Abstract
Rationale: Pulmonary arterial hypertension (PAH) is a proliferative arteriopathy associated with glucose transporter-1 (Glut1) up-regulation and a glycolytic shift in lung metabolism. Glycolytic metabolism can be detected with the positron emission tomography (PET) tracer 18F-fluorodeoxyglucose (FDG).
Objectives: The precise cell type in which glycolytic abnormalities occur in PAH is unknown. Moreover, whether FDG-PET is sufficiently sensitive to monitor PAH progression and detect therapeutic regression is untested. We hypothesized that increased lung FDG-PET reflects enhanced glycolysis in vascular cells and is reversible in response to effective therapies.
Methods: PAH was induced in Sprague-Dawley rats by monocrotaline or chronic hypoxia (10% oxygen) in combination with Sugen 5416. Monocrotaline rats were treated with oral dichloroacetate or daily imatinib injections. FDG-PET scans and pulmonary artery acceleration times were obtained weekly. The origin of the PET signal was assessed by laser capture microdissection of airway versus vascular tissue. Metabolism was measured in pulmonary artery smooth muscle cell (PASMC) cultures, using a Seahorse extracellular flux analyzer.
Measurements and Main Results: Lung FDG increases 1–2 weeks after monocrotaline (when PAH is mild) and is normalized by dichloroacetate and imatinib, which both also regress medial hypertrophy. Glut1 mRNA is up-regulated in both endothelium and PASMCs, but not airway cells or macrophages. PASMCs from monocrotaline rats are hyperproliferative and display normoxic activation of hypoxia-inducible factor-1α (HIF-1α), which underlies their glycolytic phenotype.
Conclusions: HIF-1α-mediated Glut1 up-regulation in proliferating vascular cells in PAH accounts for increased lung FDG-PET uptake. FDG-PET is sensitive to mild PAH and can monitor therapeutic changes in the vasculature.
Keywords: hypoxia-inducible factor-1α (HIF-1α), glucose transporter-1 (Glut1), glycolysis, imatinib, Sugen 5416
At a Glance Commentary
Scientific Knowledge on the Subject
The current study was inspired by a report that identified increased 18F-fluorodeoxyglucose (FDG) uptake in the lungs of patients with idiopathic pulmonary arterial hypertension (PAH) and suggested this technique could have utility to monitor disease progression. However, it is unclear whether FDG-positron emission tomography (PET) could detect mild PAH or monitor disease regression with therapy. Moreover, the molecular pathway leading to enhanced glycolysis is largely unstudied.
What This Study Adds to the Field
We describe the use of the PET tracer FDG to monitor progression and regression of pulmonary vascular disease in two experimental models of PAH: monocrotaline and SU5416/hypoxia. The FDG signal increases early in the disease, when PAH is mild, and is reduced by effective therapies (dichloroacetate and imatinib), suggesting that lung FDG-PET could be used to noninvasively diagnose and monitor therapy in patients with PAH. We demonstrate that the FDG-PET signal originates in the vasculature from proliferating glycolytic smooth muscle cells and endothelium.
Pulmonary arterial hypertension (PAH) is a syndrome in which the mean pulmonary artery pressure exceeds 25 mm Hg because of obstruction and constriction of precapillary pulmonary resistance arteries. An important aspect of the pathophysiology of PAH is increased proliferation and reduced apoptosis of pulmonary arterial endothelial and smooth muscle cells (PASMCs) (1–8), leading to plexiform lesions and increased muscularization, respectively.
Publications describe impaired glucose oxidation and/or a shift to glycolytic metabolism in PASMCs in rodents with experimental PAH (9), in endothelial cells of patients with PAH (10, 11), and in the pulmonary arteries of rats (12) and mice (13) exposed to chronic hypoxia. Aerobic glycolysis is also known as the Warburg effect and was shown to confer a growth advantage to proliferating cells (14). A crucial determinant of whether cells generate energy through glycolysis or aerobic mitochondrial respiration is the activity of pyruvate dehydrogenase complex (PDH). PDH catalyzes the conversion of pyruvate into acetyl coenzyme A, which is taken up in the Krebs cycle where it fuels mitochondrial oxidative metabolism. In PAH, pathological activation of pyruvate dehydrogenase kinases (PDKs) leads to phosphorylation and inhibition of PDH (9). Two isoforms, PDK1 and PDK3, are transcriptionally up-regulated by hypoxia-inducible factor (HIF)-1α (15, 16), a transcription factor that is activated in the lungs of individuals with PAH (9, 17, 18) and in experimental PAH (9). The pathological importance of the lung's glycolytic shift in PAH is supported by the observation that the PDK inhibitor dichloroacetate (DCA), which restores oxidative phosphorylation in glycolytic tissue (19), regresses many forms of pulmonary hypertension, including chronic hypoxic pulmonary hypertension (12) and experimental PAH in monocrotaline rats (20), fawn hooded rats (9), and mice overexpressing the serotonin transporter in PASMCs (21).
The glycolytic shift in PAH is not only mechanistically relevant but suggests a potential diagnostic strategy to monitor the lung vasculature. 18F-labeled deoxyglucose (FDG) is a glucose analog that is used as a radiotracer for positron emission tomography (PET). FDG is taken up in cells via the glucose transporter-1 (Glut1) and accumulates because of the lack of a 2′-hydroxyl group, which prevents further metabolism after phosphorylation. FDG uptake is increased in glycolytic cancer cells, which allows the diagnosis of cancer and the noninvasive monitoring of therapy by PET (22). Increased right ventricular FDG uptake has also been described in patients with PAH (23) and rats (24), reflecting a shift from oxidative toward glycolytic metabolism (24). This right ventricular FDG-PET signal is reduced by therapies that decrease pressure overload (such as prostaglandins [23]) or enhance glucose oxidation (such as DCA [24]).
The current study was inspired by a report by Xu and colleagues, which identified increased FDG uptake in the lungs of patients with idiopathic PAH and suggested this technique could be used to monitor disease progression (11). However, in this important observational study it was unclear whether FDG-PET could detect mild PAH or monitor disease regression with therapy. Moreover, although Xu and colleagues found evidence of enhanced glycolysis in endothelial cells, the precise tissue (airway vs. pulmonary artery) and cellular origins (PASMCs [9] versus endothelium [11]) of the FDG-PET signal was unclear. We address these questions by using a well-established rat PAH model, induced by injection of monocrotaline (MCT) (25, 26). This model has the advantage of allowing serial observations of disease progression and regression and permits correlation of FDG-PET with histology, hemodynamics, and measurement of cell metabolism. In addition, we studied the Sugen 5416 (SU5416)/hypoxia model (27) to evaluate whether our findings in the MCT model can be extrapolated to this model as well.
Using PET, we found that lung FDG uptake increases with the earliest onset of PAH and decreases with DCA or imatinib therapy. Increased lung FDG is associated with activation of HIF-1α and up-regulation of glycolytic genes in the lung vasculature, not the airways or macrophages. The glycolytic signal detected by PET appears to originate from HIF-1α– and proliferating cell nuclear antigen (PCNA)–positive cells in the pulmonary arterial wall. Increased glycolysis persists in cultures of PASMCs from MCT rats and is associated with increased lactate production and up-regulation of PDK3. FDG-PET may be useful in diagnosing and monitoring PAH as it reflects the metabolic state in small pulmonary arteries.
Methods
For details on Methods, see the online supplement.
Animal Studies
The University of Chicago (Chicago, IL) Institutional Animal Care and Use Committee approved all experimental protocols. Pulmonary hypertension was induced in Sprague-Dawley rats (Charles River Laboratories, Chicago, IL).
Monocrotaline Model
Rats received an intraperitoneal injection of MCT (60 mg/kg; Sigma-Aldrich, St. Louis, MO). Seven rats were imaged weekly by Doppler echocardiography and PET. Therapies consisted of drinking water containing DCA (0.75 g/L; Sigma-Aldrich) from the time of the MCT injection (n = 10 rats) or imatinib (50 mg/kg/d; Enzo Life Sciences, Farmingdale, NY) starting 10 days after MCT injection (n = 6 rats).
SU5416 Plus Hypoxia Model
Rats received a single subcutaneous injection of SU5416 (20 mg/kg) and were exposed to normobaric hypoxia for 3 weeks (10% oxygen). Lung FDG uptake was measured in rats with confirmed pulmonary hypertension (i.e., pulmonary artery acceleration time [PAAT] < 20 ms; n = 6).
FDG-PET and Computed Tomography
Pulmonary glucose uptake was measured by PET after FDG injection (240 μCi; IBA, Romeoville, IL) as previously described (24). Organ localization was improved by augmenting three-dimensional (3D) PET images with 3D micro-computed tomography (CT) data using a FLEX Triumph trimodality imaging system (Gamma Medica Ideas, Northridge, CA). CT data were acquired and 30 minutes after FDG injection, a 30-minute PET acquisition was started. FDG uptake statistics were computed from the registered PET data volume using Amira 5.2 (Visage Imaging, San Diego, CA). Averaged FDG uptake was normalized to body weight, injected dose, and radioactive decay.
Oxygen Consumption and Metabolic Measurements
Extracellular acidification (which is proportional to lactate production) and oxygen consumption were measured with an XF24 extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA). Fifty thousand PASMCs were plated per well of an XF24 microplate and used for measurements the next day in XF assay medium supplemented with glucose (4.5 g/L).
Laser Capture Microdissection
Cryosections were stained with an Arcturus HistoGene frozen section staining kit (Applied Biosystems, Carlsbad, CA). At least 20 small (<100 μm) pulmonary arteries were collected with a Palm MicroBeam system (Carl Zeiss, Thornwood, NY) and RNA was isolated with a PicoPure RNA isolation kit (Applied Biosystems).
The online supplement contains expanded animal protocols, PET-FDG, and laser capture microscopy methodology. In addition, it contains methods for hemodynamic measurements, rat PASMC culture, RNA isolation, qRT-PCR, lung histology analysis, and PDH activity, all of which were performed as previously described (17, 28).
Statistics
Values are stated as means ± SEM. When applicable, normality was confirmed by Kolmogorov-Smirnov test. Intergroup differences were assessed by unpaired Student t test, and analysis of variance (ANOVA) with Tukey post hoc analysis was used for comparing multiple groups. Serial echocardiography and PET measurements were compared with baseline, using a repeated-measure ANOVA with post hoc Dunnett comparison analysis. Correlation was tested by linear regression analysis. Both the P value indicating whether the slope is significantly different from zero and the r2 value were calculated. A P value less than 0.05 was considered statistically significant. Statistical significance is indicated in the figures as follows: *P < 0.05; **P < 0.01; and ***P < 0.001. All statistical analyses were performed with Prism 5.0a (GraphPad Software, La Jolla, CA).
Results
FDG Uptake Correlates with Disease Progression
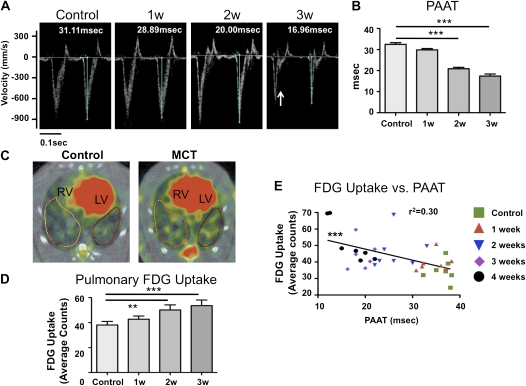
Rats were imaged weekly after MCT injection, using echocardiography and FDG-PET. Serial Doppler measurements of PAAT, a parameter that varies inversely with mean pulmonary artery pressure, showed no change until 1 week after MCT injection, whereupon there was a weekly shortening of PAAT, consistent with evolving PAH (Figures 1A and 1B). This change became significantly different from the baseline measurement after 2 weeks. The greatest decrease in PAAT occurred between Week 1 and Week 2, with smaller decreases thereafter. Systolic notching of the pulmonary artery Doppler envelope, a well-established indicator of severe pulmonary hypertension (28), was observed after 3 weeks (Figure 1A).
Figure 1.
Time course of pulmonary arterial hypertension disease progression after monocrotaline (MCT) administration. (A) Pulmonary arterial acceleration time was measured by pulsed-wave Doppler echocardiography. Measurements were made before MCT injection and weekly thereafter. The arrow at the 3-week time point indicates systolic notching of the pulmonary artery Doppler envelope, typical of severe pulmonary hypertension. (B) Pulmonary artery acceleration time (PAAT) is inversely related to the mean pulmonary artery pressure and decreases during the development of pulmonary hypertension. Starting from Week 2, a significant reduction in PAAT is observed versus baseline measurements. (C) Representative positron emission tomography (PET) scans. Note the increased 18F-fluorodeoxyglucose (FDG) uptake in the right ventricle (RV) and the lung parenchyma of MCT animals. LV = left ventricle. (D) Quantification of pulmonary FDG uptake measured with PET. Starting from Week 2, significantly higher lung FDG uptake was observed. (E) Correlation analysis demonstrates the inverse relationship between PAAT and FDG uptake. Seven rats were imaged at each time point.
Serial FDG-PET scans reveal progressively increasing FDG uptake in the lungs of MCT animals, which exceeded control levels within 2 weeks of MCT injection (Figures 1C and 1D; and see Figure E1 in the online supplement). Thus evidence of a metabolic shift in lung metabolism occurred simultaneously with onset of pulmonary hypertension (beginning between 1 and 2 wk after MCT). Figure 1E shows that there is a significant correlation between FDG uptake and PAAT measurements (r2 = 0.3, P < 0.001).
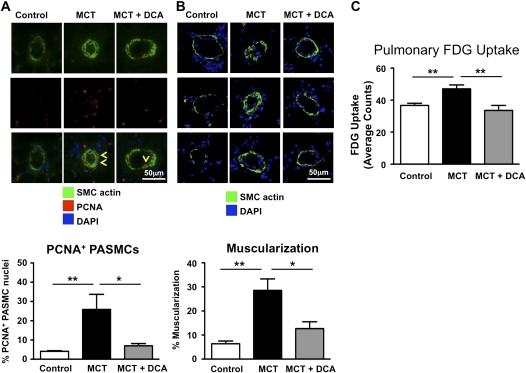
DCA Reverses Increased Muscularization and PET Imaging Is Able to Detect Therapeutic Benefits
We observed increased PASMC proliferation in MCT PAH, and this was prevented by DCA therapy, evident as a smaller percentage of PASMCs in the small precapillary resistance vessels stained for the proliferation marker PCNA (Figure 2A). In agreement, pulmonary artery muscularization decreased after DCA treatment (Figure 2B). Concordant with the regression of vascular disease and reduction in proliferation, PET measurements demonstrated a significant reduction in pulmonary FDG uptake in response to DCA treatment of MCT animals (Figure 2C).
Figure 2.
Dichloroacetate (DCA) treatment decreased pulmonary artery smooth muscle cell (PASMC) proliferation and pulmonary artery muscularization and reduced 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) signal intensity. (A) To measure proliferation in vivo, we stained lung sections with smooth muscle cell actin, to identify PASMCs, and proliferating cell nuclear antigen (PCNA), to identify proliferating cells. Yellow arrowheads point at PCNA-positive PASMCs. For each of the 3 groups, we analyzed approximately 400 PASMC nuclei. (B) Muscularization of small pulmonary arteries (diameter < 100 μm) is increased after monocrotaline (MCT) treatment. DCA treatment reverses this muscularization. For each of the 3 groups, we measured approximately 80–100 blood vessels. (C) Treatment of MCT animals with dichloroacetate (DCA) can clearly be identified by PET imaging, demonstrating the usefulness of PET imaging to monitor therapeutic efficacy (at least 10 animals were imaged per group).
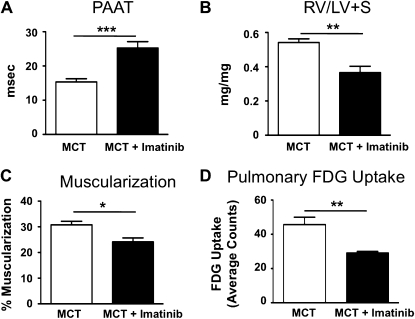
PET Imaging Is Able to Detect Therapeutic Benefits of Imatinib Treatment
Because DCA directly affects metabolism by inhibiting PDK, thereby increasing pyruvate uptake into the Krebs cycle and augmenting glucose oxidation, we also wanted to assess the effects of a nonmetabolic therapy on FDG-PET. We used imatinib mesylate, a tyrosine kinase inhibitor, which inactivates platelet-derived growth factor receptor β and can be used to treat MCT-induced pulmonary hypertension (29). We confirm improved PAAT and decreased right ventricular fractional weight and pulmonary artery muscularization in animals treated with imatinib (Figures 3A–3C). Moreover, pulmonary FDG uptake was markedly lower after imatinib treatment (Figure 3D), confirming that FDG uptake reflects the severity of pulmonary vascular remodeling.
Figure 3.
Efficacy of imatinib treatment correlates with 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) signal intensity. (A) Ten days after monocrotaline (MCT) injection, rats were randomized to daily imatinib treatment (50 mg/kg/d) or saline injections. After a total of 3 weeks, echocardiography confirmed a low pulmonary artery acceleration time (PAAT) in the untreated MCT animals, indicative of the development of pulmonary hypertension. Imatinib treatment significantly increases PAAT (n = 6 animals per group). (B and C) Right ventricular fractional weight (RV/LV+S; i.e., right ventricular weight to left ventricular plus septum weight) and the degree of muscularization of the small pulmonary arteries (<100 μm) were lowered by imatinib treatment. (D) Pulmonary FDG uptake is significantly lower in rats treated with imatinib (n = 6 animals per group), indicating that FDG uptake can also be used to monitor therapeutic efficacy of nonmetabolic drugs.
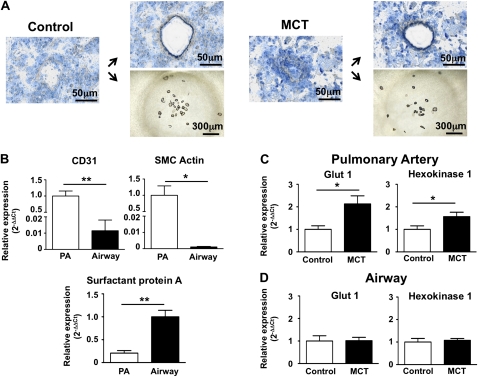
Increased Glycolytic Gene Expression in Small Pulmonary Arteries
To identify the tissue origin of the increased pulmonary FDG signal, laser capture microscopy was used to specifically collect pulmonary arteries or airway tissue (Figure 4A). CD31 or SMC actin (vascular markers) versus surfactant protein A, (airway marker) were used to confirm the relative purity of vascular and airway acquisitions. Consistent with effective tissue dissection, CD31 and SMC actin expression were high in vascular specimens and almost absent in airway tissue; conversely, airway samples were enriched in surfactant protein A compared with blood vessels (Figure 4B). In MCT pulmonary arteries there was significant up-regulation of Glut1, which mediates FDG uptake, and hexokinase-1, which phosphorylates glucose, thereby retaining it within the cell (Figure 4C). These markers of increased glycolysis were not up-regulated in airway tissues (Figure 4D).
Figure 4.
Laser capture microscopy (LCM) confirms the vascular origin of the glycolytic signal in the lungs of monocrotaline (MCT) rats. (A) Small pulmonary precapillary resistance vessels (<100 μm in diameter) or pieces of airway tissue were collected by LCM. (B) Gene expression analysis of vascular and airway preparations. Both the endothelial marker CD31 and the smooth muscle cell (SMC) marker SMC actin were almost absent in airway tissue. Conversely, airway tissue is enriched in surfactant protein A compared with blood vessels. (C) Compared with pulmonary blood vessels derived from control animals, MCT blood vessels contain a higher expression of glucose transporter-1 (Glut1) and hexokinase-1 (n = 6–9 animals per group). (D) No changes in glycolytic genes are observed in airway tissue (n = 6–9 animals per group).
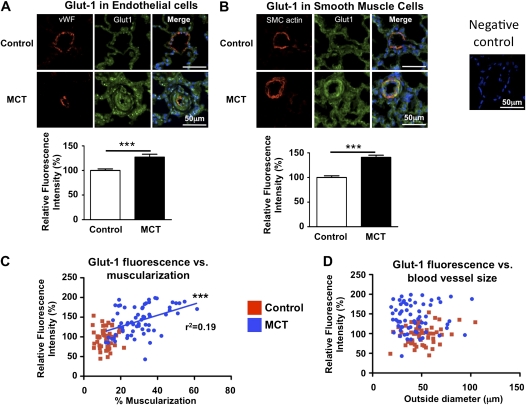
To confirm these findings at the protein level and to determine the cell type(s) within the pulmonary artery in which Glut1 was up-regulated, we used immunohistochemistry for von Willebrand factor (endothelial cells) and smooth muscle cell actin in combination with Glut1 to measure Glut1 staining intensity specifically in endothelial and smooth muscle cells, respectively. Glut1 intensity increased 27 ± 6% in endothelial cells (Figure 5A) and 41 ± 4% in smooth muscle cells (Figure 5B). Correlation analysis between Glut1 intensity and degree of muscularization (Figure 5C) or blood vessel size (Figure 5D) demonstrates that only the degree of remodeling/muscularization is strongly correlated with Glut1 staining intensity (i.e., those vessels with the most medial hypertrophy had the greatest Glut1 intensity).
Figure 5.
Confocal microscopy confirms up-regulation of glucose transporter-1 (Glut1) in both pulmonary artery smooth muscle cells (PASMCs) and endothelium. (A) Small precapillary pulmonary blood vessels (diameter < 100 μm) were identified by von Willebrand factor (vWF) staining and Glut1 intensity was measured specifically in the regions that were stained with vWF (n > 40 blood vessels for each group). (B) Smooth muscle cell–specific measurements of Glut1 intensity in pulmonary blood vessels (n > 55 for each group). (C) Correlation analysis of blood vessel muscularization and Glut1 demonstrates that there is a significant correlation between severity of disease (increased muscularization) and Glut1 intensity in the monocrotaline (MCT) animals. (D) Correlation analysis of blood vessel size (outer diameter) and Glut1 intensity demonstrates that the size of the pulmonary blood vessels does not correlate with Glut1 intensity.
Inflammatory Cells Are Not Up-regulating Glut1 Expression and Pulmonary FDG Uptake Remains Elevated in the Absence of Inflammatory Cells
The MCT model has an important inflammatory component (e.g., see Figure E2D) and inflammation might contribute to increased pulmonary FDG uptake. Therefore, we isolated pulmonary macrophages by bronchoalveolar lavage and both RNA expression analysis (Figure E2A) and immunohistochemistry (Figure E2B) did not show up-regulation of Glut1 in isolated macrophages. In support of this, immunohistochemistry of lung sections did not show increased Glut1 staining intensity of inflammatory cells (Figure E2C). Together, these data indicate that there is no glycolytic shift in pulmonary macrophages. Nevertheless, increased abundance of inflammatory cells in vivo might contribute to increased FDG uptake. We addressed this by measuring FDG uptake in MCT rats before and after intravenous injection of GdCl3 (10 mg/kg). GdCl3 accumulates and induces apoptosis in phagocytic cells and therefore an intravenous injection selectively removes circulating monocytic cells (30). Our results confirm that after 4 days, this results in a significant decrease in pulmonary macrophages from 11.5 ± 0.6 macrophages per high-power field in untreated MCT rats to 1.3 ± 0.3 macrophages per high-power field in the lungs of MCT rats treated with GdCl3 (P < 0.001; Figure E2D). In the absence of inflammatory cells, FDG uptake remains unchanged (Figure E2E).
Glycolytic Switch in the SU5416/Hypoxia Model
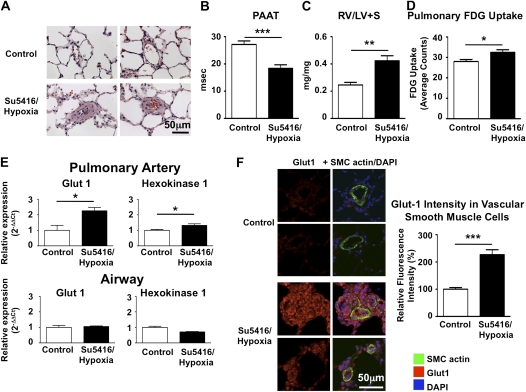
The MCT model allows one to study excessive PASMC proliferation, but the lack of plexiform lesions demonstrate that the MCT model is not recapitulating the entire human disease pathology. Therefore, we repeated part of our experiments in the SU5416/chronic hypoxia model (27). This model leads to vascular remodeling and in some instances even obstruction of small precapillary resistance vessels (Figure 6A). The severity of disease was confirmed by echocardiography and postmortem weighing of the right ventricle (Figures 6B and 6C). Pulmonary FDG uptake was significantly increased (Figure 6D) after injection of SU5416 plus hypoxia. Laser capture microscopy shows a clear induction of Glut1 and hexokinase-1 in the small pulmonary arteries, but not airway tissue (Figure 6E). Immunostaining of pulmonary lung sections confirms Glut1 up-regulation in pulmonary arteries, especially in blood vessels containing plexiform lesions (Figure 6F).
Figure 6.
Glycolytic shift in the vasculature of rats with pulmonary hypertension due to SU5416 plus hypoxia. (A) Hematoxylin and eosin staining of lung sections of control rats and rats treated with a single subcutaneous injection of SU5416 followed by 3 weeks of hypoxia (10% oxygen). Remodeled small pulmonary arteries can be observed throughout the lungs. (B and C) Pulmonary artery acceleration time (PAAT) was decreased and right ventricular fractional weight (RV/LV+S; i.e., right ventricular weight to left ventricular plus septum weight) increased in SU5416/hypoxia rats. (D) Pulmonary 18F-fluorodeoxyglucose (FDG) uptake was increased in rats with SU5416/hypoxia-induced pulmonary hypertension (n = 4 controls and 6 SU5416/hypoxia rats). (E) Laser capture microscopy reveals up-regulation of glucose transporter-1 (Glut1) and hexokinase-1 in small pulmonary arteries (diameter < 100 μm), but not in airway tissue. (F) Increased Glut1 immunostaining intensity in the remodeled pulmonary arteries of SU5416/hypoxia rats. Sections were costained for Glut1 (red) and smooth muscle cell actin (green). For each group, 5 animals were studied, with 20 blood vessels measured per animal. DAPI = 4′,6-diamidino-2-phenylindole.
Isolated PASMCs Maintain Their Glycolytic Phenotype In Vitro
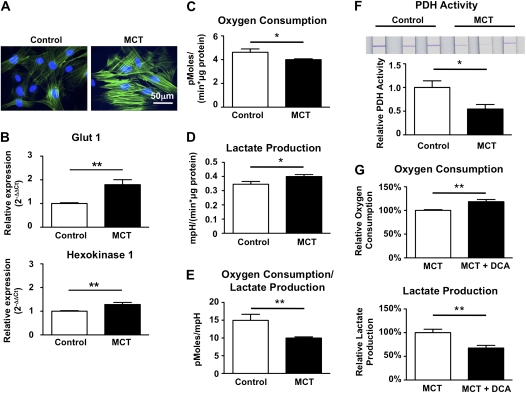
PASMCs were cultured from control or MCT animals and cells were characterized at an early passage (passage 2 or 3). SMC actin staining was used to confirm the smooth muscle cell phenotype of the cells in culture (Figure 7A).
Figure 7.
Isolated pulmonary arterial hypertension (PAH) pulmonary artery smooth muscle cells (PASMCs) retain a glycolytic metabolic pattern. (A) PASMCs were freshly cultured from explants and smooth muscle cell actin staining was used to confirm the smooth muscle cell phenotype of the cultured cells. (B) Gene expression was analyzed at passage 2. Compared with control PASMCs, PASMCs from monocrotaline (MCT) animals have higher expression levels of glucose transporter-1 (Glut1) and hexokinase-1 (n = 7 cell lines per group). (C) The oxygen consumption rate (OCR) was significantly different between PASMCs isolated from control animals and animals 3 weeks after MCT injection. Values are normalized to protein concentrations in the respective wells. (D) Increased lactate production in MCT PASMCs. mpH = milli-pH units. (E) The ratio of OCR to lactate production is significantly decreased in MCT animals. (F) Pyruvate dehydrogenase activity is decreased in isolated PASMCs derived from MCT animals compared with control PASMCs (n = 7 cell lines per group). (G) Overnight treatment with 10 mM dichloroacetate (DCA) significantly increases oxygen consumption and decreases lactate production in MCT PASMCs (10 independent wells per group).
PASMCs maintain their glycolytic gene expression profile in vitro, evident in persistent up-regulation of both Glut1 and hexokinase-1 (Figure 7B). There was a reduction in oxygen consumption rates in MCT PASMCs (Figure 7C) and increased lactate production in MCT versus control PASMCs (Figure 7D). Thus the ratio of oxygen consumption to lactate production was decreased in MCT PAH (Figure 7E). We confirmed that nonmitochondrial oxygen consumption, measured after inhibition of complex III with antimycin A, is not different between both groups (data not shown).
Cells with oxidative metabolism use PDH to convert pyruvate into acetyl coenzyme A to fuel the mitochondrial Krebs cycle, whereas glycolytic cells convert pyruvate to lactate. PDH activity is decreased in PASMCs from MCT animals (Figure 7F; n = 7 cell lines per group), which likely explains the increased lactate production. A similar reduction in PDH activity was observed in total lung tissue (Figure E3). PDH is inactivated after phosphorylation by pyruvate dehydrogenase kinases (PDKs). As shown in Figure 7G, overnight treatment with a 10 mM concentration of the PDK inhibitor DCA significantly increases oxygen consumption and decreases lactate production in MCT PASMCs.
Activation of HIF-1α in Pulmonary Vascular Cells
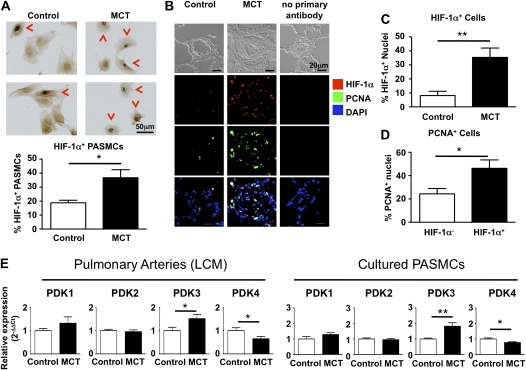
To assess the mechanism for the glycolytic shift in MCT PASMCs, we measured expression of activated HIF-1α (defined as HIF-1α localized within the nucleus). The percentage of PASMCs stained positive for nuclear HIF-1α was roughly twice as high in MCT versus control PASMCs (Figure 8A). To confirm that HIF-1α activation also occurs in vivo, we analyzed lung tissue sections for HIF-1α positivity and found that pulmonary arteries from MCT animals have a large number of HIF-1α positive cells in the intima and media (Figures 8B and 8C). We combined this staining with PCNA to determine whether HIF-1α activation is associated with higher proliferation rates. Because blood vessels in the control animals have a low amount of cells with HIF-1α activation or PCNA positivity, we calculated the percentage of proliferating cells (PCNA positive) for the HIF-1α–negative and –positive subpopulations present in pulmonary arteries of the MCT animals. There is a significant enrichment of proliferating cells in the HIF-1α positive cells (Figure 8D). Finally, we investigated whether the glycolytic shift in MCT PASMCs is due to up-regulation of any of the four known PDK isoforms. Interestingly, we observed significant up-regulation of PDK3 both in small pulmonary arteries isolated by laser capture microscopy and in cultured PASMCs.
Figure 8.
Hypoxia-inducible factor-1α (HIF-1α) is activated and pyruvate dehydrogenase kinase-3 (PDK3) is up-regulated in isolated pulmonary artery smooth muscle cells (PASMCs) and pulmonary arteries from monocrotaline (MCT) animals. (A) Immunohistochemistry for HIF-1α on isolated PASMCs. Nuclear accumulation of HIF-1α is indicated by red arrowheads and indicates HIF-1α activation (n = 5 different cell lines for each, with six high-power fields counted per cell line). A higher proportion of PASMCs derived from MCT animals have nuclear localization of HIF-1α. (B) Lung sections were stained for HIF-1α and proliferating cell nuclear antigen (PCNA). Note the large number of HIF-1α– and PCNA-positive nuclei in a pulmonary artery of an MCT animal. DAPI = 4′,6-diamidino-2-phenylindole. (C) Quantification of the percentage of HIF-1α–positive nuclei present in the intima or media of pulmonary arteries. (D) HIF-1α–positive nuclei are more likely to be proliferating. (E) To identify how increased glycolysis is established, we measured the expression levels of the four known pyruvate dehydrogenase kinase (PDK) isoforms and found a significant up-regulation of PDK3 in both pulmonary arteries isolated with laser capture microscopy (n = 10 lungs per group) and in cultured PASMCs (seven cell lines per group).
Discussion
This study offers five new discoveries that clarify the timing, reversibility, and cellular origins/mechanisms of the glycolytic shift that has been recognized in human and experimental PAH. First, we clarify the temporal relationship between increased lung FDG-PET and onset of PAH, showing that both occur virtually simultaneously (1–2 wk after MCT; Figure 1). Second, we provide the first evidence that PET imaging of increased pulmonary FDG uptake is sufficiently sensitive to allow both early detection of PAH and monitoring of disease regression in response to DCA or direct inhibition of platelet-derived growth factor signaling with imatinib. The fall in lung FDG-PET signal is associated with decreased muscularization of the pulmonary arteries (Figures 2 and 3). Third, we used laser capture microscopy and immunohistochemistry to demonstrate that increased pulmonary FDG uptake reflects increased Glut1 expression in both pulmonary artery endothelial and smooth muscle cells in vivo (Figures 4 and 5). A similar observation was made in the SU5416/hypoxia model (Figure 6). Furthermore, analysis of inflammatory cells collected by bronchoalveolar lavage shows no glycolytic shift in these cells and pulmonary macrophage depletion does not decrease pulmonary FDG uptake, suggesting that pulmonary FDG uptake is independent of inflammation (Figure E2). Fourth, we used the Seahorse extracellular flux analyzer to validate that the increased FDG seen in vivo was actually the result of increased glycolysis, which persisted in cultured MCT PASMCs (Figure 7). Moreover, PDH activity was reduced in PASMCs from MCT animals and PDH activation with DCA decreases lactate formation (Figure 7G), confirming that PDH is not fully active in MCT PASMCs. Finally, we show that activation of HIF-1α occurs in MCT PAH (Figure 8), which is associated with increased vascular cell proliferation, consistent with prior findings in chronic hypoxic pulmonary hypertension (31) and PAH in fawn hooded rats (9) and humans (9, 17, 18). We also observed a significant up-regulation of the HIF-1α−inducible PDK3 isoform in small pulmonary arteries and in cultured PASMCs from MCT animals, suggesting a molecular connection between HIF-1α activation and increased glycolysis.
These discoveries suggest that monitoring lung FDG uptake allows noninvasive assessment of the status of the pulmonary vasculature in PAH and reflects the metabolic, proliferative diathesis of endothelial and smooth muscle cells. Increased lung FDG uptake has been noted in a small cohort of patients with PAH, and at least some of the signal originates from endothelial cells (11). However, it remained unclear whether the FDG-PET signal is also derived from PASMCs, airway cells, and/or inflammatory cells. The rat monocrotaline model is characterized by the rapid development of pulmonary hypertension within 3–4 weeks. Using Doppler of the pulmonary artery, the first signs of increased pulmonary artery pressures (shortening of the PAAT [28]) can be identified after 2 weeks. FDG imaging demonstrates a parallel rise in FDG uptake and pulmonary artery pressure (evident from shortening PAAT). The ability to detect these changes early in the development of PAH suggests FDG-PET has the potential as a noninvasive test for early diagnosis and monitoring of PAH (Figures 1C and 1D), as previously suggested (11).
Because there is a clear correlation between the expression level of Glut1 and FDG signal intensity in tumors (32), we were interested in studying this transporter in lung sections to determine which cell type is responsible for the increased FDG uptake that we observed. Using immunohistochemistry, we confirmed that Glut1 is up-regulated both in endothelial cells (as has been described previously in patients with PAH [11]) and smooth muscle cells (Figure 5). There is a clear correlation between the degree of muscularization and Glut1 staining intensity, suggesting that the more remodeled blood vessels are responsible for the observed increased FDG uptake (Figure 5C). This finding also indicates the important contribution of PASMCs to the FDG-PET signal in this model of PAH. To prove the general applicability of our findings, we also investigated the SU5416/hypoxia model, which is characterized by endothelial proliferation in addition to PASMC proliferation (27). Laser capture microscopy shows induction of Glut1 in the small pulmonary arteries, and the pulmonary FDG uptake was increased (Figure 6D). In combination, both the MCT and SU5426/hypoxia models share a glycolytic shift in the pulmonary blood vessels that can be detected by PET imaging.
Interestingly, using a Seahorse extracellular flux analyzer, we confirmed that PASMCs isolated from MCT animals maintain their glycolytic/lactate-producing phenotype in vitro (Figures 7D and 7E). Previous publications describe that endothelial cells of patients with pulmonary arterial hypertension also maintain this glycolytic phenotype in vitro (10, 11). Expression levels of Glut1 and hexokinase-1 are up-regulated in early-passage PASMCs derived from MCT animals, which allows these cells to take up and retain a larger amount of glucose (Figure 7B), indicating a glycolytic shift. Indeed, PDH activity, which regulates uptake of pyruvate into the Krebs cycle, is decreased in MCT PASMCs, whereas activation of PDH with DCA leads to increased oxygen consumption and decreased lactate production (Figure 7G).
To establish the basis for this increased glycolysis, we measured HIF-1α activation in PASMCs derived from control and MCT animals, because this transcription factor is known to be pathologically activated in PAH and because HIF-1α regulates the expression of Glut1. We observed that a higher percentage of PASMCs from MCT animals stain positive for HIF-1α (Figure 8A), in agreement with findings in PASMCs from human patients with PAH and spontaneously hypertensive fawn hooded rats (9, 17). Remarkably, this nuclear localization and activation of HIF-1α persist in culture where the Po2 is less than 100 mm Hg. Analysis of lung sections reveals that MCT animals have a larger number of HIF-1α–positive nuclei in vivo (Figures 8B and 8C). Because Glut1 expression is regulated by HIF-1α (33), the increased level of Glut1 is likely explained by the larger number of PASMCs containing activated HIF-1α. Costaining for PCNA reveals that HIF-1α–positive cells are more likely to be proliferating (Figure 8D), consistent with the emerging hypothesis that glycolysis is permissive of proliferation (see review in Reference 34). Two PDK isoforms, PDK1 and PDK3, have been shown to be up-regulated by HIF-1α in lymphoma cells and stromal/cancer cell lines, respectively (15, 16). In our experiments, we observed significant up-regulation of PDK3 in small pulmonary arteries and in cultured PASMCs from MCT animals, suggesting that HIF-1α mediates the glycolytic shift in PASMCs by activating PDK3 (Figure 8E).
Limitations
Because this study focused on the diagnostic utility and cellular origins of the lung FDG-PET signals in PAH, we did not explore the mechanism of HIF-1α activation in this study. However, prior work from our group demonstrates that HIF-1α activation can result from changes in redox state acquired through epigenetic silencing of superoxide dismutase-2 (17). In addition, deficiency of p53 can alter HIF-1α activation and pulmonary arterial remodeling in hypoxic pulmonary hypertension (35). Interestingly, activation of HIF-1α promotes proliferation in PASMCs from fawn hooded rats, a rat strain that spontaneously develops pulmonary hypertension (17), and in the lungs of patients with PAH (9). The importance of glycolytic energy metabolism as a permissive factor for PASMC proliferation was demonstrated in mice lacking malonyl-CoA decarboxylase (13).
Conclusions
We demonstrate that the development of pulmonary hypertension is associated with increased reliance of pulmonary vascular cells (endothelium and PASMCs) on glycolytic energy metabolism, which can be imaged in vivo by PET-FDG imaging. These findings are consistent with assessments of the origins and significance of the FDG-PET signal in cancer as reflecting the activity of hyperproliferative, glycolytic cells (22). PET imaging can detect early changes in the vasculature and holds promise for better characterizing therapeutic efficacy at the cellular level.
Supplementary Material
Acknowledgments
The authors thank James Vosicky for assistance in performing PET imaging.
Footnotes
Supported in part by the Harold Hines Jr. Chair in Medicine (S.L.A.), NIH-RO1-HL071115 (S.L.A.), 1RC1HL099462-01 (S.L.A.), NIH/NCRR S10 RR022520 (C.C.), and the American Heart Association.
Author Contributions: C.W., G.M., C.R.H., P.T.T., J.J.R., E.M., T.T., P.B.W., L.P., J.P., and C.C., data acquisition, analysis, and interpretation; C.W., G.M., and S.L.A., drafting and revising the manuscript; S.L.A., hypothesis generation and funding.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201108-1562OC on January 12, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 2005;115:1479–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res 2001;88:555–562 [DOI] [PubMed] [Google Scholar]
- 3.Thomas M, Docx C, Holmes AM, Beach S, Duggan N, England K, Leblanc C, Lebret C, Schindler F, Raza F, et al. Activin-like kinase 5 (ALK5) mediates abnormal proliferation of vascular smooth muscle cells from patients with familial pulmonary arterial hypertension and is involved in the progression of experimental pulmonary arterial hypertension induced by monocrotaline. Am J Pathol 2009;174:380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouri FM, Queisser MA, Konigshoff M, Chrobak I, Preissner KT, Seeger W, Eickelberg O. Plasminogen activator inhibitor type 1 inhibits smooth muscle cell proliferation in pulmonary arterial hypertension. Int J Biochem Cell Biol 2008;40:1872–1882 [DOI] [PubMed] [Google Scholar]
- 5.Zakrzewicz A, Hecker M, Marsh LM, Kwapiszewska G, Nejman B, Long L, Seeger W, Schermuly RT, Morrell NW, Morty RE, et al. Receptor for activated C-kinase 1, a novel interaction partner of type II bone morphogenetic protein receptor, regulates smooth muscle cell proliferation in pulmonary arterial hypertension. Circulation 2007;115:2957–2968 [DOI] [PubMed] [Google Scholar]
- 6.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest 1998;101:927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, et al. An antiproliferative BMP-2/PPARγ/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 2008;118:1846–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-β1 and bone morphogenetic proteins. Circulation 2001;104:790–795 [DOI] [PubMed] [Google Scholar]
- 9.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, et al. An abnormal mitochondrial–hypoxia inducible factor-1α–Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 2006;113:2630–2641 [DOI] [PubMed] [Google Scholar]
- 10.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, et al. Hypoxia inducible-factor 1α regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 2010;176:1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA 2007;104:1342–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 2002;105:244–250 [DOI] [PubMed] [Google Scholar]
- 13.Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, Dyck JR, Michelakis ED. doi: 10.1126/scitranslmed.3001327. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Translat Med 2010;2:44ra58. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Harry G, et al. A mitochondria–K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007;11:37–51 [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. Hif-1–mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 2006;3:177–185 [DOI] [PubMed] [Google Scholar]
- 16.Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem 2008;283:28106–28114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation 2010;121:2661–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med 2004;169:764–769 [DOI] [PubMed] [Google Scholar]
- 19.Knoechel TR, Tucker AD, Robinson CM, Phillips C, Taylor W, Bungay PJ, Kasten SA, Roche TE, Brown DG. Regulatory roles of the N-terminal domain based on crystal structures of human pyruvate dehydrogenase kinase 2 containing physiological and synthetic ligands. Biochemistry 2006;45:402–415 [DOI] [PubMed] [Google Scholar]
- 20.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 2004;95:830–840 [DOI] [PubMed] [Google Scholar]
- 21.Guignabert C, Tu L, Izikki M, Dewachter L, Zadigue P, Humbert M, Adnot S, Fadel E, Eddahibi S. Dichloroacetate treatment partially regresses established pulmonary hypertension in mice with SM22α-targeted overexpression of the serotonin transporter. FASEB J 2009;23:4135–4147 [DOI] [PubMed] [Google Scholar]
- 22.Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, Joshi U, Semenza GL, Hoekstra OS, Lammertsma AA, et al. Biologic correlates of 18fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 2002;20:379–387 [DOI] [PubMed] [Google Scholar]
- 23.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, Takahashi T, Nawata J, Ido T, Watanabe J, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol 2005;45:1849–1855 [DOI] [PubMed] [Google Scholar]
- 24.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med 2010;88:47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 2009;297:L1013–L1032 [DOI] [PubMed] [Google Scholar]
- 26.Ryan J, Bloch K, Archer SL. Rodent models of pulmonary hypertension: Harmonisation with the World Health Organisation's categorisation of human PH. Int J Clin Pract 2011;65:15–34 [DOI] [PubMed] [Google Scholar]
- 27.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. doi: 10.1096/fj.00-0343com. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death–dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001;15:427–438. [DOI] [PubMed] [Google Scholar]
- 28.Urboniene D, Haber I, Fang YH, Thenappan T, Archer SL. Validation of high-resolution echocardiography and magnetic resonance imaging vs. high-fidelity catheterization in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2010;299:L401–L412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 2005;115:2811–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 2006;168:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 2001;281:L202–L208 [DOI] [PubMed] [Google Scholar]
- 32.Nakajo M, Kajiya Y, Tani A, Yoneda S, Shirahama H, Higashi M. 18FDG PET for grading malignancy in thymic epithelial tumors: significant differences in 18FDG uptake and expression of glucose transporter-1 and hexokinase II between low and high-risk tumors: preliminary study. Eur J Radiol 2012;81:146–151 [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of Glut1 mRNA by hypoxia-inducible factor-1: interaction between h-Ras and hypoxia. J Biol Chem 2001;276:9519–9525 [DOI] [PubMed] [Google Scholar]
- 34.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 2010;121:2045–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizuno S, Bogaard HJ, Kraskauskas D, Alhussaini A, Gomez-Arroyo J, Voelkel NF, Ishizaki T. p53 gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol 2011;300:L753–L761 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.