Abstract
Landmark cancer genome resequencing efforts are leading to the identification of mutated genes in many types of cancer. The extreme diversity of mutations being detected presents significant challenges to subdivide causal from coincidental mutations to elucidate how disrupted regulatory networks drive cancer processes. Given that a common early perturbation in solid tumor initiation is bypass of matrix-dependent proliferation restraints, we sought to functionally interrogate colorectal cancer candidate genes (CAN-genes) to identify driver tumor suppressors. We have employed an isogenic human colonic epithelial cell (HCEC) model to identify suppressors of anchorage-independent growth by conducting a soft agar–based short hairpin RNA (shRNA) screen within the cohort of CAN-genes. Remarkably, depletion of 65 of the 151 CAN-genes tested collaborated with ectopic expression of K-RASV12 and/or TP53 knockdown to promote anchorage-independent proliferation of HCECs. In contrast, only 5 of 362 random shRNAs (1.4%) enhanced soft agar growth. We have identified additional members of an extensive gene network specifying matrix-dependent proliferation, by constructing an interaction map of these confirmed progression suppressors with approximately 700 mutated genes that were excluded from CAN-genes, and experimentally verifying soft agar growth enhancement in response to depletion of a subset of these genes. Collectively, this study revealed a profound diversity of nodes within a fundamental tumor suppressor network that are susceptible to perturbation leading to enhanced cell-autonomous anchorage-independent proliferative fitness. Tumor suppressor network fragility as a paradigm within this and other regulatory systems perturbed in cancer could, in large part, account for the heterogeneity of somatic mutations detected in tumors.
Introduction
Normal epithelial cells require attachment to the extracellular matrix components for differentiation, proliferation, and viability. Loss of matrix anchorage results in a type of programmed cell death called anoikis that is regulated by a variety of different signaling pathways including extracellular signal regulated kinase,c-jun N-terminal kinase(JNK), andAKT(1).To become cancer cells, normal epithelial cells need to acquire the ability to grow anchorage independently, a hallmark feature of cancer and one of the most faithful in vitro indications of tumorigenicity. In contrast, how epithelial tumors acquire anchorage-independent abilities is poorly understood and therapeutic strategies exploiting this process are almost nonexisting. Advances in RNA interference techniques have enabled loss-of-function screens to identify tumor suppressors in mammalian cells. These studies have identified genes that restrain anchorage-independent growth in SV40 T antigen and human telomerase reverse transcriptase (hTERT)-immortalized partially transformed fibroblasts (2) and mammary epithelial cells (3). Lack of overlapping genes from these 2 screens and identification of different tumor suppressor profiles from in vivo lymphoma (4) and liver cancer (5) short hairpin RNA (shRNA) screens suggest that tumor suppressors are highly context dependent and emphasize the importance of loss-of-function screens, using different tissue-specific cell types.
Similar to other hallmarks of cancer, the ability to grow anchorage independently could be acquired by progressive genetic alterations and represented by some of the mutations already established to occur in tumors. Since the first published cancer genome-sequencing project (6), thousands of cancer genomes have been sequenced. One ongoing point of debate about these efforts is the cost versus benefit imbalance (7). Many point mutations, duplications, deletions, or small insertions have been reported that had not been previously associated with cancer. The functional role of the vast majority of these mutated genes in cancer initiation, progression, or maintenance is unknown. It is believed that many of these mutated genes may be incidental or passenger mutations and thus not driving oncogenic processes. Efforts to identify drivers within the cohort of mutated genes have largely been in silico (8, 9) and have not been subjected to rigorous experimental testing, using biologically relevant functional assays. To this end, we set out to identify suppressors of anchorage-independent growth within reported colorectal cancer (CRC) mutated genes (6, 10), using otherwise isogenic K-rasV12–expressing or p53 knockdown hTERT immortalized diploid human colonic epithelial cells (HCEC; ref. 11). This approach revealed a profound enrichment for driver tumor suppressors within CRC candidate cancer genes (CAN-genes).
Materials and Methods
Plasmids
CDK4 was expressed together with G418 from retroviral pSRaMSU vector. hTERT was cloned from pGRN145 to pMIN-Ub-IRES-Blast lentiviral vector coexpressing blastocidin resistance marker (both vectors were provided by Geron Corporation). pSRZ-shTP53 and pBABE-hyg-KRASV12 are described elsewhere (12). Flag-tagged MAP2K7 was in pcDNA3.1 neo backbone (a gift from L. Lum, Department of Cell Biology, Southwestern Medical School, Dallas TX). MSCV-GRD-Pac retroviral vector was kindly provided by D.W. Clapp (Indiana University School of Medicine, Indianapolis, IN) and used to express NF1 GTPase-activating protein–related domain (GRD). PTEN cDNA was obtained from mammalian gene collection and cloned into pMIN-based lentiviral vector. FBXW7 and HAPLN1 cDNAs were gifts from L. Lum and were cloned into pMIN lentiviral vector. All constructs were sequence verified.
Viral transductions
For retrovirus production, 2 μg of the appropriate vector was transfected into Phoenix A cells in 6-cm dishes with Effectene reagent (Qiagen). For lentivirus production, 1 μg of the appropriate vector together with 1 μg of helper plasmids (0.4 μg pMD2G and 0.6 μg psPAX2) were transfected into 293FT cells with Effectene reagent (Qiagen). Viral supernatants were collected 48 hours after transfections and cleared through 0.45-μm filter. Cells were infected with viral supernatants containing 4 μg/mL poybrene (Sigma) and selected with appropriate antibiotics.
Cells
HCEC growth media and tissue culture conditions are described elsewhere (11). HCECs isolated from normal colonic biopsies were immortalized by successive infections of CDK4 and hTERT followed by selection with respective antibiotics—G418 (250 μg/mL) and blastocidin (2.5 μg/mL). KRASV12 and shRNA against p53 were introduced with retroviruses, and oncogenic KRAS expression was verified as described (12, 13). Human colon cancer cell lines (HCT116, DLD-1, RKO, and LoVo) and virus-producing cell lines (293FT, Phoenix A) were cultured in basal medium supplemented with 10% serum. Fully sequenced colon cancer cell lines (VaCo) were provided by J.A. Wilson (Harold C. Simms Comprehensive Cancer Center, University of Texas Southwestern Medical Center, Dallas, TX) and cultured as described (6). Identity of all cell lines was verified by DNA fingerprinting.
Screen
A total of 481 shRNAs against CRC mutated genes were arrayed in 96-well plates and transfected into 293FT cells together with helper plasmids—pMD2G and psPAX2. Viral supernatants were collected 48 hours later and were passed through Multiscreen HTS 0.45-μm filter plates (Milipore). HCECs were infected with filtered viral supernatants containing 4 μg/mL of polybrene (Sigma) at multiplicity of infection (MOI) of approximately 1. Successfully infected cells were selected with 1 μg/mL puromycin and seeded in 0.375% Noble agar (Difco) on top of 0.5% presolidified agar in 96-well plates in “one-shRNA-one-well” format. After 21 days, wells were stained with 0.005% crystal violet and scored for macroscopically visible colonies. For the random shRNA screen, we assayed 4 plates from the whole genome shRNA library as purchased from Open Biosystems. These 4 plates were chosen because each of them contained at least 1 shRNA that scored in our primary screen. This setting ensured consistency with the primary screen and enabled us to screen 362 random shRNAs. Clone IDs for each shRNA used in this study are provided in Supplementary Tables S1 and S2, and could be used to retrieve target sequence as well as detailed information about the vectors from Open Biosystems.
Anchorage-independent colony formation assay, transient transfections, immunofluorescence, immunobloting, quantitative real-time PCR, and network analysis
See Supplementary Data.
Results and Discussion
Anchorage-independent growth suppressor screen
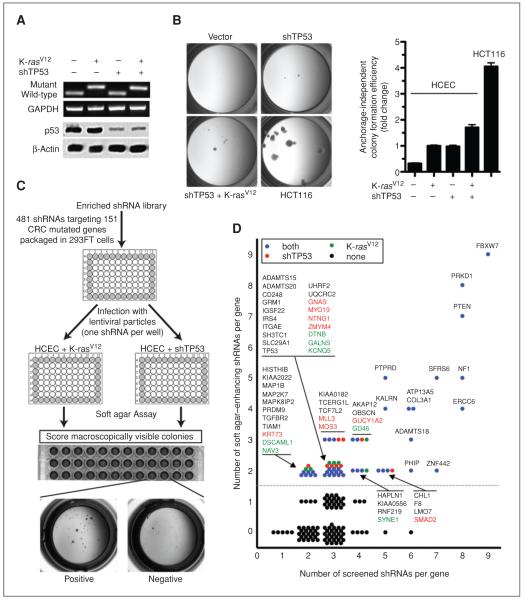
With the exception of APC truncation mutations, K-RASV12 and TP53 alterations represent 2 of the most frequent mutations in CRC (6, 10) and either alone or in combination slightly enhance colony formation efficiency of HCECs in soft agar. However, even in HCECs with both of these oncogenic alterations, soft agar growth is considerably less robust compared with that of an established CRC cell line, HCT116 (Fig. 1A and B). Furthermore, none of these HCEC derivatives formed tumors when injected either s.c. or under the renal capsule of immunocompromised mice (data not shown). Therefore, these premalignant HCECs provide a sensitized background that might allow discovery of context-dependent (i.e., cooperation with a specific oncogenic alteration) and context-independent (i.e., cooperation with both oncogenic alterations) tumor suppressors.
Figure 1.
Identification of tumor suppressors within CRC CAN-genes with an enriched shRNA library by using isogenic HCECs. A, total RNA from immortalized HCECs and their oncogenically progressed derivatives expressing K-rasV12, shTP53, or both were subjected to qRT-PCR followed by a diagnostic restriction digestion assay to detect WT and mutant KRAS alleles. Whole-cell extracts from same cells were immunoblotted for expression of p53. B, each cell line was cultured in soft agar in 96-well plates for 3 weeks and photographed with a stereomicroscope. Colonies larger than 0.1 mm in size are quantified. Bars represent 12 data points (3 separate experiments in quadruplicates), mean ± SEM. C, schematic representation of the strategy. HCECs expressing K-rasV12 or shRNA against p53 were infected with lentiviral shRNA constructs in “one-shRNA-one-well” format and the ability of cells to form macroscopic soft agar colonies was assessed after 3 weeks. D, scatter plot showing the overall results of the screens. Each circle represents a gene. Context-dependent anchorage-independent growth suppressors are shown in green (K-rasV12–specific hits) and red (shTP53-specific hits) whereas context-independent suppressors are shown in blue. All genes, except those isolated only in K-rasV12 context, were plotted according to results obtained from shTP53 context.
A soft agar–based shRNA screen against CAN-genes (6, 10) in a one-shRNA-one-well format (Fig. 1C) revealed 49 context-independent and 16 context-dependent progression suppressors (Fig. 1D; Supplementary Table S1). Although our approach by its nature was not exhaustive, the number of identified progression suppressors was unexpectedly high. To test whether the high rate of tumor suppressors discovered within CAN-genes was because of a more permissive cellular system, we assayed 4 plates from the whole genome shRNA library, each plate containing at least 1 shRNA against 1 of the confirmed genes from the primary screen. All 5 shRNAs that scored in the primary screen were also identified in this screen, whereas only 5 of 362 random shRNAs enhanced soft agar growth (Supplementary Table S2)—a background rate of 1.4% as opposed to 48% observed against CRC CAN-genes. These results provide strong evidence against off-target effects or artifacts that may have resulted from global perturbation of microRNA biogenesis and suggest that the assay itself is extremely robust.
The observation that oncogenic Ras-expressing cells are more resistant to p53-dependent apoptosis (14) may account, in part, for the large number of overlapping hits in mutant KRASV12 and TP53 knockdown backgrounds. Accordingly, KRASV12-expressing HCECs failed to activate p53-induced proapoptotic targets such as p21 and Bax despite having wild–type (WT) levels of p53 and were able to phosphorylate p53 upon radiation exposure (Supplementary Fig. S1). These findings suggest that shRNAs identified in both KRASV12 and TP53 knockdown backgrounds may cooperate with deregulated p53 signaling.
Suppressors of anchorage-independent growth identified in this screen fall under a variety of different signaling pathways and biological processes (Supplementary Table S3). Furthermore, on average 37% of all CAN-genes in any individual colon cancer are involved in matrix-dependent proliferation (Supplementary Table S4). Candidate tumor suppressors identified in this study were uniformly contributed from all samples rather than a few tumors with highly deregulated matrix-dependent proliferation.
To further minimize off-target effects, we only considered genes as candidates if 2 or more shRNAs enhanced soft agar growth. Quantitative retesting of soft agar–enhancing shRNAs confirmed all tested genes with at least 1 shRNA with comparable or greater enhancement to K-rasV12 and an attrition rate of 8% (3 of 36 shRNAs did not confirm; arrows, Supplementary Fig. S2). Importantly, shRNAs against well-known tumor suppressors (FBXW7, NF1, PTEN, TGFBR2, and TP53) scored positive whereas genes with established oncogenic gain of function (KRAS, PIK3CA, and RET) did not score in the loss-of-function assay as might have been expected. Analysis of the nonscoring, well-described tumor suppressor genes such as APC and SMAD4 showed that none of the screened shRNAs against these 2 genes was effective in decreasing protein levels (data not shown). This is an exception within the library, as analyses of 23 nonscoring shRNAs for their ability to downregulate their target gene revealed only 2 nonfunctional shRNAs (Supplementary Fig. S3). Quantitative testing of PTEN and NF1 shRNAs (Supplementary Fig. S4) or screening of approximately half of the shRNA library by using immortalized HCECs without KRASV12 or TP53 alterations did not reveal any gene that enhanced anchorage-independent growth when knocked down (data not shown). This suggests that candidate tumor suppressors identified in this screen are important for progression but not initiation of CRC.
Ectopic expression of most potent candidates
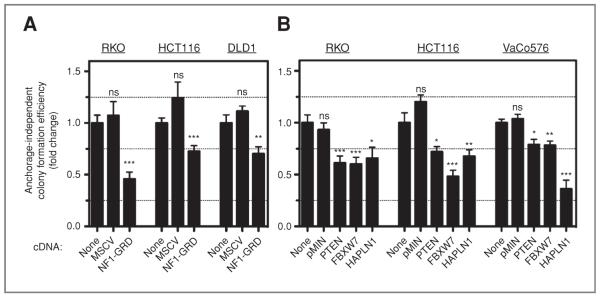
We next ectopically expressed some of the most potent candidate tumor suppressors, selected by quantitative retesting (Supplementary Fig. S2) in established colon cancer lines and measuring their ability to decrease anchorage-independent growth. Almost all tested cDNAs in all tested colon cancer lines decreased soft agar growth with varying degrees (Fig. 2). An interesting genotype–phenotype correlation emerged from NF1-GRD expression studies. NF1-GRD has been shown to be sufficient to inhibit WT KRAS (15). Accordingly, we observed a 50% reduction in anchorage-independent growth in RKO cells with 2 WT KRAS alleles whereas the reduction was only 25% in HCT116 and DLD1, which carry 1 mutant (G13D) and 1 WT allele (Fig. 2A) without any change in their monolayer growth kinetics (data not shown). shRNAs against NF1 enhanced soft agar growth in both p53 down-regulated and, surprisingly, in oncogenic KRAS-expressing HCECs with similar potencies that are proportional to the level of NF1 knockdown (Supplementary Fig. S5A). Considering the majority of KRAS transcripts in these cells are the mutant form (Fig. 1A) that cannot be inactivated by NF1, involvement of NF1 in an additional pathway to enhance soft agar growth is suggested by these observations. Recently, it has been shown that another Ras GTPase activating protein, DAB2IP2, can activate both Ras and NF-κB pathways (16). However, examination of shNF1-expressing cells did not reveal increased NF-κB activation in HCECs (Supplementary Fig. S5B). The mechanism underlying increased anchorage-independent growth in response to NF1 knockdown in oncogenic KRAS-expressing cells has yet to be identified. Importantly, we have also confirmed the ability of HAPLN1, a novel candidate tumor suppressor, to decrease anchorage-independent growth in a variety of colon cancer cell lines (Fig. 2B). The magnitude of inhibition was greatest when WT HAPLN1 was introduced into cells carrying HAPLN1 mutations (VaCo576; Fig. 2B).
Figure 2.
Functional validation of candidate tumor suppressors. Ectopic expression of candidate tumor suppressors by retroviral (A) or lentiviral (B) vectors in colon cancer cell lines leads to decreased soft agar growth. Triplicates from 2 separate experiments, 2-tailed Student t test, compared with none, mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, nonsignificant.
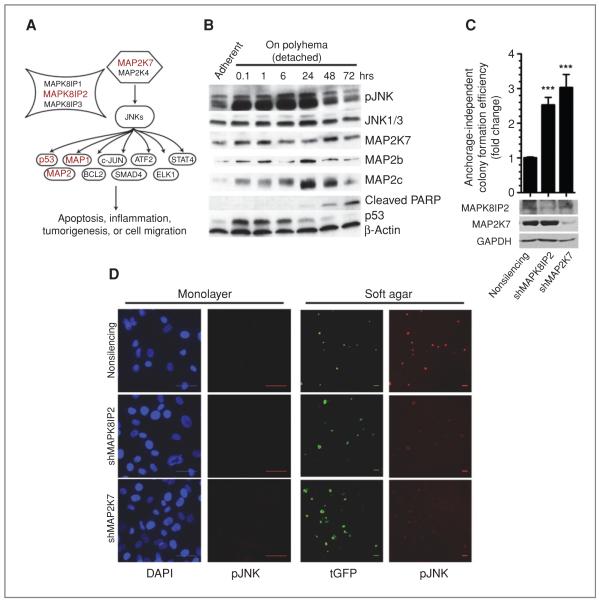
JNK signaling is a master suppressor of anchorage-independent growth
As another proof of concept, we next focused on the JNK signaling pathway not only because it was the most enriched pathway (Fig. 3A) but also because MAP2K7 and MAPK8IP2 were the most potent hits without previously established tumor suppressive functions (Supplementary Fig. S2). The role of JNK signaling in cancer is controversial with evidence for both oncogenic and tumor suppressor activities largely due to opposing roles of JNK targets (17, 18). These opposing effects are also regulated by the duration of JNK activating signal: Transient activations promote survival, whereas sustained JNK activity (1–6 hours) promotes apoptosis (19). Accordingly, switching HCECs to detached culture conditions results in rapid phosphorylation of JNK, which is sustained for 24 hours (Fig. 3B). JNK activation increases p53 levels and stabilizes MAP2, which in turn leads to apoptosis (20) as shown by increased cleaved PARP levels (Fig. 3B). shRNAs against upstream activating kinase MAP2K7 and scaffold protein MAPK8IP2 enhance anchorage-independent growth by 3- and 2.5-fold, respectively (Fig. 3C). Importantly, anchorage-independent growth–induced JNK activation was abrogated in HCECs expressing shRNAs against MAP2K7 or MAPK8IP2 (Fig. 3D). Taken together, these results suggest that HCECs normally undergo JNK-induced apoptosis in response to loss of attachment (anoikis) and that downregulation of MAP2K7 and MAPK8IP2 enhances anchorage-independent growth by preventing activation of JNK. These results were corroborated in HCT116 cells with MAP2K7 siRNA knockdown or overexpression (Supplementary Fig. S6). Interestingly, shRNAs against MAP2K7, but not MAPK8IP2, also enhanced invasion through Matrigel by 3-fold in HCECs (Supplementary Fig. S7), supporting the idea that a different set of genes is involved in invasion through basement membrane, a later stage in carcinogenesis.
Figure 3.
Identification of JNK pathway as suppressor of anchorage-independent growth. A, schematic representation of JNK pathway. shRNAs against 5 members of this pathway (red) was found to enhance soft agar growth. B, whole-cell extracts from immortalized HCECs were immunoblotted for JNK pathway members in response to loss of attachment. C, fold change in soft agar colony formation efficiency in response to MAPK8IP2 or MAP2K7 depletion in shTP53-expressing HCECs. Bars represent 16 data points from 2 separate experiments, 2-tailed Student t test, mean ± SEM. ***, P < 0.0001. D, phosphorylated (active) JNK immunofluorescence staining of cells in (C), either in monolayer or soft agar culture. Bars: 50 μm, tGFP is encoded from the shRNA vector, 24 hours after seed. DAPI, 4′,6-diamidino-2-phenylindole.
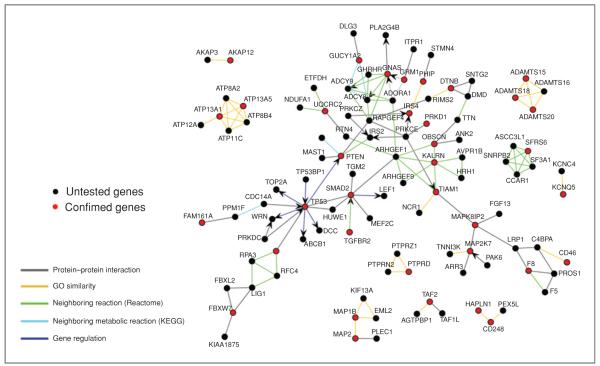
Discovery of additional tumor suppressors by using network analysis
Given the high degree of enrichment in JNK signaling pathways, we next asked whether other hits could be used as stepping stones to identify novel tumor suppressors. Initial CRC genome resequencing efforts identified approximately 850 mutated genes; however, approximately 150 of these mutated genes were included in CAN-genes and considered drivers based on frequency (6, 10). To identify relevant mutated tumor suppressors within genes that are otherwise considered “passengers,” we constructed an interaction map of the candidate tumor suppressors identified in this anchorage-independent screen with approximately 700 additional CRC mutated genes (Fig. 4). Importantly, downregulation of 5 of the 6 tested mutated genes (FBXL2, HUWE1, PAK6, PRKDC1, and TP53BP1) that interacted with confirmed hits in the primary screen also enhanced soft agar growth (Supplementary Fig. S8). These enhancements were comparable with that of ectopic expression of oncogenic K-ras in p53 down-regulated HCECs (Supplementary Fig. S2). This analysis provides a rationale framework to further test additional mutated genes to potentially identify more “druggable” colon cancer candidate tumor suppressors and show the need for biological assays to distinguish between driver and passenger genes instead of relying solely on bioinformatics.
Figure 4.
Discovering tumor suppressors from less frequently mutated genes with interaction mapping. Data on the network of interactions between the confirmed genes (red nodes) and other mutated genes are from Wood and colleagues (10). Interactions are colored according to the source and type of interactions as shown in the color key. Untested genes were included in the network plot only if they had an interaction with at least one of the confirmed genes, and genes with no interactions (confirmed or untested) were removed for the purpose of clarity.
In summary, this study functionally interrogated genes mutated in CRC and showed that cancer genome sequencing provides a valuable enrichment for cancer driver genes. More specifically our study discovered (by using a relevant transformation assay) that a remarkable fraction of CAN-genes are tumor suppressors involved in cell-autonomous anchorage-independent growth. Highly fragile tumor suppressor processes could, in part, explain the extensive heterogeneity observed in primary cancers; there are multiple independent genes abrogating various pathways that precancerous cells acquire to progress. This approach not only permits identification of causal cancer genome mutations, but also provides a roadmap for the interrogation and identification of important tumor suppressors in the much larger list of putative cancer genes and reveals the need to implement functional significance filters when identifying driver cancer genes.
Supplementary Material
Acknowledgments
We thank L. Lum and J. Minna for valuable discussions and providing reagents. We also thank W. Clapp and Geron Corporation for providing reagents.
Grant Support
This work was supported by CPRIT Training grant RP101496 to U. Eskiocak and P. Ly; AGA Research Scholar Award and UT Southwestern DOCs award to A.I. Roig; Welch Foundation I-1414, NIH CA71443, and NIH CA129451 to M.A. White; and NASA grants NNX11AC54G and NNX09AU95G to J.W. Shay.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–64. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Kolfschoten IGM, van Leeuwen B, Berns K, Mullenders J, Beijers-bergen RL, Bernards R, et al. A genetic screen identifies PITX1 as a suppressor of RAS activity and tumorigenicity. Cell. 2005;121:849–58. doi: 10.1016/j.cell.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Westbrook TF, Martin ES, Schlabach MR, Leng Y, Liang AC, Feng B, et al. A genetic screen for candidate tumor suppressors identifies REST. Cell. 2005;121:837–48. doi: 10.1016/j.cell.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Bric A, Miething C, Bialucha CU, Scuoppo C, Zender L, Krasnitz A, et al. Functional identification of tumor-suppressor genes through an in vivo RNA interference screen in a mouse lymphoma model. Cancer Cell. 2009;16:324–35. doi: 10.1016/j.ccr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, et al. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852–64. doi: 10.1016/j.cell.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 7.Ledford H. Big science: the cancer genome challenge. Nature. 2010;464:972–4. doi: 10.1038/464972a. [DOI] [PubMed] [Google Scholar]
- 8.Carter H, Chen S, Isik L, Tyekucheva S, Velculescu VE, Kinzler KW, et al. Cancer-specific high-throughput annotation of somatic mutations: computational prediction of driver missense mutations. Cancer Res. 2009;69:6660–7. doi: 10.1158/0008-5472.CAN-09-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youn A, Simon R. Identifying cancer driver genes in tumor genome sequencing studies. Bioinformatics. 2011;27:175–81. doi: 10.1093/bioinformatics/btq630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–13. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 11.Roig AI, Eskiocak U, Hight SK, Kim SB, Delgado O, Souza RF, et al. Immortalized epithelial cells derived from human colon biopsies express stem cell markers and differentiate in vitro. Gastroenterology. 2010;138:1012–21. e1–5. doi: 10.1053/j.gastro.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 12.Sato M, Vaughan MB, Girard L, Peyton M, Lee W, Shames DS, et al. Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–28. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- 13.Eskiocak U, Kim SB, Roig AI, Kitten E, Batten K, Cornelius C, et al. CDDO-Me protects against space radiation-induced transformation of human colon epithelial cells. Radiat Res. 2010;174:27–36. doi: 10.1667/RR2155.1. [DOI] [PubMed] [Google Scholar]
- 14.Ries S, Biederer C, Woods D, Shifman O, Shirasawa S, Sasazuki T, et al. Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell. 2000;103:321–30. doi: 10.1016/s0092-8674(00)00123-9. [DOI] [PubMed] [Google Scholar]
- 15.Hiatt KK, Ingram DA, Zhang Y, Bollag G, Clapp DW. Neurofibromin GTPase-activating protein-related domains restore normal growth in Nf1−/− cells. J Biol Chem. 2001;276:7240–5. doi: 10.1074/jbc.M009202200. [DOI] [PubMed] [Google Scholar]
- 16.Min J, Zaslavsky A, Fedele G, Mclaughlin SK, Reczek EE, De Raedt T, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med. 2010;16:286–94. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 18.Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007;46:591–8. doi: 10.1002/mc.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ventura J-J, Hübner A, Zhang C, Flavell RA, Shokat KM, Davis RJ. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21:701–10. doi: 10.1016/j.molcel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Soltani MH, Pichardo R, Song Z, Sangha N, Camacho F, Satya-moorthy K, et al. Microtubule-associated protein 2, a marker of neuronal differentiation, induces mitotic defects, inhibits growth of melanoma cells, and predicts metastatic potential of cutaneous melanoma. Am J Pathol. 2005;166:1841–50. doi: 10.1016/S0002-9440(10)62493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.