Abstract
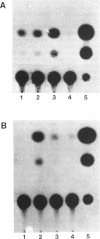
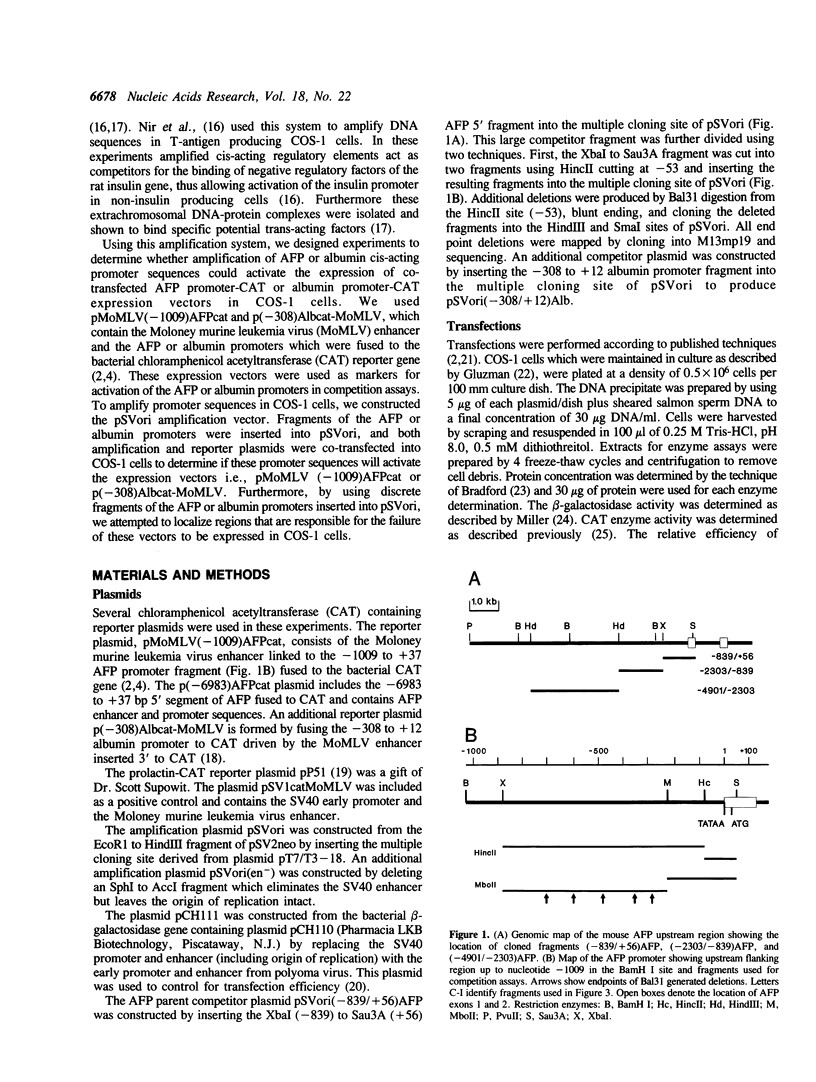
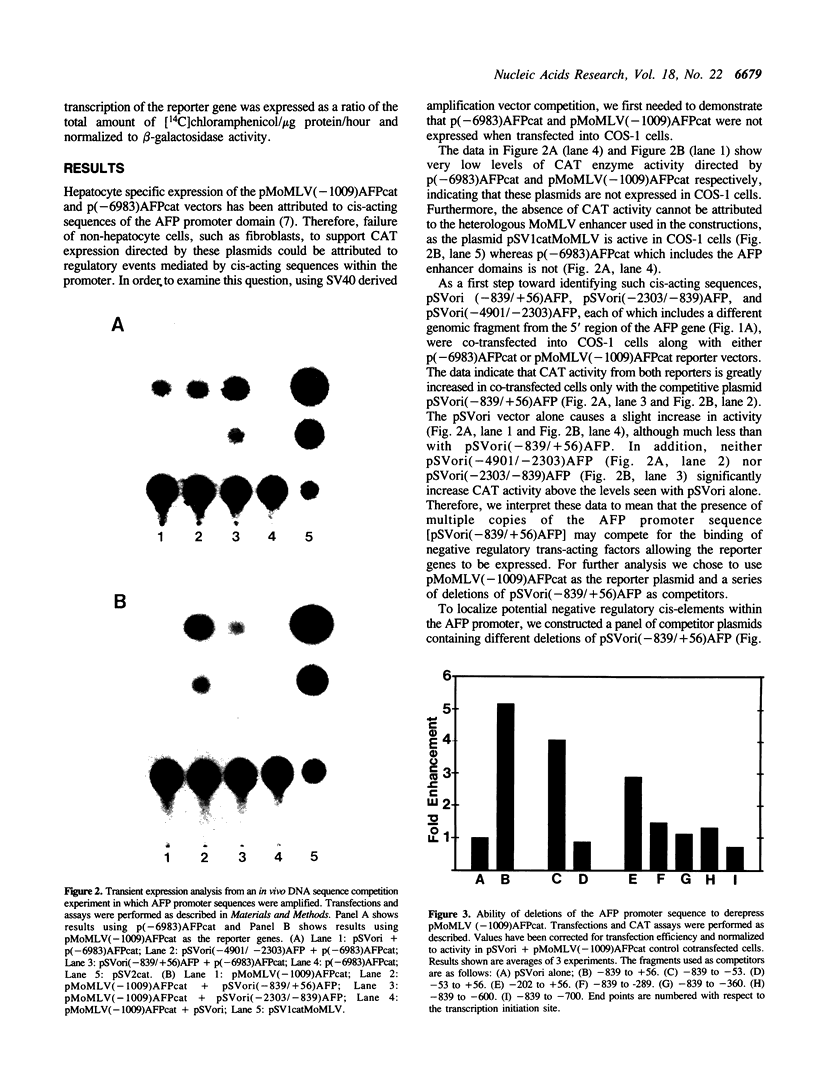
The existence of trans-acting regulatory factors has been demonstrated by in vivo competition with cis-acting sequences from both viral and eukaryotic genomes. Plasmids containing a functional SV40 origin of replication when transfected into permissive SV40 T-antigen producing COS-1 cells will amplify to high copy numbers (5,000 to 10,000) without inflicting toxic effects upon the host cell. This amplification vector (pSVori) has been used to amplify cis-acting regulatory elements which can act as competitors for positive and negative trans-acting factors in vivo. Using this amplification system we conducted experiments to determine whether amplification of alpha-fetoprotein (AFP) and albumin cis-acting promoter sequences could activate a corresponding co-transfected AFP-promoter-CAT or Alb-promoter-CAT expression vector in COS-1 cells. We used pMoMLV(-1009)AFPcat, or p(-308)Albcat-MoMLV as reporter genes and pSVori to amplify specific promoter sequences of the AFP or albumin promoter. Our experiments indicated that amplification of a region from -53 to -202 of the AFP promoter resulted in the activation of the pMoMLV(-1009)AFPcat and p(-308)Albcat-MoMLV expression vectors in COS-1 cells. Surprisingly, amplification of the albumin promoter sequences failed to activate either the pMoMLV(-1009)AFPcat or p(-308)Albcat-MoMLV plasmids.
Full text
PDF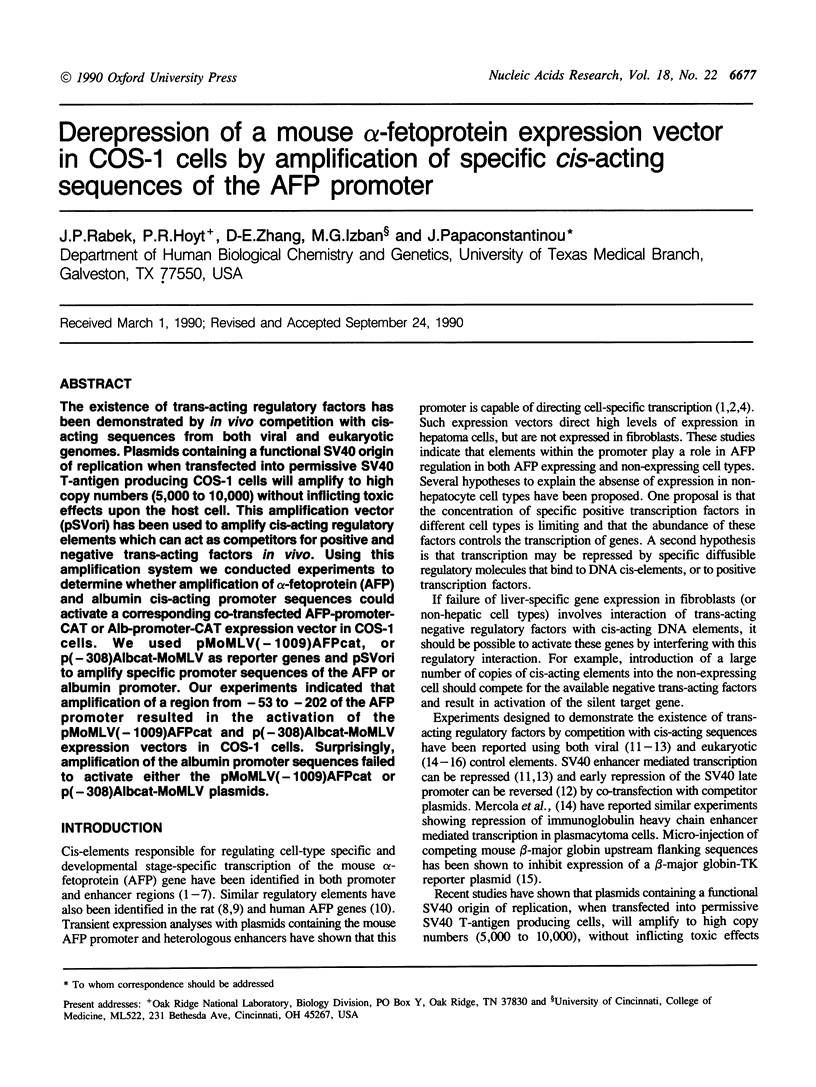


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Picardi J. Activity of simian virus 40 late promoter elements in the absence of large T antigen: evidence for repression of late gene expression. J Virol. 1986 Nov;60(2):400–404. doi: 10.1128/jvi.60.2.400-404.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumhueter S., Courtois G., Crabtree G. R. A variant nuclear protein in dedifferentiated hepatoma cells binds to the same functional sequences in the beta fibrinogen gene promoter as HNF-1. EMBO J. 1988 Aug;7(8):2485–2493. doi: 10.1002/j.1460-2075.1988.tb03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Tilghman S. M. Postnatal repression of the alpha-fetoprotein gene is enhancer independent. Genes Dev. 1989 Apr;3(4):537–546. doi: 10.1101/gad.3.4.537. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Blumenfeld M., Yaniv M. A liver-specific factor essential for albumin transcription differs between differentiated and dedifferentiated rat hepatoma cells. Genes Dev. 1988 Aug;2(8):957–974. doi: 10.1101/gad.2.8.957. [DOI] [PubMed] [Google Scholar]
- Church W. K., Papaconstantinou J., Kwan S. W., Poliard A., Szpirer C., Szpirer J. Molecular mechanisms of extinction of liver-specific functions in mouse hepatoma rat fibroblast hybrids: extinction of alpha-fetoprotein gene. Somat Cell Mol Genet. 1984 Sep;10(5):541–545. doi: 10.1007/BF01534859. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Xanthopoulos K. G., Darnell J. E., Jr A liver-specific DNA-binding protein recognizes multiple nucleotide sites in regulatory regions of transthyretin, alpha 1-antitrypsin, albumin, and simian virus 40 genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3840–3844. doi: 10.1073/pnas.85.11.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsholtz H. P., Mangalam H. J., Potter E., Albert V. R., Supowit S., Evans R. M., Rosenfeld M. G. Two different cis-active elements transfer the transcriptional effects of both EGF and phorbol esters. Science. 1986 Dec 19;234(4783):1552–1557. doi: 10.1126/science.3491428. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Godbout R., Ingram R. S., Tilghman S. M. Fine-structure mapping of the three mouse alpha-fetoprotein gene enhancers. Mol Cell Biol. 1988 Mar;8(3):1169–1178. doi: 10.1128/mcb.8.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout R., Ingram R., Tilghman S. M. Multiple regulatory elements in the intergenic region between the alpha-fetoprotein and albumin genes. Mol Cell Biol. 1986 Feb;6(2):477–487. doi: 10.1128/mcb.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guertin M., LaRue H., Bernier D., Wrange O., Chevrette M., Gingras M. C., Bélanger L. Enhancer and promoter elements directing activation and glucocorticoid repression of the alpha 1-fetoprotein gene in hepatocytes. Mol Cell Biol. 1988 Apr;8(4):1398–1407. doi: 10.1128/mcb.8.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hammer R. E., Krumlauf R., Camper S. A., Brinster R. L., Tilghman S. M. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science. 1987 Jan 2;235(4784):53–58. doi: 10.1126/science.2432657. [DOI] [PubMed] [Google Scholar]
- Izban M. G., Papaconstantinou J. Cell-specific expression of mouse albumin promoter. Evidence for cell-specific DNA elements within the proximal promoter region and cis-acting DNA elements upstream of -160. J Biol Chem. 1989 Jun 5;264(16):9171–9179. [PubMed] [Google Scholar]
- Kahn C. R., Bertolotti R., Ninio M., Weiss M. C. Short-lived cytoplasmic regulators of gene expression in cell cybrids. Nature. 1981 Apr 23;290(5808):717–720. doi: 10.1038/290717a0. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Mercola M., Goverman J., Mirell C., Calame K. Immunoglobulin heavy-chain enhancer requires one or more tissue-specific factors. Science. 1985 Jan 18;227(4684):266–270. doi: 10.1126/science.3917575. [DOI] [PubMed] [Google Scholar]
- Molné M., Houart C., Szpirer J., Szpirer C. Combinatorial control of positive and negative, upstream and intragenic regulatory DNA domains of the mouse alpha 1-foetoprotein gene. Nucleic Acids Res. 1989 May 11;17(9):3447–3457. doi: 10.1093/nar/17.9.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muglia L., Rothman-Denes L. B. Cell type-specific negative regulatory element in the control region of the rat alpha-fetoprotein gene. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7653–7657. doi: 10.1073/pnas.83.20.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Nir U., Fodor E., Rutter W. J. Capturing nuclear sequence-specific DNA-binding proteins by using simian virus 40-derived minichromosomes. Mol Cell Biol. 1988 Feb;8(2):982–987. doi: 10.1128/mcb.8.2.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir U., Walker M. D., Rutter W. J. Regulation of rat insulin 1 gene expression: evidence for negative regulation in nonpancreatic cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3180–3184. doi: 10.1073/pnas.83.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paonessa G., Gounari F., Frank R., Cortese R. Purification of a NF1-like DNA-binding protein from rat liver and cloning of the corresponding cDNA. EMBO J. 1988 Oct;7(10):3115–3123. doi: 10.1002/j.1460-2075.1988.tb03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C., Levilliers J., Ott M. O., Weiss M. C. Tissue-specific expression of the rat albumin gene: genetic control of its extinction in microcell hybrids. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2561–2565. doi: 10.1073/pnas.83.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M., Morinaga T., Urano Y., Watanabe K., Wegmann T. G., Tamaoki T. The human alpha-fetoprotein gene. Sequence organization and the 5' flanking region. J Biol Chem. 1985 Apr 25;260(8):5055–5060. [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Tilghman S. M. Transient expression of a mouse alpha-fetoprotein minigene: deletion analyses of promoter function. Mol Cell Biol. 1983 Jul;3(7):1295–1309. doi: 10.1128/mcb.3.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpirer J., Szpirer C. The control of serum protein synthesis in hepatoma-fibroblast hybrids. Cell. 1975 Sep;6(1):53–60. doi: 10.1016/0092-8674(75)90073-2. [DOI] [PubMed] [Google Scholar]
- Takada S., Obinata M. Characterization of trans-acting factor(s) regulating beta-globin gene expression by in vivo competition. Cell Differ. 1987 Jul;21(2):111–118. doi: 10.1016/0045-6039(87)90418-0. [DOI] [PubMed] [Google Scholar]
- Wang X. F., Calame K. SV40 enhancer-binding factors are required at the establishment but not the maintenance step of enhancer-dependent transcriptional activation. Cell. 1986 Oct 24;47(2):241–247. doi: 10.1016/0092-8674(86)90446-0. [DOI] [PubMed] [Google Scholar]
- Widen S. G., Papaconstantinou J. Extinction of alpha-fetoprotein gene expression in somatic cell hybrids involves cis-acting DNA elements. Mol Cell Biol. 1987 Jul;7(7):2606–2609. doi: 10.1128/mcb.7.7.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widen S. G., Papaconstantinou J. Liver-specific expression of the mouse alpha-fetoprotein gene is mediated by cis-acting DNA elements. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8196–8200. doi: 10.1073/pnas.83.21.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Ferrandon D., Rosales R., Vigneron M., Macchi M., Ruffenach F., Chambon P. One cell-specific and three ubiquitous nuclear proteins bind in vitro to overlapping motifs in the domain B1 of the SV40 enhancer. EMBO J. 1987 Oct;6(10):3005–3013. doi: 10.1002/j.1460-2075.1987.tb02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Macchi M., Rosales R., Vigneron M., Staub A., Chambon P. In vitro binding of several cell-specific and ubiquitous nuclear proteins to the GT-I motif of the SV40 enhancer. Genes Dev. 1987 Oct;1(8):794–807. doi: 10.1101/gad.1.8.794. [DOI] [PubMed] [Google Scholar]
- Yu H., Porton B., Shen L. Y., Eckhardt L. A. Role of the octamer motif in hybrid cell extinction of immunoglobulin gene expression: extinction is dominant in a two enhancer system. Cell. 1989 Aug 11;58(3):441–448. doi: 10.1016/0092-8674(89)90425-x. [DOI] [PubMed] [Google Scholar]
- Zhang D. E., Hoyt P. R., Papaconstantinou J. Localization of DNA protein-binding sites in the proximal and distal promoter regions of the mouse alpha-fetoprotein gene. J Biol Chem. 1990 Feb 25;265(6):3382–3391. [PubMed] [Google Scholar]