Abstract
Mild traumatic brain injury is the most prevalent neurological insult and frequently results in neurobehavioural sequelae. However, little is known about the pathophysiology underlying the injury and how these injuries change as a function of time. Although diffusion tensor imaging holds promise for in vivo characterization of white matter pathology, both the direction and magnitude of anisotropic water diffusion abnormalities in axonal tracts are actively debated. The current study therefore represents both an independent replication effort (n = 28) of our previous findings (n = 22) of increased fractional anisotropy during semi-acute injury, as well as a prospective study (n = 26) on the putative recovery of diffusion abnormalities. Moreover, new analytical strategies were applied to capture spatially heterogeneous white matter injuries, which minimize implicit assumptions of uniform injury across diverse clinical presentations. Results indicate that whereas a general pattern of high anisotropic diffusion/low radial diffusivity was present in various white matter tracts in both the replication and original cohorts, this pattern was only consistently observed in the genu of the corpus callosum across both samples. Evidence for a greater number of localized clusters with increased anisotropic diffusion was identified across both cohorts at trend levels, confirming heterogeneity in white matter injury. Pooled analyses (50 patients; 50 controls) suggested that measures of diffusion within the genu were predictive of patient classification, albeit at very modest levels (71% accuracy). Finally, we observed evidence of recovery in lesion load in returning patients across a 4-month interval, which was correlated with a reduction in self-reported post-concussive symptomatology. In summary, the corpus callosum may serve as a common point of injury in mild traumatic brain injury secondary to anatomical (high frequency of long unmyelinated fibres) and biomechanics factors. A spatially heterogeneous pattern of increased anisotropic diffusion exists in various other white matter tracts, and these white matter anomalies appear to diminish with recovery. This macroscopic pattern of diffusion abnormalities may be associated with cytotoxic oedema following mechanical forces, resulting in changes in ionic homeostasis, and alterations in the ratio of intracellular and extracellular water. Animal models more specific to the types of mild traumatic brain injury typically incurred by humans are needed to confirm the histological correlates of these macroscopic markers of white matter pathology.
Keywords: mild traumatic brain injury, DTI, longitudinal
Introduction
In spite of the large number of individuals who sustain a mild traumatic brain injury (TBI) each year (Faul et al., 2010), the pathophysiology of mild TBI and its role in producing neurobehavioural symptomatology is still actively debated. Since complex cognitive processes such as attention, executive functions and memory are dependent on intact white matter tracts between frontal, parietal and medial temporal lobes (Grieve et al., 2007), putative white matter pathology provides an intriguing link to the neurobehavioural symptomatology that is frequently observed during the semi-acute stage (i.e. first weeks) of mild TBI (Belanger et al., 2007; Bigler, 2008). Indeed, accumulating evidence from both animal (Mac Donald et al., 2007a; Spain et al., 2010) and human (Blumbergs et al., 1994; Bigler, 2010; Niogi and Mukherjee, 2010) studies suggest subtle white matter abnormalities that are better captured by diffusion tensor imaging (DTI) than by conventional imaging. However, the direction of tissue water diffusivity abnormalities (as measured by DTI) has proven difficult to characterize (Niogi and Mukherjee, 2010). Specifically, different studies report increased (Bazarian et al., 2007; Wilde et al., 2008; Mayer et al., 2010; Henry et al., 2011), reduced (Arfanakis et al., 2002; Inglese et al., 2005; Miles et al., 2008; Smits et al., 2011) or unchanged (Zhang et al., 2010; Messe et al., 2011) white matter diffusivity during semi-acute mild TBI.
A commonly applied DTI metric is fractional anisotropy, which indirectly measures preferential water diffusion along white matter tracts and thus serves as a surrogate marker of tissue integrity. Animal studies of healthy, non-myelinated fibres and computer simulations suggest a more prominent role for axonal membranes in determining anisotropic diffusion rather than myelin (Beaulieu, 2002), with the ratio of intracellular to extracellular fluid also a contributing factor (Peled, 2007). Moreover, axonal pathology is more pronounced in the acute phase of injury (Mac Donald et al., 2007a; Dikranian et al., 2008; Spain et al., 2010), with evidence of injury progression over a 4–6-week period that correlates with cognitive dysfunction (Spain et al., 2010). These traumatic axonal injuries can occur either as a direct result of rotational forces or from complex secondary cellular processes involving mechanoreceptor dysregulation, ionic flux and alterations to the cytoskeleton (Albensi et al., 2000; Povlishock and Katz, 2005; Van Putten et al., 2005).
In contrast to animal work, differences in injury severity, injury type, measurement time post-injury, use of representative versus persistently symptomatic samples, and sample type (e.g. athlete versus military versus typical emergency room patient) all potentially contribute to the heterogeneity of findings in human diffusivity studies. For example, studies examining patients with chronic (months to years post-injury) mild TBI generally report decreased fractional anisotropy (Niogi and Mukherjee, 2010). However, patients with chronically symptomatic mild TBI reflect only a minority (3–20%) of the overall mild TBI population and thus represent a specific sample with potentially different clinical characteristics (Dikmen et al., 2001; Iverson, 2005). Similarly, sample-specific attributes (athletes or combat-exposed military personnel) may increase the likelihood of temporally proximal repetitive concussive or sub-concussive injuries (Zhang et al., 2006; Levin et al., 2010; Mac Donald et al., 2011), as well as introduce differences in general health and other complicating psychiatric factors (Hoge et al., 2008). As a result, directly recruiting sequential patients from the emergency room and scanning during the semi-acute injury phase may be the best strategy for collecting a sample that best generalizes to the overall population of mild brain injuries (Mayer et al., 2010).
As recently demonstrated with other neuroimaging modalities (Vagnozzi et al., 2010; Mayer et al., 2011; Yeo et al., 2011), imaging studies with longitudinal components are also critical for clarifying the recovery course of diffusivity abnormalities and the resolution of clinical symptoms. Animal models suggest that anisotropic measures are a good predictor of time post-injury (Mac Donald et al., 2007b), but these findings have not been easily replicated in human ‘cross-sectional’ studies (Inglese et al., 2005; Rutgers et al., 2008). Preliminary longitudinal work by our group (Mayer et al., 2010) and others (Arfanakis et al., 2002) suggests partial normalization of diffusion indices, although in each study sample sizes were small (n < 10) and the direction of ‘normalization’ varied. A recent longitudinal study of a military sample that included both semi-acute and post-semi-acute patients reported that while lower relative anisotropy in blast-related TBI persisted into follow-up, initial mean and axial diffusivity scores normalized across a 6–12-month interval (Mac Donald et al., 2011).
In addition to variability introduced by clinical factors, the analytical approach adopted for quantifying white matter abnormalities may also contribute to discrepancies in semi-acute mild TBI DTI studies. Specifically, both region of interest and voxel-based analyses have previously been used to characterize white matter pathology during semi-acute mild TBI. Voxel-based analyses emphasizing the alignment of white matter tracts [tract-based spatial statistics (TBSS)] have also been introduced. In general, both region of interest and voxel-wise analyses are based on the implicit assumption that clinically heterogeneous patients have a homogenous (i.e. high degree of spatial overlap) pattern of white matter abnormalities, although this assumption is much stronger for voxel-wise approaches. However, the validity of the spatial homogeneity assumption is questionable, which motivated us in the current study to adopt a new approach for quantifying diffusion abnormalities through a metric similar to lesion-load (White et al., 2009). Specifically, clusters of abnormally high or low anisotropic diffusion were determined on a voxel-wise basis and then summed to represent total burden of distributed pathology.
The current study thus had three primary goals. First, we attempted to independently replicate our previous finding of increased fractional anisotropy in a new cohort of patients with mild TBI (n = 28) using identical experimental protocols. Replication efforts are critical for clarifying the distinction between sample characteristics discussed earlier and the reproducibility of the methodologies used to examine their effects, especially given that previously published studies on semi-acute mild TBI have included relatively modest sample sizes (5 ≤ n ≤ 22). Secondly, data were combined across our original and replication samples (hereafter referred to as the pooled sample) to examine questions of recovery of function (through both cross-sectional and longitudinal analyses) and whether differences in fractional anisotropy were associated with indices of injury severity or type. Finally, a new metric for quantifying diffusion abnormalities that is less reliant on common injury sites across patients with mild TBI was introduced. Based on prior results, we predicted that patients with mild TBI in the replication sample would again show increased anisotropy in region of interest but not voxel-based analyses, and that there would be evidence of recovery of function at both short-term (i.e. a negative correlation with days post-injury in cross-sectional analyses) and longitudinal analyses.
Materials and Methods
Participants and clinical data: replication sample
A total of 29 patients with mild TBI and 29 gender-, age- and education-matched healthy controls were recruited through a case-control design. Specifically, the majority of controls were directly recruited to match patients in terms of gender, age (± 2 years) and education (± 2 years). One patient and one control were eliminated during quality assurance protocols (see following sections) leaving a total of 28 patients with mild TBI (16 males, 12 females; 28.21 ± 10.58 years old; 13.11 ± 2.04 years of education) and 28 healthy controls (27.36 ± 10.22 years old; 13.86 ± 1.63 years of education). All patients were recruited from local Albuquerque Emergency Rooms and were evaluated clinically (clinical exam = 15.71 ± 4.45 days post-injury) and with neuroimaging (imaging exam = 15.57 ± 4.29 days post-injury) within 21 days of injury. Clinical and imaging protocols occurred within 3 days of each other for the majority (82%) of patients (see Supplementary Table 1).
Inclusion criteria for the mild TBI sample were based on the American Congress of Rehabilitation Medicine [Glasgow Coma Score of 13–15, loss of consciousness (if present) < 30 min, post-traumatic amnesia (if present) < 24 h]. At minimum, all participants experienced an alteration in mental status, although the majority of the sample (18/28) also experienced a loss of consciousness. Mild TBI participants and controls were excluded if there was a history of neurological disease, psychiatric disturbance, additional closed-head injuries with >5 min loss of consciousness, a head injury within the last year, learning disorder, ADHD, or a history of substance or alcohol abuse. Informed consent was obtained from all participants according to institutional guidelines at the University of New Mexico.
Our clinical protocol was identical to our previous publication (please see Mayer et al., 2010, for details), and included a full neuropsychological and symptom assessment. This included measures of effort (Test of Memory Malingering), premorbid intelligence (Wechsler Test of Adult Reading), attention, working memory, processing speed, executive function and memory (composite indices). Self-reported symptomatology was also assessed with the Neurobehavioural Symptom Checklist, the Beck Depression Inventory—Second Edition and the State-Trait Anxiety Index. A modified Rivermead scale was also administered to capture current and previous head injuries and associated symptomatology. The neuropsychological data from one healthy control were excluded secondary to potential confounds (practice effects from an unrelated experiment). Additionally, one healthy control had abnormally low scores on the Test of Memory Malingering (T-score < 0) in conjunction with normal performance on other more demanding neuropsychological measures. Therefore, this subject’s Test of Memory Malingering score was excluded from further analyses.
Quality assurance
Data from the replication sample were combined with the data from our original sample (22 patients with mild TBI; 21 healthy controls) along with an additional unmatched healthy control to create a total sample of 51 mild TBI and 51 healthy controls. As head motion also introduces bias among DTI scalar measurements (Storey et al., 2007; Ling et al., 2012), quality assurance measures were also performed on all subjects (original and replication samples). One patient with mild TBI and one healthy control from the replication sample were identified as motion outliers [3 standard deviations (SD) greater than their cohorts] and were therefore eliminated from all (replication and the pooled) DTI analyses. There were no differences in head motion between mild TBI and healthy control groups in the replication (all P > 0.10) or original cohort (Ling et al., 2012). For the complete quality assurance protocol and results, see Supplementary material.
The current protocol did not include phantom data collection. Therefore, the number of days between each subject’s first and second scans in the study was used as a covariate in all DTI analyses, comparing original and replication cohorts to control for any potential changes in scanner drift.
Image acquisition and statistical analyses
Image acquisition was also identical to the previous protocol (Mayer et al., 2010). High-resolution T1-weighted anatomic images were acquired with a 5-echo multi-echo MPRAGE sequence (voxel size = 1.00 × 1.00 × 1.00 mm). T2-weighted images were collected with a fast spin echo sequence (voxel size = 1.15 × 1.15 × 1.5 mm). Two DTI scans (b = 800 s/mm2; voxel size = 2 × 2 × 2 mm) were acquired using a twice-refocused spin echo sequence with 30 diffusion gradients and the b = 0 experiment repeated five times.
DTI image analyses were performed using a mixture of AFNI and FSL software, and analyses were identical to our previous publication with the following best-practice updates: (i) the two DTI image data sets were concatenated prior to estimating the tensor; (ii) images were co-registered using an alignment of 12 rather than six degrees of freedom and (iii) the rotational effects of this alignment were applied to the gradient table. Both the original and replication samples were analysed with this new pipeline for the pooled data. Region of interest analyses were performed for the genu, splenium and body of the corpus callosum, as well as the superior longitudinal fasciculus, the anterior and posterior corona radiata, the superior corona radiata, the uncinate fasciculus and the anterior and posterior limbs of the internal capsule for both the right and left hemispheres based on the intersection of the Johns Hopkins University (JHU) white matter labels atlas from FSL (Mori and van Zijl, 2007) and each participant’s white matter tracts (i.e. a T1 image-segmented white matter mask). Multivariate tests were selected for region of interest analyses as a result of known correlations both within and across hemispheric white matter tracts.
In addition to region of interest analyses, two different voxel-wise analyses were performed. In the first analysis, each subject’s fractional anisotropy data were non-linearly aligned (using FNIRT from the FSL package) to a whole-brain fractional anisotropy template (FMRIB58_FA) and then blurred with a 6-mm full-width at half-maximum kernel prior to group analyses. To reduce the number of comparisons and restrict the analysis to white matter, all data were masked by the group fractional anisotropy mask (values ≥ 0.25). For the second voxel-wise analyses (TBSS), each subject’s fractional anisotropy data were aligned to a skeleton and all group-wise comparisons were also restricted to the skeleton (Smith et al., 2006). For both voxel-wise analyses, a permutation-based method (FSL Randomize) was used for group comparisons in conjunction with Threshold-Free Cluster Enhancement to correct for family-wise error (P < 0.05).
Finally, we also conducted a cluster analysis to capture the overall burden of white matter diffusion abnormalities, separately summing clusters of abnormally high and low anisotropy, regardless of specific location (White et al., 2009). Specifically, the mean and SD of fractional anisotropy was first calculated for each voxel from the spatially normalized (whole-brain fractional anisotropy template) pooled sample of matched healthy controls (n = 50). Next, individual subject data (both mild TBI and matched healthy controls) were transformed to signed z-scores on a voxel-wise basis using the calculated means and standard deviations from the overall healthy control sample. Individual subject z-score maps were then thresholded based on 2 SD above (z ≥ 2) or below (z ≤ −2) the healthy control mean, and masked by both the JHU atlas and the subject’s fractional anisotropy (fractional anisotropy ≥ 0.25). To further reduce false positives, contiguous voxels were required to form a cluster with a minimum of 16 native voxels (0.128 ml).
To derive an index score of diffusion abnormalities similar to lesion load, the total number of abnormal clusters and the associated total voxel count (total volume across all clusters) was then calculated by summing across all white matter tracts separately for abnormally high or low diffusion criteria. The square root of both values was then derived to more closely approximate a normal distribution.
Results
Original versus replication cohorts
There were no significant differences between healthy controls from the original and replication samples (Supplementary Material). The patient replication sample was evaluated at more days post-injury for both imaging [3.62 days difference; t(1,48) = 2.55, P = 0.014] and clinical [3.81 days difference; t(1,47) = 2.81, P = 0.007] exams, but was otherwise similar.
Replication sample results
Neuropsychological and clinical measures
Summary results (major demographics and domain scores) are presented in Table 1. There were no differences (P > 0.10) between patients with mild TBI and helathy controls on major demographic variables, indicating that the matching procedure was effective. A total of 19/28 healthy controls and 21/28 patients with mild TBI completed the modified Rivermead questionnaire, with a higher proportion (χ2 = 4.61, P = 0.032) of patients with mild TBI (10/21) reporting previous mild injury/injuries relative to the healthy controls (3/19). Healthy controls performed worse than patients with mild TBI on a measure of effort [Test of memory Malingering; t(1,32.36) = −2.09, P = 0.045], although performance across both groups was in the average range or above (T-score = 47.5 for healthy controls). Independent samples t-test indicated that patients had significantly lower estimates of premorbid intelligence Wechsler Test of Adult Reading than controls [t(1,54) = 3.02, P = 0.004]. Given the known relationship between intelligence and other neuropsychological measures as well as diffusion metrics (Grieve et al., 2007), Wechsler Test of Adult Reading was used as a covariate in all subsequent analyses (replication and pooled).
Table 1.
Neuropsychological and clinical summary measures
| Replication |
Pooled | ||
|---|---|---|---|
| Mild TBI | Controls | ||
| Mean (SD) | Mean (SD) | Cohen’s d | |
| Demographic | |||
| Age | 28.2 (10.6) | 27.4 (10.2) | 0.051 |
| Education | 13.1 (2) | 13.9 (1.6) | −0.362 |
| HQ | 81.2 (39.7) | 79.2 (44.7) | −0.029 |
| Neuropsychology | |||
| Attentiona | 52.8 (4.38) | 53.61 (6.13) | −0.234 |
| Memorya | 52.2 (6.96) | 51.96 (7.34) | −0.189 |
| Working memorya | 52.13 (6.22) | 53.4 (5.47) | −0.048 |
| Processing speeda | 43.37 (5.47) | 46.74 (7.07) | −0.322 |
| Executive functiona | 50.41 (5.55) | 49.4 (4.37) | −0.055 |
| WTAR | 50.2 (8.6) | 56.4 (6.5) | −0.690 |
| TOMM | 54.9 (4.3) | 47.5 (17.1) | 0.429 |
| Self-report | |||
| Emotional | 47.8 (8.5) | 42.8 (6.2) | 0.787 |
| NBSI-Som | 8.6 (7.9) | 1.6 (2.5) | 1.226 |
| NBSI-Cog | 4.5 (3.9) | 1.5 (2.5) | 0.975 |
| Days post-injury | |||
| Imaging | 15.6 (4.3) | N/A | N/A |
| Neuropsychology | 15.6 (4.2) | N/A | N/A |
a Means, standard deviations and effect sizes for neuropsychological indices reported following correction for Wechsler Test of Adult Reading as covariate.
HQ = handedness quotient; N/A = not applicable; NBSI-Cog = Neurobehavioural Symptom Inventory cognitive complaints (Som = somatic complaints); TOMM = Test of Memory Malingering; WTAR = Wechsler Test of Adult Reading.
The multivariate effect of group was not significant for the multivariate analysis of covariance (MANCOVA) analyses of cognitive composites (P > 0.10), although the univariate effect for processing speed showed a trend for slowed processing speed in the mild TBI group [F(1,53) = 3.33, P = 0.074]. In contrast, a significant multivariate effect of group [F(3,52) = 6.50, P = 0.001] was observed during a MANCOVA examining self-report measures (Table 1), with univariate effects suggesting significantly greater complaints for patients with mild TBI in the domains of emotional [F(1,54) = 6.36, P = 0.015], cognitive [F(1,54) = 12.02, P = 0.001] and somatic [F(1,54) = 20.15, P = 0.000] symptoms. Supplementary Table 2 provides a complete listing of neuropsychological data for the replication sample.
Structural imaging data
Eighteen of 28 patients with mild TBI had a CT scan at the time of their emergency room visit, but none of the CT scans were deemed to contain trauma-related pathology by a non-blinded neuroradiologist. In addition, T1- and T2-weighted MRI images were reviewed by a neuroradiologist blinded to patient diagnosis. Three patients (negative CT scan) had a finding that may have been secondary to trauma. Therefore, there were a total of 3/28 patients with visible lesions using conventional neuroimaging techniques.
Diffusion tensor imaging scalar analyses
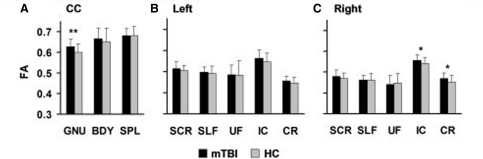
Three MANCOVAs were performed to examine group differences in fractional anisotropy within five left and right hemisphere tracts and three divisions of the corpus callosum (Fig. 1). Significant univariate results are also reported for comparison purposes with our original sample. In the replication sample, the multivariate effect of group was not significant for the corpus callosum or the right or left hemisphere white matter tracts (P > 0.10). Univariate results indicated a significant effect of group within the genu [F(1,53) = 5.28, P = 0.026, Cohen’s d = 0.67] of the corpus callosum, whereas non-significant trends were observed for the right corona radiata (F(1,53) = 3.88, P = 0.054; d = 0.57) and right internal capsule [F(1,53) = 3.87, P = 0.054; d = 0.57]. For all structures, examination of the means indicated that fractional anisotropy was elevated for the mild TBI group, with follow-up tests indicating significantly decreased radial diffusivity (RD) for subjects with mild TBI within the genu [F(1,53) = 4.60, P = 0.037, d = −0.38] with a non-significant trend in the right internal capsule [F(1,53) = 3.52, P = 0.066, d = −0.32]. There were no differences in these tracts for axial diffusivity (P > 0.10).
Figure 1.
Fractional anisotropy (FA) values from all regions of interest. This figure presents the mean FA values from the replication cohort during their first visit. Fractional anisotropy values are corrected for differences in premorbid intelligence, patients with mild TBI (mTBI) represented by black bars and healthy controls (HC) by grey bars. Error bars represent the standard deviation of the sample. (A) Regions of interest include (A) the genu (GNU), body (BDY) and splenium (SPL) of the corpus callosum (CC), as well as the superior corona radiata (SCR), the superior longitudinal fasciculus (SLF), the uncinate fasciculus (UF), the corona radiata (CR), and the internal capsule (IC) from the left (B) and right (C) hemispheres. Significant effects are denoted with double asterisks, statistical trends with a single asterisk.
Similar to our previous supplementary analyses (Mayer et al., 2010), there were no significant group differences in fractional anisotropy based on either of the two voxel-wise analyses (TBSS or voxel-wise white matter comparison) following recommended correction for family wise error.
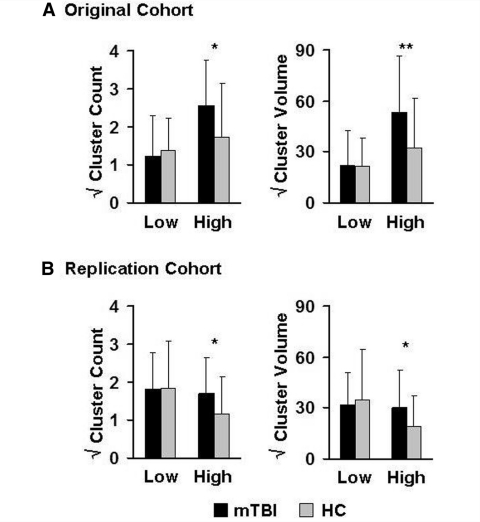
The total number of clusters with high (z ≥ 2) or low (z ≤ −2) anisotropic diffusion and associated total cluster volume were also directly compared across patients with mild TBI and controls with ANCOVAs. Results indicated non-significant trends for both an increased number of clusters with high anisotropy [F(1,53) = 3.87, P = 0.055, d = 0.58] as well as total cluster volume [F(1,53) = 3.64, P = 0.062, d = 0.56] for patients with mild TBI in the replication sample relative to controls (Fig. 2B). In contrast, there were no significant differences between the groups on either cluster measure corresponding to low anisotropy (P > 0.10). An identical series of analyses were also conducted with the original cohort data (Fig. 2A), with results showing similar effects in terms of both increased number of clusters with high anisotropy [F(1,40) = 4.06, P = 0.051, d = 0.64] as well as total cluster volume [F(1,40) = 4.68, P = 0.037, d = 0.69] and non-significant (P > 0.10) results for clusters with low anisotropy.
Figure 2.
Cluster analysis. This figure shows the mean square root of the number of clusters and their associated volumes for which individual subject z-scores exhibited low (z ≤ −2 SD below the pooled healthy controls mean) or high (z ≥ 2 SD above the healthy controls mean) anisotropy. All values are corrected for differences in premorbid intelligence, with mild traumatic brain injury patients (mild TBI) represented by black bars and healthy controls (HC) by grey bars (error bar = SD). (A) Results for the original cohort of patients and controls, whereas (B) represents the replication cohort. Significant effects are denoted with double asterisks, statistical trends with a single asterisk.
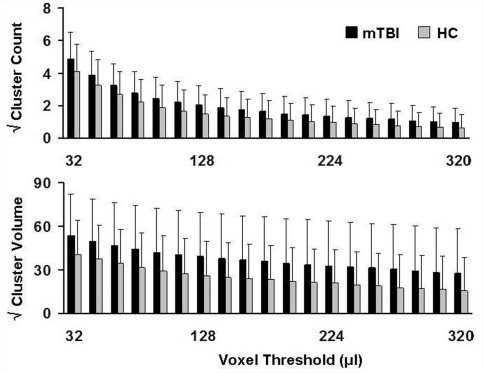
To ensure that these findings were not solely related to our minimal cluster volume (0.128 ml), the data were reanalysed using spatial volumes ranging from 0.032 to 0.320 ml in incrementing steps of 0.016 ml (Fig. 3). However, results indicated direction and magnitude of increased anisotropic diffusion were generally consistent regardless of the minimal cluster volume threshold. A full range of spatial volumes is presented in Supplementary Fig. 1.
Figure 3.
Cluster results as a function of voxel threshold. The y-axis represents the square root of the mean number (top) and mean volume (bottom panel) for clusters exhibiting high anisotropy (z ≥ 2.0) in patients with mild traumatic brain injury (mild TBI; black bars) and healthy controls (HC; grey bars). The x-axis represents different minimal cluster volume thresholds, ranging between 32 and 320 µl. Error bars represent the standard deviation. A full range of voxel thresholds (16–1008 µl) are shown in Supplementary Fig. 1.
Pooled sample results
Region of interest analyses
Pooled analyses (ANCOVAs) were restricted to summary results from the cluster analyses (cluster number/total cluster volume with high anisotropic diffusion) or region of interest that showed significant effects or trends in either the original (splenium and left superior corona radiata, uncinate fasciculus, internal capsule and corona radiata), replication (right internal capsule and corona radiata) or both original and replication cohorts (genu of corpus callosum). Significantly higher fractional anisotropy in the genu [F(1,97) = 12.02, P = 0.001, Cohen’s d = 0.74] and left corona radiata [F(1,97) = 4.80, P = 0.031, d = 0.47] was observed for patients with mild TBI relative to the healthy control group, with non-significant trends in the left superior corona radiata [F(1,97) = 3.16, P = 0.079, d = 0.38] and right corona radiata [F(1,97) = 2.92, P = 0.091, d = 0.36]. Additionally, the number of clusters exhibiting high anisotropy [F(1,97) = 6.41, P = 0.013, d = 0.54] as well as total cluster volume [F(1,97) = 6.63, P = 0.012, d = 0.55] was also significant for pooled sample results.
Effects of clinical characteristics
Several cross-sectional analyses were conducted to examine the effects of recovery (days post-injury), injury severity (based on American Academy of Neurology criteria) or type (motor vehicle accident versus falls/assaults/struck by object), and self-reported post-concussive symptoms for the cluster statistics (cluster number and total cluster volume) and four regions of interest (genu, left and right corona radiata, and left superior corona radiata) that showed significant or trend differences for fractional anisotropy in the pooled sample ANCOVAs.
The first series of analyses examined the correlation between days post-injury and fractional anisotropy. Although all six variables showed the predicted negative relationship between anisotropy and days post-injury (−0.200 ≤ r ≤ −0.022), none of the findings were statistically significant (P > 0.10). Moreover, voxel-wise analyses also indicated that there was no relationship between time post-injury and fractional anisotropy (Supplementary material).
To examine effects of injury severity on fractional anisotropy, we first reclassified the subjects with mild TBI into two groups based on American Academy of Neurology classification (37 mild TBI Grade 3; 13 Grade 1 or 2). The 13 Grade 1 and 2 subjects were then matched with 13 subjects from the Grade 3 group on age, gender, and education and compared. However, there were no significant differences (P > 0.10) between injury severity and measures of increased anisotropic diffusion in region of interest or cluster indices. A similar procedure was utilized to match the 13 patients with mild TBI from a motor vehicle accident to 13 mild TBI from other injury mechanisms (n = 37); however, these results were also negative (all P > 0.10).
Linear regressions were conducted to investigate potential relationships between self-reported concussive symptoms and intelligence on measures of increased anisotropic diffusion. The overall model was not significant for any of the regressions, although cognitive (t = 1.77, P = 0.084) and somatic (t = −1.88, P = 0.066) complaints were weakly associated with fractional anisotropy in the genu.
Finally, we attempted to objectively classify patients and controls using metrics of anisotropic diffusion through binary logistic regression. In the first model, estimates of premorbid intelligence were entered first followed by fractional anisotropy from the genu, bilateral corona radiata and left superior corona radiata. Results indicated that the estimate of premorbid intelligence (Wald = 9.86, P = 0.002) was significantly able to discriminate between healthy controls (66% accuracy) and patients with mild TBI (64%). The inclusion of fractional anisotropy from regions of interest significantly improved classification accuracy across both groups, although the increase in accuracy was marginal (healthy controls = 70%; mild TBI = 72%) and only significant for the genu (Wald = 7.35, P = 0.007). The second model utilized a similar hierarchical structure (premorbid intelligence entered first) with the cluster statistics (positive cluster number and total cluster volume) entered as a second step. However, neither metric significantly improved prediction of patient/control status (Wald P > 0.10).
Longitudinal comparisons
Twenty-six patients with mild TBI from the pooled sample returned for their longitudinal visit. Returning patients were more educated [t(1,48) = −2.40, P = 0.020] and performed better on the memory composite index [t(1,47) = −2.40, P = 0.020] relative to the non-returning cohort. There were no other significant differences in key demographic variables, clinical variables, cluster statistics or fractional anisotropy of the genu for returning and non-returning patients (P > 0.10). Twenty-six returning healthy controls (total returning controls = 42) were selected on gender, age and education to best match with returning patients, with no significant differences in key demographic variables across the groups.
Longitudinal analyses were first conducted to examine changes in self-reported symptomatology as a function of recovery. The main effects of group and time were significant across all composite indices (all P < 0.05). The Group × Time interaction was also significant for cognitive [F(1,49) = 7.84, P = 0.007], somatic [F(1,49) = 6.64, P = 0.013] and emotional [F(1,49) = 5.19, P = 0.027] complaints, with simple effects testing suggested that the level of post-concussive complaints decreased as a function of time (Visit 1 to Visit 2) within the mild TBI subjects (all P < 0.05) but not healthy controls (all P > 0.10) group.
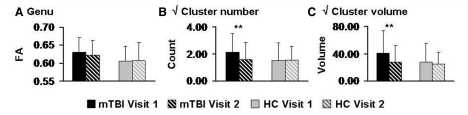
Three 2 × 2 (Group × Time) mixed measures ANCOVAs were then performed to examine how the number of clusters with high anisotropy, total cluster volume and fractional anisotropy within the genu changed as a function of recovery (Fig. 4). Longitudinal analyses were restricted to these metrics as they exhibited reliable differences across both the original and replication cohorts. Results indicated that the main effects of group and time were not significant for any of the analyses (P > 0.10). A trend for the Group × Time interaction [F(1,49) = 3.68, P = 0.061] was observed for the total number of positive clusters. Simple effect testing indicated that whereas healthy control subjects exhibited no change in the total number of clusters with high anisotropy (P > 0.10), clusters with high anisotropy were significantly reduced at Visit 2 for the patients with mild TBI [t(1,25) = 2.40, P = 0.024]. Even though the omnibus interaction term was not significant, the total cluster volume also decreased in Visit 2 for patients with mild TBI [t(1,25) = 3.07, P = 0.005] but not controls (P > 0.10).
Figure 4.
Longitudinal measures of high anisotropy. This figure presents longitudinal changes in anisotropy measures for patients with mild traumatic brain injury (mild TBI; black bars) and healthy controls (HC; grey bars). Solid bars indicate data from Visit 1 whereas bars with diagonals show Visit 2 data, with all error bars equivalent to the standard deviation. (A) Mean fractional anisotropy (FA) of the genu of the corpus callosum, whereas the number of clusters with high anisotropic diffusion (z ≥ 2.0) and their respective volumes are presented in (B) and (C), respectively. Significant effects are denoted with double asterisks.
Finally, a multiple regression was conducted to determine whether changes (Visit 1 to Visit 2) in the number of clusters with increased anisotropy was associated with self-reported cognitive, somatic or emotional symptomatology for the subjects with mild TBI. Although the overall model was not significant (P > 0.10), a reduction in severity of somatic complaints significantly predicted a reduction in the number of clusters with increased anisotropy (t = 2.33, P = 0.029).
Exploratory analyses
A primary aim of the current study was to replicate our previous findings of increased fractional anisotropy in a limited selection of regions of interest. However, several other white matter tracts have also been implicated in studies of both semi-acute and chronic mild TBI. Therefore, exploratory analyses were also conducted to compare fractional anisotropy from all 48 regions of interest in the JHU atlas across the patients and controls in the pooled sample. Results indicated significantly higher fractional anisotropy in the mild TBI group for 7/48 white matter tracts (uncorrected P < 0.05), with an additional 4/48 tracts showing a non-significant trend (uncorrected P < 0.10). Fractional anisotropy was increased for mild TBI subjects in varying degrees of magnitude for 32/37 remaining tracts, resulting in a significant signed test (P = 0.001). Sample means, standard deviations, effect sizes, uncorrected P-values and number of patients/controls with significant clusters of high anisotropy are presented for all 48 regions of interest (Supplementary material).
Discussion
A clear picture of the diffusion abnormalities associated with mild TBI has not yet emerged from human neuroimaging studies (Niogi and Mukherjee, 2010), perhaps because of different sample characteristics and the implicit assumption that white matter injuries will occur in the same location across patients whose injuries reflect diverse mechanical deformations. As noted above, previous studies utilizing region of interest techniques have reported both reduced fractional anisotropy in the internal capsule and splenium (Huisman et al., 2004), centrum semiovale, corpus callosum and internal capsule (Miles et al., 2008), as well as increased fractional anisotropy in the corpus callosum (Bazarian et al., 2007; Mayer et al., 2010; Henry et al., 2011), left superior corona radiata, left corona radiata and left uncinate fasciculus (Mayer et al., 2010) during the semi-acute stage of mild TBI. Similarly, findings have also been mixed for studies utilizing voxel-based techniques (Mayer et al., 2010; Cubon et al., 2011; Messe et al., 2011; Smits et al., 2011). Therefore, a principal goal of the current study was to replicate our previous finding of increased anisotropic diffusion (fractional anisotropy) and reduced radial diffusion (Mayer et al., 2010) in an independent cohort of patients using nearly identical clinical and experimental procedures.
Results from our replication cohort confirmed a general pattern of increased fractional anisotropy and reduced radial diffusivity during the semi-acute phase of mild TBI; however, the spatial location of these diffusion abnormalities varied across cohorts. Specifically, several left hemisphere tracts exhibited increased diffusion anisotropy in the original but not replication cohort, whereas two right hemisphere tracts exhibited trends for increased diffusion in the replication but not original cohort. Only the genu of the corpus callosum exhibited a significant effect across both cohorts, suggesting that this structure may serve as a common point of injury regardless of initial biomechanical factors. Approximately 80% of fibres in the corpus callosum are unmyelinated, rendering them more vulnerable to traumatic axonal injury (Reeves et al., 2005), which likely contributes to the frequent occurrence of histopathological abnormalities in this structure following TBI (Gentry et al., 1988). In addition, modelling studies indicate that the inter-hemispheric fibres of the corpus callosum may be more susceptible to mechanical strain as a result of brain deformation compared to long intra-hemispheric fibre bundles (Viano et al., 2005). Finally, individual subject-based finite element modelling has recently been used to show that mean and maximum strain rates directly correlate with changes in anisotropic diffusion in the corpus callosum of 10 recently concussed athletes (McAllister et al., 2012).
In contrast to our region of interest analyses, two separate voxel-wise comparisons (TBSS and a voxel-based white matter analysis) did not reveal any group differences in anisotropic diffusion following recommended corrections for false positives. Null findings in voxel-wise comparisons have been previously reported (Levin et al., 2010; Mayer et al., 2010; Zhang et al., 2010; Cubon et al., 2011; Messe et al., 2011), although others have reported significant group differences by using alternative thresholding strategies (Henry et al., 2011; Smits et al., 2011). These findings suggest that the putative combination of heterogeneity in injury location and strict corrections for reducing false positives may limit the utility of voxel-based approaches in mild TBI research. In addition, known differences in individual white matter morphology must be corrected through spatial smoothing and complex registration algorithms, both of which can introduce variability in the data (Jones et al., 2005; Van Hecke et al., 2009; Moraschi et al., 2010). TBSS (Smith et al., 2006) attempts to mitigate these two issues through registration to a tract-based skeleton, but in doing so limits the number of voxels examined to the core of white tracts. This may be particularly detrimental for detecting abnormalities in grey-white matter boundaries, where pathology is frequently secondary to differences in tissue rigidity (Ducreux et al., 2005; Le et al., 2005).
From a methodological perspective, region of interest analyses somewhat relax the assumption of spatial homogeneity of injury by smoothing (i.e. averaging) over a priori defined white matter tracts. However, the manual selection of regions of interest can affect the reliability and reproducibility of results as well as be quite time-consuming. Our region of interest methodology mitigates the first two concerns by utilizing an automatic atlas-driven approach and reduces partial-voluming effects (intersection with individual subject’s white matter mask). A larger concern is that region of interest analyses may reduce the sensitivity for detecting the relatively sporadic lesions that exist even within tracts exposed to similar deformation forces (Browne et al., 2011; Greer et al., 2011).
To overcome the respective limitations of both region of interest and voxel-based methods, an approach for identifying diffuse traumatic axonal injuries on a voxel-wise basis was developed (White et al., 2009). Specifically, we defined individual clusters of both increased and decreased diffusion anisotropy based on our healthy control data to more accurately model a prediction of spatially heterogeneous diffuse lesions. Results indicated trends for a larger number of clusters with increased diffusion and greater total volume of these clusters for patients with mild TBI across both the original and replication cohorts. These findings are similar to a co-occurring publication demonstrating spatially heterogeneous white matter injuries following blast injury (Davenport et al., 2012). However, the Davenport and colleagues report regions of reduced anisotropic diffusion, potentially reflecting a relatively more chronic injury. Exploratory analyses confirmed that 42 out of the 48 regions of interest showed increased anisotropy for patients with mild TBI relative to controls to some degree, with six tracts exhibiting a moderate or greater effect size (Cohen’s d ≥ 0.40). Thus, current results provide evidence of diffusion abnormalities in several other tracts that may be targeted for future studies.
As previously discussed by our group and others (Bazarian et al., 2007; Wilde et al., 2008; Mayer et al., 2010), increased fractional anisotropy in white matter may be reflective of changes in the concentrations of intra and extracellular water (cytotoxic oedema). Specifically, the mechanical forces of mild TBI result in the stretching of axons and related supporting structures such as oligodendrocytes (Povlishock and Katz, 2005; Browne et al., 2011), altering the function of gated ion channels, and resulting in increased intracellular and decreased extracellular water (Rosenblum, 2007). The decrease in extracellular water may lead to a decrease in diffusivity perpendicular to the axon (radial diffusivity), secondary to more tightly compacted axons and the resultant changes in tortuosity of water (Sotak, 2002; Rosenblum, 2007). Other potential physiological bases for increased fractional anisotropy include changes in myelin structure. However, myelin plays a relatively minor role in anisotropic diffusion in comparison with axonal membranes (Beaulieu, 2002) and animal models of concussion more consistently indicate axonal rather than myelin pathology (Spain et al., 2010).
Human diffusion studies (i.e. DTI) are also influenced by a variety of other non-trauma-related factors including head motion (Ling et al., 2012), individual differences such as intelligence level (Grieve et al., 2007), smoking history (Gons et al., 2011) and emotional status (Korgaonkar et al., 2011), all factors that likely account for the variability in anisotropic diffusion that is observed within both patient and control groups in many studies (Ling et al., 2012). The contributions of some of these factors can partially be mitigated through careful selection of appropriate control populations through careful case-control. For example, in the current study all controls were directly matched on gender, and 47/51 controls were matched to within 2 years (age and education) of their respective patients. As discussed in the ‘Introduction’ section, sample characteristics also likely contribute to the differences in findings that are observed across different studies of mild TBI (Niogi and Mukherjee, 2010). Patients with positive CT scans (complicated mild TBI) tend to experience more neurobehavioural symptoms and poorer prognoses relative to non-complicated patients (Iverson, 2006; Lange et al., 2009). Future studies are needed to determine whether poor outcome is related to the presence of a CT detectable lesion or may result from more diffuse injuries such as those reported in the current study.
DTI also measures underlying pathology on a relatively macroscopic level (i.e. standard voxel volume of 0.008 ml) and there are several morphological changes, metabolic processes and inflammatory responses that occur following TBI on a cellular level (Albensi et al., 2000; Van Putten et al., 2005; Barkhoudarian et al., 2011). Therefore, it is a considerable challenge to directly relate macroscopic measures of diffusion (DTI) with cell histology. The current findings of increased anisotropic diffusivity appear to be inconsistent with animal models of TBI, which have consistently indicated reduced anisotropic white matter water diffusion in the acute and semi-acute injury stages (Mac Donald et al., 2007a, b; Budde et al., 2011; van de Looij et al., 2012). Although other animal studies of blast (Rubovitch et al., 2011) and cortical impact injuries (Budde et al., 2011; Xu et al., 2011) indicate ‘increased’ fractional anisotropy, these changes are more prevalent in grey rather than white matter regions. It is important to note that animal injury models frequently induce cortical contusions or other alterations (e.g. enlarged ventricles) of sufficient severity that they are visible with magnetic resonance, and these lesions are rarely observed in human mild TBI (Hughes et al., 2004). This suggests the need for animal models that more closely parallel the types of injuries typically observed in human mild TBI (Rubovitch et al., 2011).
For example, a recent study carefully attempted to replicate the mechanical forces necessary to result in axonal deformations that are typically present in human mild TBI in a swine model (Browne et al., 2011). Although DTI metrics were not obtained, histopathological results indicated widespread axonal swelling secondary to a disruption in axonal transport following mild TBI. It has been demonstrated that fast axonal transport and associated microtubule structure have little influence on anisotropic diffusion after pharmacological lesions (Beaulieu and Allen, 1994); however, the pathology of mechanical trauma is different from pharmacological injury. Seminal studies of Weiss and Hiscoe (1948) suggest that axonal constriction results in disrupted slow axonal transport, with accumulating neurofilament proteins and axoplasm ultimately resulting in cell distension upstream of the injury site (cited in Koehnle and Brown, 1999; Miller and Heidemann, 2008). Of note, cell distension should theoretically result in increased radial diffusivity (and thus reduced fractional anisotropy) ‘within a single axon’. It is not clear how axonal swelling affects macroscopic diffusion measurements that may be influenced by partial compression of adjacent axons and the reduction of extracellular fluid. Clearly, more research is needed to understand disruptions in slow axonal transport and how it relates to the macroscopic measures of diffusion used in human studies. This may be especially relevant given the emerging literature on the role of disrupted slow axonal transport in neurodegenerative disorders and tauopathies (Roy et al., 2005), with equivalent findings of tauopathies in human histopathological studies of repetitive mild TBI (McKee et al., 2009).
A critical goal of all biomarker research is to provide an objective, sensitive and specific measure that is associated with symptomatology following injury. Similar to our previous finding (Mayer et al., 2010), we were able to objectively classify mild TBI patients from controls based on measures of anisotropic diffusion from the genu of the corpus callosum in our pooled sample. However, the sensitivity and specificity of classification were relatively modest (healthy controls = 70%; mild TBI = 72%), likely a result of the considerable variability that was present across both patient and control groups. Finally, the relationship between our metrics of increased anisotropic diffusion (both cluster metrics and region of interest) and clinical measures of injury severity (based on American Academy of Neurology), injury type and post-concussive symptomatology were relatively weak. Collectively, current and previous (Niogi and Mukherjee, 2010) results suggest that although diffusion abnormalities may provide unique objective information about mild TBI that is not available through other standard assessment techniques, by itself it may only provide a weak biomarker of injury.
The second major goal of the current study was to examine how diffusion abnormalities changed as patients transitioned from the semi-acute to more chronic injury stages. Previous cross-sectional studies examining the evolution of anisotropic diffusion across multi-year periods have not reported significant differences between semi-acute and patients with chronic mild TBI (Inglese et al., 2005). Similarly, our moderately sized cohort of 50 patients did not exhibit any evidence of changes in increased anisotropic diffusion during the semi-acute injury phase (2–21-day post-injury). The lack of correlation with days post-injury suggests that white matter abnormalities may not evolve as quickly as alterations in neurometabolic functioning (Vagnozzi et al., 2010; Yeo et al., 2011), and hence, may represent part of the neuroanatomical substrate for the repetitive injuries.
The current study also incorporated a more powerful longitudinal design to examine the recovery of diffusion abnormalities. In our initial study we showed evidence for a partial normalization of fractional anisotropy within the splenium and corona radiata for the mild TBI group across a 4–5-month interval. Other studies have reported a continued reduction in anisotropic values during longitudinal studies in more severely injured (Bendlin et al., 2008; Kumar et al., 2009) and mild TBI (Arfanakis et al., 2002; Mac Donald et al., 2011) populations. The current study provided mixed evidence of recovery in a cohort of 26 patients, with the number of clusters and total volume of voxels with increased diffusion decreasing as patients recovered from their injury. Importantly, these reductions were also associated with a reduction in self-reported post-concussive symptomatology (somatic complaints). However, there were no significant changes in fractional anisotropy values of the genu, indicating that measuring the longitudinal recovery of anisotropic diffusion may be challenging in the presence of considerable individual heterogeneity.
The current study had several limitations. First, despite the implementation of several protocols to maximize participant retention (i.e. regular telephone and mail contact; alternative contact information), a significant number of patients did not return for their follow-up visit. The primary reasons for attrition were inability to contact patients (71%), patient no-shows (21%) and inability of patient to complete second visit secondary to personal circumstances (8%). Our attrition rate is similar to previously published TBI studies (Jorge et al., 2004; Rapoport et al., 2006; Jacobs et al., 2010) and there were no differences between returning and non-returning patients on key demographic, clinical and imaging variables. However, patient attrition may have biased the sample to a more or less symptomatic population. Secondly, our anatomical imaging protocol was rather limited for classifying lesions. Future studies should include a more detailed anatomical protocol (e.g. susceptibility weighted imaging) to better characterize additional lesion types such as petechial haemorrhages that are also seen in mild TBI. Thirdly, we did not assess patients for litigation, although the rate of litigation is likely to be very low among patients with mild TBI prospectively recruited from the emergency room.
In conclusion, we independently replicated our previous finding of increased fractional anisotropy and reduced radial diffusivity during the semi-acute stage of mild TBI in the genu of the corpus callosum. The corpus callosum has been previously established as a common point of trauma, and may be more susceptible to pathology regardless of actual injury focus secondary to anatomical (high frequency of unmyelinated fibres) and biomechanical factors. In contrast, the lack of replication between our previous (Mayer et al., 2010) and our current results across several other white matter tracts argues forcefully for the heterogeneous nature of diffusion abnormalities, potentially resulting from the unique circumstances that characterize each injury. This heterogeneity can be partially overcome by using methods that minimize the assumption of spatial homogeneity of injury, resulting in a finding of increased anisotropic diffusion across both our original and replication samples.
Funding
National Institutes of Health (R24-HD050836, R21-NS064464-01A1 and 3 R21 NS064464-01S1 to A.M.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
Special thanks to Diana South and Cathy Smith for assistance with data collection.
Glossary
Abbreviations
- DTI
diffusion tensor imaging
- TBI
traumatic brain injury
- TBSS
tract-based spatial statistics
References
- Albensi BC, Knoblach SM, Chew BG, O’Reilly MP, Faden AI, Pekar JJ. Diffusion and high resolution MRI of traumatic brain injury in rats: time course and correlation with histology. Exp Neurol. 2000;162:61–72. doi: 10.1006/exnr.2000.7256. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007;24:1447–59. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, et al. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42:503–14. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc. 2008;14:1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Neuroimaging in Mild Traumatic Brain Injury. Psychol Inj and Law. 2010;2010:36–49. [Google Scholar]
- Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–6. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- Browne KD, Chen XH, Meaney DF, Smith DH. Mild traumatic brain injury and diffuse axonal injury in Swine. J Neurotrauma. 2011;28:1747–55. doi: 10.1089/neu.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–60. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28:189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, Armstrong MT, Sponheim SR. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage. 2012;59:2017–24. doi: 10.1016/j.neuroimage.2011.10.050. [DOI] [PubMed] [Google Scholar]
- Dikmen S, Machamer J, Temkin N. Mild head injury: facts and artifacts. J Clin Exp Neuropsychol. 2001;23:729–38. doi: 10.1076/jcen.23.6.729.1019. [DOI] [PubMed] [Google Scholar]
- Dikranian K, Cohen R, Mac DC, Pan Y, Brakefield D, Bayly P, et al. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp Neurol. 2008;211:551–60. doi: 10.1016/j.expneurol.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducreux D, Huynh I, Fillard P, Renoux J, Petit-Lacour MC, Marsot-Dupuch K, et al. Brain MR diffusion tensor imaging and fibre tracking to differentiate between two diffuse axonal injuries. Neuroradiology. 2005;47:604–8. doi: 10.1007/s00234-005-1389-1. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention; 2010. [Google Scholar]
- Gentry LR, Thompson B, Godersky JC. Trauma to the corpus callosum: MR features. AJNR Am J Neuroradiol. 1988;9:1129–38. [PMC free article] [PubMed] [Google Scholar]
- Gons RA, van Norden AG, de Laat KF, van Oudheusden LJ, van Uden IW, Zwiers MP, et al. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain. 2011;134:2116–24. doi: 10.1093/brain/awr145. [DOI] [PubMed] [Google Scholar]
- Greer JE, McGinn MJ, Povlishock JT. Diffuse traumatic axonal injury in the mouse induces atrophy, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. J Neurosci. 2011;31:5089–105. doi: 10.1523/JNEUROSCI.5103-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol. 2007;28:226–35. [PMC free article] [PubMed] [Google Scholar]
- Henry LC, Tremblay J, Tremblay S, Lee A, Brun C, Lepore N, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28:2049–59. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–63. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hughes DG, Jackson A, Mason DL, Berry E, Hollis S, Yates DW. Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology. 2004;46:550–8. doi: 10.1007/s00234-004-1227-x. [DOI] [PubMed] [Google Scholar]
- Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25:370–6. [PMC free article] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Iverson GL. Complicated vs uncomplicated mild traumatic brain injury: acute neuropsychological outcome. Brain Inj. 2006;20:1335–44. doi: 10.1080/02699050601082156. [DOI] [PubMed] [Google Scholar]
- Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry. 2005;18:301–17. doi: 10.1097/01.yco.0000165601.29047.ae. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Beems T, Stulemeijer M, van Vugt AB, van der Vliet TM, Borm GF, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma. 2010;27:655–68. doi: 10.1089/neu.2009.1059. [DOI] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–54. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61:42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- Koehnle TJ, Brown A. Slow axonal transport of neurofilament protein in cultured neurons. J Cell Biol. 1999;144:447–58. doi: 10.1083/jcb.144.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Grieve SM, Koslow SH, Gabrieli JD, Gordon E, Williams LM. Loss of white matter integrity in major depressive disorder: Evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum Brain Mapp. 2011;32:2161–71. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma. 2009;26:481–95. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- Lange RT, Iverson GL, Franzen MD. Neuropsychological functioning following complicated vs. uncomplicated mild traumatic brain injury. Brain Inj. 2009;23:83–91. doi: 10.1080/02699050802635281. [DOI] [PubMed] [Google Scholar]
- Le TH, Mukherjee P, Henry RG, Berman JI, Ware M, Manley GT. Diffusion tensor imaging with three-dimensional fibre tractography of traumatic axonal shearing injury: an imaging correlate for the posterior callosal “disconnection” syndrome: case report. Neurosurgery. 2005;56:189. [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma. 2010;27:683–94. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Ling J, Merideth F, Caprihan A, Pena A, Teshiba T, Mayer AR. Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Hum Brain Mapp. 2012;33:50–62. doi: 10.1002/hbm.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007a;27:11869–76. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007b;205:116–31. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364:2091–100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–50. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32:1825–35. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Ford JC, Ji S, Beckwith JG, Flashman LA, Paulsen K, et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann Biomed Eng. 2012;40:127–40. doi: 10.1007/s10439-011-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–35. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messe A, Caplain S, Paradot G, Garrigue D, Mineo JF, Soto AG, et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Mapp. 2011;32:999–1011. doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22:115–22. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- Miller KE, Heidemann SR. What is slow axonal transport? Exp Cell Res. 2008;314:1981–90. doi: 10.1016/j.yexcr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Moraschi M, Hagberg GE, Di PM, Spalletta G, Maraviglia B, Giove F. Smoothing that does not blur: effects of the anisotropic approach for evaluating diffusion tensor imaging data in the clinic. J Magn Reson Imaging. 2010;31:690–7. doi: 10.1002/jmri.22040. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Human white matter atlas. Am J Psychiatry. 2007;164:1005. doi: 10.1176/ajp.2007.164.7.1005. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil. 2010;25:241–55. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- Peled S. New perspectives on the sources of white matter DTI signal. IEEE Trans Med Imaging. 2007;26:1448–55. doi: 10.1109/TMI.2007.906787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, Herrmann N, Shammi P, Kiss A, Phillips A, Feinstein A. Outcome after traumatic brain injury sustained in older adulthood: a one-year longitudinal study. Am J Geriatr Psychiatry. 2006;14:456–65. doi: 10.1097/01.JGP.0000199339.79689.8a. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol. 2005;196:126–37. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI. Cytotoxic edema: monitoring its magnitude and contribution to brain swelling. J Neuropathol Exp Neurol. 2007;66:771–8. doi: 10.1097/nen.0b013e3181461965. [DOI] [PubMed] [Google Scholar]
- Roy S, Zhang B, Lee VM, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- Rubovitch V, Ten-Bosch M, Zohar O, Harrison CR, Tempel-Brami C, Stein E, et al. A mouse model of blast-induced mild traumatic brain injury. Exp Neurol. 2011;232:280–9. doi: 10.1016/j.expneurol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008;29:514–9. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smits M, Houston GC, Dippel DW, Wielopolski PA, Vernooij MW, Koudstaal PJ, et al. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2011;53:553–63. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury – a review. NMR Biomed. 2002;15:561–9. doi: 10.1002/nbm.786. [DOI] [PubMed] [Google Scholar]
- Spain A, Daumas S, Lifshitz J, Rhodes J, Andrews PJ, Horsburgh K, et al. Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. J Neurotrauma. 2010;27:1429–38. doi: 10.1089/neu.2010.1288. [DOI] [PubMed] [Google Scholar]
- Storey P, Frigo FJ, Hinks RS, Mock BJ, Collick BD, Baker N, et al. Partial k-space reconstruction in single-shot diffusion-weighted echo-planar imaging. Magn Reson Med. 2007;57:614–9. doi: 10.1002/mrm.21132. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–42. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- van de Looij Y, Mauconduit F, Beaumont M, Valable S, Farion R, Francony G, et al. Diffusion tensor imaging of diffuse axonal injury in a rat brain trauma model. NMR Biomed. 2012;25:93–103. doi: 10.1002/nbm.1721. [DOI] [PubMed] [Google Scholar]
- Van Hecke W, Sijbers J, DeBacker S, Poot D, Parizel PM, Leemans A. On the construction of a ground truth framework for evaluating voxel-based diffusion tensor MRI analysis methods. Neuroimage. 2009;46:692–707. doi: 10.1016/j.neuroimage.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Van Putten HP, Bouwhuis MG, Muizelaar JP, Lyeth BG, Berman RF. Diffusion-weighted imaging of edema following traumatic brain injury in rats: effects of secondary hypoxia. J Neurotrauma. 2005;22:857–72. doi: 10.1089/neu.2005.22.857. [DOI] [PubMed] [Google Scholar]
- Viano DC, Casson IR, Pellman EJ, Zhang L, King AI, Yang KH. Concussion in professional football: brain responses by finite element analysis: part 9. Neurosurgery. 2005;57:891–916. doi: 10.1227/01.neu.0000186950.54075.3b. [DOI] [PubMed] [Google Scholar]
- White T, Schmidt M, Karatekin C. White matter ‘potholes’ in early-onset schizophrenia: a new approach to evaluate white matter microstructure using diffusion tensor imaging. Psychiatry Res. 2009;174:110–5. doi: 10.1016/j.pscychresns.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–55. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Xu S, Zhuo J, Racz J, Shi D, Roys S, Fiskum G, et al. Early microstructural and metabolic changes following controlled cortical impact injury in rat: a magnetic resonance imaging and spectroscopy study. J Neurotrauma. 2011;28:2091–102. doi: 10.1089/neu.2010.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo RA, Gasparovic C, Merideth F, Ruhl D, Doezema D, Mayer AR. A longitudinal proton magnetic resonance spectroscopy study of mild traumatic brain injury. J Neurotrauma. 2011;28:1–11. doi: 10.1089/neu.2010.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp Brain Res. 2010;204:57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM. Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol. 2006;27:2000–4. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.