials (MMN and novelty P3) in permanent vegetative or minimally conscious states. Clin Neurophysiol. 2010;121:1032–42. doi: 10.1016/j.clinph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Friston K. Beyond phrenology: what can neuroimaging tell us about distributed circuitry? Annu Rev Neurosci. 2002;25:221–50. doi: 10.1146/annurev.neuro.25.112701.142846. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–53. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–9. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Schnakers C, Rodriguez-Moreno D, Kalmar K, Schiff N, Hirsch J. Behavioral assessment in patients with disorders of consciousness: gold standard or fool's gold? Prog Brain Res. 2009;177:33–48. doi: 10.1016/S0079-6123(09)17704-X. [DOI] [PubMed] [Google Scholar]
- Hamalainen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hauk O. Keep it simple: a case for using classical minimum norm estimation in the analysis of EEG and MEG data. Neuroimage. 2004;21:1612–21. doi: 10.1016/j.neuroimage.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Pace-Schott EF, Stickgold R. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci. 2000;23:793–842. doi: 10.1017/s0140525x00003976. [DOI] [PubMed] [Google Scholar]
- Holler Y, Bergmann J, Kronbichler M, Crone JS, Schmid EV, Golaszewski S, et al. Preserved oscillatory response but lack of mismatch negativity in patients with disorders of consciousness. Clin Neurophysiol. 2011;122:1744–54. doi: 10.1016/j.clinph.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–40. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet. 1972;1:734–7. doi: 10.1016/s0140-6736(72)90242-5. [DOI] [PubMed] [Google Scholar]
- Kahkonen S, Komssi S, Wilenius J, Ilmoniemi RJ. Prefrontal TMS produces smaller EEG responses than motor-cortex TMS: implications for rTMS treatment in depression. Psychopharmacol. 2005a;181:16–20. doi: 10.1007/s00213-005-2197-3. [DOI] [PubMed] [Google Scholar]
- Kahkonen S, Komssi S, Wilenius J, Ilmoniemi RJ. Prefrontal transcranial magnetic stimulation produces intensity-dependent EEG responses in humans. Neuroimage. 2005b;24:955–60. doi: 10.1016/j.neuroimage.2004.09.048. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Samuels MA. Neuropathology of the persistent vegetative state. A review. J Neuropathol Exp Neurol. 1994;53:548–58. doi: 10.1097/00005072-199411000-00002. [DOI] [PubMed] [Google Scholar]
- Komssi S, Huttunen J, Aronen HJ, Ilmoniemi RJ. EEG minimum-norm estimation compared with MEG dipole fitting in the localization of somatosensory sources at S1. Clin Neurophysiol. 2004a;115:534–42. doi: 10.1016/j.clinph.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Komssi S, Kahkonen S, Ilmoniemi RJ. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum Brain Mapp. 2004b;21:154–64. doi: 10.1002/hbm.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komssi S, Savolainen P, Heiskala J, Kahkonen S. Excitation threshold of the motor cortex estimated with transcranial magnetic stimulation electroencephalography. Neuroreport. 2007;18:13–6. doi: 10.1097/WNR.0b013e328011b89a. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B, Lang S, Mezger G, Schmalohr D, Schneck M, Semmler A, et al. Information processing in severe disorders of consciousness: vegetative state and minimally conscious state. Clin Neurophysiol. 2005;116:2441–53. doi: 10.1016/j.clinph.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Landsness E, Bruno MA, Noirhomme Q, Riedner B, Gosseries O, Schnakers C, et al. Electrophysiological correlates of behavioural changes in vigilance in vegetative state and minimally conscious state. Brain. 2011;134:2222–32. doi: 10.1093/brain/awr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci. 2005;9:556–9. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–46. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- Lee L, Harrison LM, Mechelli A. A report of the functional connectivity workshop, Dusseldorf 2002. Neuroimage. 2003;19:457–65. doi: 10.1016/s1053-8119(03)00062-4. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol. 1980;48:609–21. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Litvak V, Komssi S, Scherg M, Hoechstetter K, Classen J, Zaaroor M, et al. Artifact correction and source analysis of early electroencephalographic responses evoked by transcranial magnetic stimulation over primary motor cortex. Neuroimage. 2007;37:56–70. doi: 10.1016/j.neuroimage.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Luaute J, Maucort-Boulch D, Tell L, Quelard F, Sarraf T, Iwaz J, et al. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology. 2010;75:246–52. doi: 10.1212/WNL.0b013e3181e8e8df. [DOI] [PubMed] [Google Scholar]
- Lv J, Simpson DM, Bell SL. Objective detection of evoked potentials using a bootstrap technique. Med Eng Phys. 2007;29:191–8. doi: 10.1016/j.medengphy.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Maandag NJ, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, et al. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci USA. 2007;104:20546–51. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus S, Bruno MA, Schnakers C, Giacino JT, Laureys S. The problem of aphasia in the assessment of consciousness in brain-damaged patients. Prog Brain Res. 2009;177:49–61. doi: 10.1016/S0079-6123(09)17705-1. [DOI] [PubMed] [Google Scholar]
- Majerus S, Gill-Thwaites H, Andrews K, Laureys S. Behavioral evaluation of consciousness in severe brain damage. Prog Brain Res. 2005;150:397–413. doi: 10.1016/S0079-6123(05)50028-1. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Kessler J. Massive impairment in executive functions with partial preservation of other cognitive functions: the case of a young patient with severe degeneration of the prefrontal cortex. Exp Brain Res. 2000;133:94–102. doi: 10.1007/s002210000404. [DOI] [PubMed] [Google Scholar]
- Massimini M, Boly M, Casali A, Rosanova M, Tononi G. A perturbational approach for evaluating the brain's capacity for consciousness. Prog Brain Res. 2009a;177:201–14. doi: 10.1016/S0079-6123(09)17714-2. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, et al. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci USA. 2007;104:8496–501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Murphy M, Huber R, Riedner B, Casarotto S, et al. Cortical reactivity and effective connectivity during REM sleep in humans. Cogn Neurosci. 2010;1:176–83. doi: 10.1080/17588921003731578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Tononi G, Huber R. Slow waves, synaptic plasticity and information processing: insights from transcranial magnetic stimulation and high-density EEG experiments. Eur J Neurosci. 2009b;29:1761–70. doi: 10.1111/j.1460-9568.2009.06720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataro M, Jurado MA, Garcia-Sanchez C, Barraquer L, Costa-Jussa FR, Junque C. Long-term effects of bilateral frontal brain lesion: 60 years after injury with an iron bar. Arch Neurol. 2001;58:1139–42. doi: 10.1001/archneur.58.7.1139. [DOI] [PubMed] [Google Scholar]
- McCubbin J, Yee T, Vrba J, Robinson SE, Murphy P, Eswaran H, et al. Bootstrap significance of low SNR evoked response. J Neurosci Methods. 2008;168:265–72. doi: 10.1016/j.jneumeth.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Laureys S, Owen AM. The vegetative state. BMJ. 2010b;341:c3765. doi: 10.1136/bmj.c3765. [DOI] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med. 2010a;362:579–89. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Akaishi R, Yamada Y, Okuda J, Toma K, Sakai K. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat Neurosci. 2009;12:85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Paus T. Inferring causality in brain images: a perturbation approach. Philos Trans R Soc Lond B Biol Sci. 2005;360:1109–14. doi: 10.1098/rstb.2005.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci. 2009;29:7679–85. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of Physicians. Medical aspects of the persistent vegetative state (1). The Multi-Society Task Force on PVS. N Engl J Med. 1994;330:1499–508. doi: 10.1056/NEJM199405263302107. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–3. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Ribary U, Moreno DR, Beattie B, Kronberg E, Blasberg R, et al. Residual cerebral activity and behavioural fragments can remain in the persistently vegetative brain. Brain. 2002;125:1210–34. doi: 10.1093/brain/awf131. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Perrin F, Schabus M, Hustinx R, Majerus S, Moonen G, et al. Detecting consciousness in a total locked-in syndrome: an active event-related paradigm. Neurocase. 2009;15:271–7. doi: 10.1080/13554790902724904. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Perrin F, Schabus M, Majerus S, Ledoux D, Damas P, et al. Voluntary brain processing in disorders of consciousness. Neurology. 2008;71:1614–20. doi: 10.1212/01.wnl.0000334754.15330.69. [DOI] [PubMed] [Google Scholar]
- Seth AK, Dienes Z, Cleeremans A, Overgaard M, Pessoa L. Measuring consciousness: relating behavioural and neurophysiological approaches. Trends Cogn Sci. 2008;12:314–21. doi: 10.1016/j.tics.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Schiff ND. Central thalamic deep brain stimulation for cognitive neuromodulation - a review of proposed mechanisms and investigational studies. Eur J Neurosci. 2010;32:1135–44. doi: 10.1111/j.1460-9568.2010.07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Baseline brain energy supports the state of consciousness. Proc Natl Acad Sci USA. 2009;106:11096–101. doi: 10.1073/pnas.0903941106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C, Maltez JC, Trindade E, Arriaga A, Ducla-Soares E. Evaluation of L1 and L2 minimum norm performances on EEG localizations. Clin Neurophysiol. 2004;115:1657–68. doi: 10.1016/j.clinph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Koch C. The neural correlates of consciousness: an update. Ann N Y Acad Sci. 2008;1124:239–61. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- Virtanen J, Ruohonen J, Naatanen R, Ilmoniemi RJ. Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation. Med Biol Eng Comput. 1999;37:322–6. doi: 10.1007/BF02513307. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–65. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Wijnen VJ, van Boxtel GJ, Eilander HJ, de Gelder B. Mismatch negativity predicts recovery from the vegetative state. Clin Neurophysiol. 2007;118:597–605. doi: 10.1016/j.clinph.2006.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.