Abstract
BP180 (collagen XVII) is the target antigen in several autoimmune diseases including bullous pemphigoid (BP). Both IgE and IgG class autoantibodies have been shown to be pathogenic in BP; however, studies designed to elucidate the patho-mechanisms mediated specifically by the IgE-class autoantibodies are limited by the low levels (ng/mL) of IgE in human sera. In this report, we developed mouse IgE class monoclonal antibodies (MAbs) against the immunodominant NC16A domain of the human BP180 protein and characterized two of the resultant MAbs, designated 395A5 and 395D2. Epitope mapping studies revealed that both MAbs target segment 2 of NC16A, as was described for IgE and IgG class BP autoantibodies. Also similar to BP IgE, MAb 395A5 showed indirect immunofluorescence labeling of the basement membrane zone (BMZ) of human skin, stimulated histamine release from mast cells when triggered with NC16A, and induced keratinocyte production of IL-8. The 395D2 MAb was also able to trigger antigen-specific histamine release from mast cells; however, in contrast to BP IgE and 395A5, 395D2 did not label the cutaneous BMZ, nor did it induce IL-8 production in keratinocytes. In summary, these studies underscore the importance of functionally characterizing MAbs generated for use in human disease models. The 395A5 IgE class murine MAb was shown to share several key functional properties with the pathogenically active IgE produced by BP patients. We therefore expect that this MAb will prove to be a useful tool for dissecting the mechanisms used by BP180-NC16A-specific IgE antibodies in the induction of BP skin lesions.
Introduction
BP180, also termed type XVII collagen or BPAG2, BPAG2, BP180 is a component of hemidesmosomal adhesion complex that is critical for maintaining adhesion of the epidermis to the underlying dermis of the skin. BP180 is the cellular target of autoantibodies in a family of subepidermal autoimmune blistering diseases, including mucous membrane pemphigoid, lichen planus pemphigoides, linear IgA dermatosis, pemphigoid gestationis, and bullous pemphigoid (BP). In BP, both IgG- and IgE-class autoantibodies specific for BP180 are found in the circulation and bound to the basement membrane zone (BMZ) of affected skin.(1–4) These two classes of autoantibodies, which have been shown to target the same cluster of epitopes located within the non-collagenous 16A region (NC16A) of BP180, are produced by the vast majority of BP patients (IgG, 94% of patients; IgE, >85%).(1,5–7)
Early work probing the pathogenesis of BP in in vitro and in vivo models demonstrated a role for IgG class autoantibodies(6,8–10); however, none of these IgG-based models were able to recapitulate the early phase of lesion development in the human disease. The first evidence that BP IgE autoantibodies can fill this gap came from studies employing a model in which human skin was grafted onto nude mice.(11) In these studies, injection of physiologic concentrations of BP IgE (6–47 ng in a volume of 100 μL) into the xenografts resulted in urticarial plaque formation, eosinophil influx, mast cell degranulation and spontaneous subepidermal blistering, thus replicating the early phase of BP lesion development that was lacking in the IgG-based BP models.(6,8,9)
In another model of IgE autoimmunity, Zone and co-workers(12) generated MAbs specific for part of the shed ectodomain of BP180, termed LABD97, which is the antigenic target in linear IgA disease.(13,14) Injection of these hybridomas into human skin grafted onto mice reproduced many of the features of BP180-specific IgE in vivo. Although the NC16A region of BP180 is the major antigenic target in BP, these studies showed that antibodies to BP180 sites outside of the immunodominant region can also be pathogenic.
To further define the pathogenic mechanisms elicited by the BP180-specific IgE, affinity purification of these autoantibodies and removal of IgG are necessary. However, the concentration of IgE in BP sera––as in normal sera––is typically quite low (in the range of 50–150 ng/mL),(15,16) and the difficulty in obtaining sufficient quantities of blood from untreated BP patients to carry out these IgE purifications has hampered the rate of progress in this area of research. To circumvent this problem, we have generated and characterized IgE class MAbs specific for the immunodominant NC16A region of human BP180. We report here that these MAbs replicate many of the effects of the human IgE autoantibodies in vitro. These MAbs should facilitate further investigations into the pathogenic mechanisms of IgE class autoantibodies in autoimmune skin diseases targeting BP180.
Materials and Methods
Expression and purification of recombinant forms of NC16A region of human BP180
For the epitope mapping studies, we used glutathione S-transferase (GST) fusion proteins containing a series of overlapping segments of the BP180 NC16A domain. The cDNA cloning, expression, and purification of these recombinant proteins were described previously.(5,17,18) Briefly, cDNA encoding the entire NC16A domain, or overlapping segments thereof, were obtained by PCR amplification and cloned into the pGEX-2T vector (GE Healthcare Life Sciences, Piscataway, NJ). The relative positions of the BP180 subdomains have been described(5) and are referred to as NC16A1, NC16A2, NC16A2.5, NC16A3, and NC16A1-3. The corresponding GST fusion proteins and GST alone were expressed in Escherichia coli (Rosetta strain) and purified from bacterial lysates using glutathione-agarose affinity chromatography, as described.(5)
Immunization of mice and monoclonal antibody production
The immunization and cell fusion required to generate IgE MAbs specific for the NC16A region of BP180 were performed in collaboration with Open Biosystems (Lafayette, CO). Briefly, female Swiss Webster mice (∼10 weeks of age, n=5) were subcutaneously immunized on days 0, 14, 28, and 42 with purified recombinant full-length NC16A (10 μg) suspended at a 1:1 ratio in Imject® Alum (Pierce, Rockford IL). Sera were obtained 13 days after the second, third, and fourth immunizations (days 27, 41, and 55) and screened initially for murine IgE production by ELISA (Bethyl Laboratories, Montgomery, TX). IgE positive sera were tested by immunoblot for reactivity to purified recombinant NC16A-GST and GST (control).(7) After transfer of proteins to nitrocellulose membranes, sera were diluted 1:5 and incubated overnight at 4°C as primary antibodies. Reactivity was determined using anti-mouse IgE-HRP (Bethyl Laboratories). Mice with the strongest specific reactivity (n=3) received a final booster immunization (20 μg) 3 days prior to cell fusion. The spleens were harvested and fused to the mouse myeloma cell line P3x63Ag8.653.(19)
Hybridoma supernatants were screened for IgE production using a mouse IgE ELISA (Bethyl Laboratories). Positive supernatants were diluted 1:2 and screened for specific reactivity to NC16A by immunoblot, as described above. Those with the highest NC16A-specifc reactivity were expanded and cloned by limiting dilution. The 395 clone was selected, recloned, and rescreened for NC16A-specific IgE by immunoblot. For antibody production, 200×106 cells were placed in the cell chamber of a bioreactor (Integra Biosciences, Hudson, NH) in CD hybridoma medium supplemented with 10 nM 2-mercaptoethanol and L-glutamine (all from Gibco, Carlsbad, CA). Antibodies were purified from cell supernatants, quantitated by ELISA, and specificity was confirmed by immunoblot.
To confirm the isotype of our IgE MAbs, expression of immunoglobin heavy chain cDNA was confirmed by RT-PCR using immunoglobin epsilon (CH1) and gamma (CHG1 or CHG2,3) specific primers.(20) Poly-A+ RNA from the 395 hybridomas and HD18 (a IgG murine hybridoma control) was purified using the FastTrack 2.0 kit (Invitrogen, Carlsbad, CA) and RT-PCR was carried out using the SuperScript III One-Step RT-PCR system (Invitrogen) with appropriate combinations of the following primers. VH1 (forward): 5′-ATGGCCSAGGTSMARCTGCAGSAGTCWGG, along with one of the following reverse primers: CHE1: 5′-AGCCTAGGGTCATGGAAGCTG; CHG1: 5′-CTCAATTTTCTTGTCCACCTTGGTGC, or CHG2,3: 5′-CTCGATTCTCTTGATCAACTCAGTCT. The resulting PCR products were subcloned into the pScript vector and sequence analysis was performed. Sequence analysis confirmed that the cDNA was derived from an epsilon chain mRNA. Amplification of the 395 RNA with either of the two gamma-specific primers yielded no product. RNA from clone HD-18 (previously isotyped as CHG1(21)) yielded a cDNA product only when the CHG1 primer was used.
Purification of IgE from human sera
Sera were obtained from patients with well-characterized clinical, histological, and immunological features of BP, as well as from age and sex-matched controls. This study was approved by the University of Iowa IRB and was carried out in adherence to the Declaration of Helsinki Guidelines. IgE was purified from a BP serum known to have a high level of NC16A-specific IgE by ELISA (BP IgE) or a control (normal human IgE, NH IgE) serum, using two-step affinity chromatography.(2) First, the IgG was removed with a protein G column and the IgE was purified from the flow-through using human ɛ heavy chain-specific monoclonal antibody (HB-235; ATCC, Manassas, VA) coupled to Affi-Gel HZ (Bio-Rad, Hercules, CA). Final IgE concentrations were determined by ELISA (Immunology Consultants Laboratory, Newberg, OR).
Immunofluorescence microscopy
Cryosections (5 μm thick) of normal human skin were incubated with undiluted supernatants from 395A5 or 395D2, IgELB4 (anti-trinitrophenol IgE isotype control; ATCC), or a 1:10 dilution of BP or normal human serum. Sections were extensively washed and incubated with FITC-conjugated anti-mouse or anti human IgE as indicated. Slides were viewed with an epifluorescence Nikon photomicroscope.
Immunoblotting
Human BP sera and murine MAbs were tested by standard immunoblot analysis for reactivity with the above-described recombinant forms of human BP180.(5) Briefly, bacterial lysates or affinity purified recombinant proteins were fractionated by SDS-PAGE and transferred to nitrocellulose. HRP-conjugated anti-human IgG (gamma chain-specific) or IgE (epsilon chain-specific) and ECL detection were utilized to visualize bound antibodies (Amersham Pharmacia Biotech, Piscataway, NJ).
Histamine release assay
The histamine release assay was optimized using a rat basophil line expressing both human and rat high affinity IgE receptors, FcɛRI, RBL-SX-38 (gift from JP Kinet(22)), and the untransfected parental RBL cells (ATCC). We first established that the RBL-SX-38 cells were able to bind both human and murine IgE by flow cytometry (data not shown). For experiments, cells (104/well) were cultured overnight (37°C, 5% CO2) in a 96-well plate. Next, the medium was aspirated and cells were incubated for 30 min with serial dilutions of 395A5, 395D2 or IgELB4 MAbs, BP, or NH IgE in order to load the surface FcɛRI. These cells were then incubated with optimal concentrations of either GST-NC16A or GST control protein. At various times after protein addition, the culture supernatants were collected, and histamine content, as an index of degranulation, was measured by EIA (Immunotech Beckman Coulter, Brea, CA).
Keratinocyte IL-8 production
Primary cultures of human keratinocytes, obtained at passage 2 (Cascade Biologics, Portland, OR), were maintained at <80% confluence in keratinocyte serum-free medium (KSFM kit, Gibco).(2) For experiments, 2×104 cells/well were plated into 24-well tissue culture plates and incubated overnight. The medium was aspirated and fresh medium containing BP or NH IgE or 395A5, 395D2, or IgELB4 MAbs (all at 100 ng/mL) was added to duplicate cultures and incubated as indicated. Supernatants were collected on ice by careful aspiration, clarified by centrifugation (6000 rpm, 10 min, 4°C), and frozen at −20°C until analyzed. IL-8 content was determined using a commercially available ELISA kit as directed (R&D Systems, Minneapolis, MN).
Results
Isolation of mouse IgE class monoclonal antibodies specific for BP180
Swiss Webster mice were immunized s.c. with a GST fusion protein of the immunodominant NC16A region of the BP180 protein.(17) After four injections over 8 weeks, the sera of immunized mice were screened for specific reactivity to the NC16A moiety and hybridoma cells were generated through fusion of immune spleen cells with the myeloma cell line P3x63Ag8.653.(19) The antibodies produced by the hybridomas were screened first for IgE production by ELISA and next for NC16A specificity on immunoblots containing both GST-NC16A fusion protein and GST as a negative control. From the five immunized mice, four hybridomas maintained production of NC16A-specific IgE and those were expanded and cloned by limiting dilution. Clones were tested for the production of IgE and absence of all other isotypes by ELISA, and expression of immunoglobin epsilon heavy chain cDNA was confirmed by RT-PCR analysis.(20)
NC16A reactivity and epitope mapping
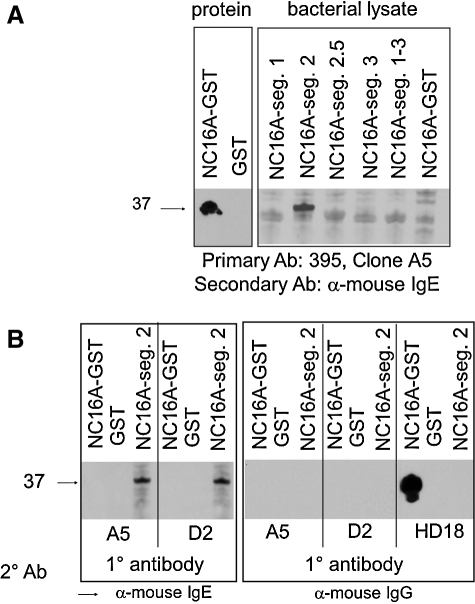
The reactivity of the 395 hybridoma subclone supernatants with lysates of bacteria expressing full-length NC16A-GST or GST control examined by immunoblot (Fig. 1, left panel). For epitope mapping, a 45 amino acid stretch of NC16A (NC16A1-3) was subdivided into three non-overlapping segments (NC16A1, NC16A2, and NC16A3). Also included in this analysis was a 17 amino acid segment (NC16A2.5) that partially overlaps NC16A2 and NC16A3 and contains a known BP autoantibody epitope that spans the NC16A2/A3 junction.(5,7) Using anti-mouse IgE secondary antibodies, only two clones (395A5 and 395D2) retained specific reactivity to NC16A-GST (37 kDa) but not the GST control (Fig. 1A, right panel). The IgE produced by both 395A5 and 395D2 was shown to be directed against the 15 amino acid NC16A2 peptide (Fig. 2, left panel). No specific reactivity is observed with either clone using an IgG-specific secondary antibody. The A2 subdomain of NC16A had previously been identified as an immunodominant site recognized by IgE and IgG autoantibodies isolated from BP sera.(5,7)
FIG. 1.
Mapping of the bullous pemphigoid (BP) IgE MAb reactive sites by immunoblot. (A) Supernatants of our 395 hybridoma, Clones A5 and D2, were screened for IgE reactivity to either affinity-purified NC16A protein or lysates of bacteria expressing one of five subregions: NC16A1, NC16A2, NC16A2.5, NC16A3, or NC16A1–3. Proteins or lysates were electrophoresed and transferred to a nitrocellulose membrane. Membranes were probed with hybridoma supernatants (diluted 1:2) as the primary antibody. Anti-human IgE was used to detect specific reactivity to purified NC16A protein (left panel) and the NC16A segment 2 subdomain (right panel). 395 clone A5 is shown. Similar results were obtained with 395 clone D2. (B) To ensure specificity, 395 clone A5 and 395 clone D2 were used as primary antibodies on protein blots containing equimolar amounts of NC16A-GST, GST, and NC16A segment 2, followed by secondary detection with either anti-mouse IgE or IgG. HD18 (control) is mouse IgG specific for NC16A.
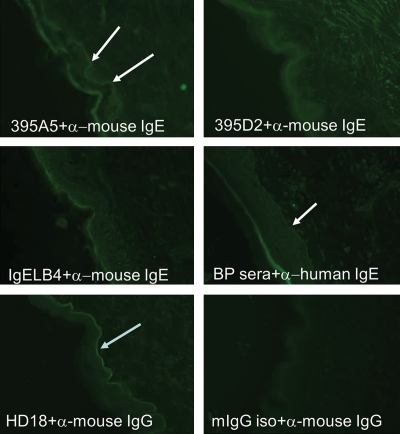
FIG. 2.
395A5, but not 395D2, MAbs bind to the basement membrane zone of human skin. Cryosections of normal human skin were incubated with MAbs 395A5, 395D2, IgELB4 (IgE isotype control), a BP serum (diluted 1:10), a BP180 specific IgG MAb (HD18), or a murine IgG isotype control. Antibody binding to the BMZ was visualized with anti-mouse IgE, anti-mouse IgG, anti-human IgE, or anti-human IgG, as indicated. Antibody binding to BP180 in human skin was evidenced by linear staining at the basement membrane zone (arrows) using 395A5 MAbs, BP sera, and HD18, but was absent with the 395D2 MAb or the isotype controls.
To confirm specific binding of secondary antibodies the supernatants of 395 clones A5 and D2 were used as primary antibodies on protein blots containing equimolar amounts of affinity purified GST, GST-NC16A, and GST-NC16A2, and were followed by secondary detection with either anti-mouse IgE or IgG. HD18 is mouse IgG1 specific for NC16A.(21) Immunoreactivity of both 395A5 and 395D2 to NC16A and NC16A2 was observed using a secondary antibody specific for murine IgE but not for IgG (Fig. 1B). Interestingly, loading equimolar amounts of each protein revealed stronger reactivity to the NC16A2 subdomain than was observed against full-length NC16A.
Binding to basement membrane zone of human skin
Epidermal blistering diseases are characterized by autoantibody deposition in the skin. In BP, both IgG and IgE are bound to the BMZ––the histologic location of the BP180 protein. One type of BP model utilizes human skin cryosections in combination with IgG autoantibodies purified from patient serum to study the mechanisms of BP pathology.(23,24) To be of use in any model of BP, the 395 IgE MAbs must also bind native BP180. To examine this, cryosections of normal human skin were incubated with MAbs 395A5, 395D2, IgELB4, a BP serum (diluted 1:10) known to have a high level of NC16A-specific IgE by ELISA, HD18, or a murine IgG isotype control. Antibody binding to the BMZ was visualized with anti-mouse IgE, anti-mouse IgG, anti-human IgE, or anti-human IgG, as indicated. Linear staining at the BMZ (arrows) was evident with both the 395A5 and BP sera but absent with the 395D2 MAb or the isotype control, IgELB4 (Fig. 2). HD18 also produced bright linear staining at the BMZ that was not observed with the mIgG isotype control.
Stimulation of keratinocyte secretion of IL-8
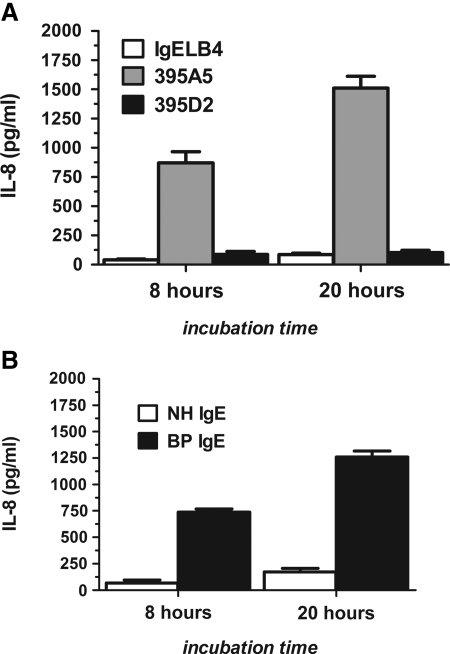
Both BP IgG and IgE autoantibodies have been shown to elicit a pro-inflammatory response in keratinocytes as measured by the secretion of IL-8,(2,25) a chemokine known to be critical for disease pathogenesis.(26) Therefore, the effects of the 395A5 and 395D2 MAbs on keratinocyte IL-8 production was compared with that of BP IgE. Keratinocytes were incubated with 100 ng/mL of 395A5, 395D2, IgELB4 MAbs, BP IgE, or NH IgE; and IL-8 was measured in the culture supernatants by ELISA. As previously reported,(2) keratinocyte treatment with BP IgE resulted in a dramatic increase in IL-8 compared to NH IgE. Likewise, treatment of keratinocytes with 395A5, but not IgELB4 or 395D2, MAbs stimulated secretion of IL-8 in the same manner as the human BP IgE autoantibodies (Fig. 3).
FIG. 3.
395A5, but not 395D2, MAbs trigger IL-8 production by human keratinocytes. Primary human keratinocytes were treated with 100 ng/mL 395A5, 395D2, IgELB4, or IgE purified from a BP patient (BP IgE) or normal control serum (NH IgE) for 20 h. Cell-free supernatants were collected and IL-8 was measured by ELISA. (A) Treatment keratinocytes with 395A5 MAbs, but not 395D2 or IgELB4, triggered release of IL-8. (B) Similarly, BP IgE, but not NH IgE, triggered IL-8 secretion.
Induction of basophil histamine release
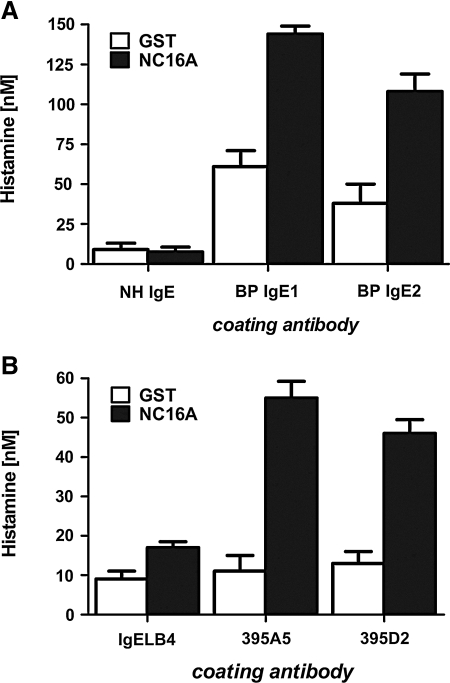
The efficacy of IgE antibodies in releasing histamine is variable depending on its affinity for its antigen.(27) Basophils from BP patients have been demonstrated to release histamine after crosslinking endogenous surface bound IgE by exposure to recombinant NC16A.(16) Thus, IgE autoantibodies in these patients are high enough in affinity to produce degranulation. To determine if the 395 MAbs can also mediate degranulation, histamine release was examined from RBL-SX basophils after coating with BP sera or with IgE from 395A5, 395D2, or IgELB4 MAbs, and then incubating with either NC16A-GST or GST control. As seen in Figure 4, coating of the RBL-SX cells with either of the 395 MAbs or BP sera (Fig. 4A, B, respectively) resulted in an NC16A-specific release of histamine.
FIG. 4.
395A5 and 395D2 MAbs mediate NC16A-specific histamine release. RBL-SX-38 basophils were coated with 395A5, 395D2, or IgELB4 MAbs or IgE purified from two BP patients (BP IgE1 or BP IgE2) or normal control serum (NH IgE) for 30 min and washed, and degranulation was triggered by exposure to 100 nM NC16A or GST control protein for 30 min. (A) MAbs 395A5 and 395D2 triggered greater histamine release in response to NC16A protein, but not GST control protein, similar to BP IgE1 and IgE2 (B). Control antibodies did not trigger NC16A-specific histamine release.
Discussion
The clinical relevance of IgE autoantibodies in BP was recently demonstrated by the resolution of disease, despite the continued presence of BP180-specific IgG, after treatment with omalizumab (anti-IgE that prevents IgE receptor binding) in a patient unresponsive to conventional therapy.(15) In vivo and in vitro models of BP have been established to dissect the mechanisms of the autoantibody-induced pathology. These studies suggest that BP IgE can initiate disruption of hemidesmosomes through antigen binding(2,28) and trigger degranulation of immune cells through FcɛRI receptor engagement.(16) However, more complete studies (in vitro and in vivo) examining the downstream events after IgE autoantibody binding to the immunodominant NC16A region of BP180 are limited by inherent variability associated with BP IgE prepared from individual patient sera and the low yields achieved using the currently available technology. In addition, these studies utilized total IgE since affinity purifying adequate amounts NC16A-specific IgE from peripheral blood is not possible using current methods. For these reasons, previous investigations could not definitively conclude that the observed effects of BP IgE were due specifically to anti-BP180 autoantibodies or those of an alternate specificity.
In this study, we used the NC16A region of the BP180 protein as immunogen and produced two specific IgE class MAbs by hybridoma technology. The mice were immunized subcutaneously to increase the likelihood of IgE-class antibody production; however, this route is known to generate other classes as well.(19) In addition to the clones (A5 and D2) described herein, five additional IgE clones with initial IgE reactivity were obtained; however, they did not maintain sufficient MAb production to be of use.
The IgE MAbs were subjected to epitope mapping and characterized for their ability to bind to the BMZ of human skin, stimulate IL-8 release from human keratinocytes, and trigger degranulation of basophils in a manner similar to affinity purified BP IgE. The specific reactivity of both clones A5 and D2 mapped to the same region of BP180 (NC16A region 2) as the majority of BP patient antibodies of either the IgE or IgG class.(5,7) The ability of the 395A5 IgE MAb to replicate all of the in vitro findings of BP IgE clearly demonstrates its utility in current BP models. In contrast, the 395D2 IgE MAb clone did not bind native BP180 in human skin cryosections or stimulate IL-8 production by keratinocytes but was able to induce degranulation of mast cells. This suggests that the 395D2 clone is unable to bind NC16A within the context of the entire BP180 protein, which could be due to differences in secondary or tertiary structure. These findings underscore the need for functional characterization of MAbs generated for use in models of human disease. In summary, the 395A5 IgE MAb should prove to be a useful tool for further exploration of the mechanisms behind NC16A-specific IgE autoantibody-mediated tissue damage in BP, as well as in other autoimmune skin diseases targeting the BP180 hemidesmosomal antigen.
Acknowledgments
This project was funded by a VA Merit Review Award grant (JAF), the NIH grant Short-term Training for Students in the Health Professions at the Carver College of Medicine (EAV), and an NIH grant AR040410 (GJG). Technical assistance was provided by Steven L. Eliason.
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Messingham KA. Noe MH. Chapman MA. Giudice GJ. Fairley JA. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J Immunol Methods. 2009;346:18–25. doi: 10.1016/j.jim.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messingham KN. Srikantha R. DeGueme AM. Fairley JA. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol. 2011;187:553–560. doi: 10.4049/jimmunol.1001753. [DOI] [PubMed] [Google Scholar]
- 3.Provost TT. Tomasi TB., Jr Immunopathology of bullous pemphigoid. Basement membrane deposition of IgE, alternate pathway components and fibrin. Clin Exp Immunol. 1974;18:193–200. [PMC free article] [PubMed] [Google Scholar]
- 4.Yayli S. Pelivani N. Beltraminelli H. Wirthmüller U. Beleznay Z. Horn M. Borradori L. Detection of linear IgE deposits in bullous pemphigoid and mucous membrane pemphigoid: a useful clue for diagnosis. Br J Dermatol. 2011;165:1133–1137. doi: 10.1111/j.1365-2133.2011.10481.x. [DOI] [PubMed] [Google Scholar]
- 5.Zillikens D. Rose PA. Balding SD. Liu Z. Olague-Marchan M. Diaz LA. Giudice GJ. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol. 1997;109:573–579. doi: 10.1111/1523-1747.ep12337492. [DOI] [PubMed] [Google Scholar]
- 6.Nishie W. Sawamura D. Goto M. Ito K. Shibaki A. McMillan JR. Sakai K. Nakamura H. Olasz E. Yancey KB. Akiyama M. Shimizu H. Humanization of autoantigen. Nat Med. 2007;13:378–383. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- 7.Fairley JA. Fu CL. Giudice GJ. Mapping the binding sites of anti-BP180 immunoglobulin E autoantibodies in bullous pemphigoid. J Invest Dermatol. 2005;125:467–472. doi: 10.1111/j.0022-202X.2005.23853.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z. Diaz LA. Troy JL. Taylor AF. Emery DJ. Fairley JA. Giudice GJ. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z. Sui W. Zhao M. Li Z. Li N. Thresher R. Giudice GJ. Fairley JA. Sitaru C. Zillikens D. Ning G. Marinkovich MP. Diaz LA. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. J Autoimmun. 2008;31:331–338. doi: 10.1016/j.jaut.2008.08.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gammon WR. Merritt CC. Lewis DM. Sams WM., Jr Carlo JR. Wheeler CE., Jr An in vitro model of immune complex-mediated basement membrane zone separation caused by pemphigoid antibodies, leukocytes, and complement. J Invest Dermatol. 1982;78:285–290. doi: 10.1111/1523-1747.ep12507222. [DOI] [PubMed] [Google Scholar]
- 11.Fairley JA. Burnett CT. Fu C-L. Larson DL. Fleming MG. Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid ige reproduces the early phase of lesion development in human skin grafted to nu//nu mice. J Invest Dermatol. 2007;127:2605–2611. doi: 10.1038/sj.jid.5700958. [DOI] [PubMed] [Google Scholar]
- 12.Zone JJ. Taylor T. Hull C. Schmidt L. Meyer L. IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J Invest Dermatol. 2007;127:1167–1174. doi: 10.1038/sj.jid.5700681. [DOI] [PubMed] [Google Scholar]
- 13.Egan CA. Martineau MR. Taylor TB. Meyer LJ. Petersen MJ. Zone JJ. IgA antibodies recognizing LABD97 are predominantly IgA1 subclass. Acta Dermato-Venereol. 1999;79:343–346. doi: 10.1080/000155599750010229. [DOI] [PubMed] [Google Scholar]
- 14.Egan CA. Reddy D. Nie Z. Taylor TB. Schmidt LA. Meyer LJ. Petersen MJ. Hashimoto T. Marinkovich MP. Zone JJ. IgG anti-LABD97 antibodies in bullous pemphigoid patients' sera react with the mid-portion of the BPAg2 ectodomain. J Invest Dermatol. 2001;116:348–350. doi: 10.1046/j.1523-1747.2001.01246.x. [DOI] [PubMed] [Google Scholar]
- 15.Fairley JA. Baum CL. Brandt DS. Messingham KAN. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol. 2009;123:704–705. doi: 10.1016/j.jaci.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimson OG. Giudice GJ. Fu CL. Van den Bergh F. Warren SJ. Janson MM. Fairley JA. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol. 2003;120:784–788. doi: 10.1046/j.1523-1747.2003.12146.x. [DOI] [PubMed] [Google Scholar]
- 17.Giudice GJ. Emery DJ. Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243–250. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- 18.Van den Bergh F. Fu CL. Olague-Marchan M. Giudice GJ. The NC16A domain of collagen XVII plays a role in triple helix assembly and stability. Biochem Biophys Res Commun. 2006;350:1032–1037. doi: 10.1016/j.bbrc.2006.09.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olaru F. Mihai S. Petrescu I. Zillikens D. Sitaru C. Generation and characterization of monoclonal antibodies against the intracellular domain of hemidesmosomal type XVII collagen. Hybridoma. 2006;25:158–162. doi: 10.1089/hyb.2006.25.158. [DOI] [PubMed] [Google Scholar]
- 20.Kaul S. Scheurer S. Danz N. Schicktanz S. Vieths S. Hoffmann A. Monoclonal IgE antibodies against birch pollen allergens: novel tools for biological characterization and standardization of allergens. J Allergy Clin Immunol. 2003;111:1262–1281. doi: 10.1067/mai.2003.1510. [DOI] [PubMed] [Google Scholar]
- 21.Pohla-Gubo G. Lazarova Z. Giudice GJ. Liebert M. Grassegger A. Hintner H. Yancey KB. Diminished expression of the extracellular domain of bullous pemphigoid antigen 2 (BPAG2) in the epidermal basement membrane of patients with generalized atrophic benign epidermolysis bullosa. Exp Dermatol. 1995;4:199–206. doi: 10.1111/j.1600-0625.1995.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 22.Weigand TW. Williams PB. Dreskin SC. Jouvin MH. Kinet JP. Tasset D. High affinity oligonucleotide ligands to human IgE inhibit binding to Fcepsilon receptor I. J Immunol. 1996;57:221–230. [PubMed] [Google Scholar]
- 23.Sitaru C. Schmidt E. Petermann S. Munteanu LS. Brocker EB. Zillikens D. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol. 2002;118:664–671. doi: 10.1046/j.1523-1747.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- 24.Sitaru C. Mihai S. Otto C. Chiriac MT. Hausser I. Dotterweich B. Saito H. Rose C. Ishiko A. Zillikens D. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest. 2005;115:870–878. doi: 10.1172/JCI21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt E. Reimer S. Kruse N. Jainta S. Brocker EB. Marinkovich MP. Giudice G J. Zillikens D. Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J Invest Dermatol. 2000;115:842–848. doi: 10.1046/j.1523-1747.2000.00141.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z. Giudice GJ. Zhou X. Swartz SJ. Troy JL. Fairley JA. Till GO. Diaz LA. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100:1256–1263. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mita H. Yasueda H. Akiyama K. Affinity of IgE antibody to antigen influences allergen-induced histamine release. Clin Exp Allergy. 2000;30:1583–1589. doi: 10.1046/j.1365-2222.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- 28.Iwata H. Kamio N. Aoyama Y. Yamamoto Y. Hirako Y. Owaribe K. Kitajima Y. IgG from patients with bullous pemphigoid depletes cultured keratinocytes of the 180-kDa bullous pemphigoid antigen (type XVII collagen) and weakens cell attachment. J Invest Dermatol. 2009;129:919–926. doi: 10.1038/jid.2008.305. [DOI] [PubMed] [Google Scholar]