Abstract
Recent advances in therapy for non–small cell lung carcinoma have shown that a personalized approach to treatment has the potential to significantly reduce lung cancer mortality. Concurrently, endoscopic ultrasound transbronchial needle aspiration has emerged as an accurate and sensitive tool for the diagnosis and staging of this disease. As knowledge of the molecular mechanisms that drive lung cancer progression increases, the amount of information that must be derived from a tumor specimen will also increase. Recent clinical studies have demonstrated that small specimens acquired by endoscopic ultrasound transbronchial needle aspiration are sufficient for molecular testing if specimen acquisition and processing are done with these needs in mind. Optimum use of this procedure requires a coordinated effort between the bronchoscopist and the cytopathologist to collect and triage specimens for diagnostic testing. When feasible, rapid onsite evaluation should be performed to assess the specimen for both diagnostic quality and quantity and to allocate the specimen for cell-block and possible immunohistochemistry and molecular studies. It is necessary for pulmonologists and bronchoscopists to understand the rationale for histologic and molecular testing of lung cancer diagnostic specimens and to ensure that specimens are acquired and processed in a fashion that provides information from small cytologic specimens that is sufficient to guide treatment in this era of targeted therapy.
Keywords: lung cancer, bronchoscopy, cytology, EGFR, molecular testing
Lung cancer remains the leading cause of cancer mortality in the world, with 157,000 deaths expected in the United States in 2010 (1). Despite the large death toll, there are reasons to be cautiously optimistic for a future with fewer lung cancer deaths. Recent advances in clinical and bench research directed toward diagnostics and therapy have led to significant and often dramatic impacts on patient outcomes (2). These developments suggest that a personalized approach to treating lung cancer has the potential to significantly reduce lung cancer mortality. Current standards of care for advanced non–small cell lung carcinoma (NSCLC) treatment assign therapy on the basis of histology and on the basis of epidermal growth factor receptor (EGFR) status for lung adenocarcinoma. This paradigm shift away from homogenous therapy of NSCLC converges with the increased use of bronchoscopic approaches for lung cancer diagnosis and staging, thus enhancing the important role for pulmonary physicians in lung cancer management. It is essential that the bronchoscopist understand the importance of acquiring and processing diagnostic specimens in a manner that provides sufficient information to guide treatment in this era of personalized therapy. This article reviews the rationale for acquiring specific histologic and molecular data from lung cancer biopsies, and recommends multidisciplinary procedures for specimen acquisition and processing that can optimize the yield of pulmonary diagnostic procedures in lung cancer. Some of the results of these studies have been previously reported in abstract form (3).
Histology Dependence of NSCLC
Until recently, all NSCLC patients were treated without regard for histologic subtype. The first data suggesting the importance of histologic dependence were shown in the Phase I–II trials of the vascular endothelial growth factor antibody bevacizumab (4). These results indicated that the drug was effective, but toxicity was increased in patients with squamous histology. Subsequent trials (e.g., ECOG4599) were restricted to patients with nonsquamous histology (5), and current Food and Drug Administration (FDA) approval is applicable only to these patients.
More recent clinical studies have shown a major treatment-by-histology (squamous vs. nonsquamous) interaction in the responsiveness of advanced stage IV NSCLC to pemetrexed. Scagliotti and colleagues (6) performed an interaction analysis of the three pivotal Phase III studies leading to the approval of pemetrexed in the first-line, second-line, and maintenance settings. The results demonstrated clear-cut and very significant treatment-by-histology interactions with regard to progression-free and overall survival, thus confirming the superior efficacy of pemetrexed in nonsquamous NSCLC patients. These findings have prompted a major shift in lung cancer management toward targeted therapy, thus mandating that diagnostic specimens be acquired and processed in a fashion that permits the histologic subtyping of NSCLC (7).
Adenocarcinoma: EGFR Mutation and EML4-ALK Translocation
The oncogene addiction hypothesis suggests that precise targeting of discrete genetic alterations in tumors will kill tumor cells and result in clinical response. This principle has been demonstrated in lung adenocarcinoma with agents targeted toward the EGFR and to anaplastic lymphoma kinase (ALK).
EGFR is a 170-kD tyrosine kinase receptor that is overexpressed in 40–80% of NSCLC (8) and can be targeted with small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib and gefitinib, which bind to the ATP-binding pocket of the receptor. The sequencing of tumor samples from responding patients in clinical trials of TKIs led to the discovery of somatic mutations in the EGFR tyrosine kinase domain (9, 10). The activating and oncogenic mutations reported to date are in exons 18–21, affecting the tyrosine kinase portion of EGFR. Most mutations are either small deletions of exon 19 affecting a three-amino acid (LRE) sequence or a point mutation L858R on exon 21. Mutations affecting exon 20, such as small insertions in T790M, account for 3–5% of all EGFR mutations, and although they are activating, they are also associated with primary resistance to EGFR TKIs. The prevalence of EGFR mutations varies by ethnicity, from 5–20% in whites to 20–40% in the Asian population; they seem uncommon in black patients (11, 12). Recent clinical trials from Japan from Maemondo and colleagues (13) and from the West Japan Oncology Group (14) have shown that TKI therapy in EGFR mutant adenocarcinoma results in response rates of greater than 70% and results in longer progression-free survival compared with conventional chemotherapy in advanced lung adenocarcinoma. Together, these studies strongly support the role of molecular testing in lung adenocarcinoma for identification of EGFR mutant cancers (15) and suggest that TKI treatment is the preferred first-line therapy for advanced EGFR mutant tumors. The American Cancer Society has issued a Provisional Clinical Opinion that patients with NSCLC who are being considered for first-line therapy with an EGFR TKI should have their tumor tested for EGFR mutations (16).
Because key lung cancer mutations are mutually exclusive, an alternative to EGFR mutation testing is K-ras mutation testing. K-ras mutations are present in approximately 30% of lung adenocarcinomas and are confined to three codons, which lowers the cost and complexity of mutation sequencing. K-ras mutation–positive tumors are resistant to TKI therapy, thus a positive test obviates the need for EGFR mutation testing. Adenocarcinomas that are negative for EGFR and K-ras mutations can be screened for the presence of chromosomal translocation of the ALK gene. ALK gene translocations, mainly EML4-ALK, were recently identified as a new oncogenic mechanism in NSCLC present in 3–5% of tumors (17). Kwak and colleagues (18) published the first human experience with ALK inhibition through the use of the dual ALK-MET inhibitor, crizotinib (PF-02341066) in patients with ALK-translocated, advanced lung carcinoma. In 82 patients with fluorescence in situ hybridization (FISH) testing–confirmed translocations, crizotinib at a dose of 250 mg orally twice a day led to a dramatic 57% response rate and a 6-month progression-free survival of 72%. Subsequently, crizotinib has been approved by the US FDA for the treatment of patients with NSCLC tumors that harbor the ALK translocation as detected by an FDA-approved FISH test. Sequential testing for EGFR, K-ras, and ALK is reasonable, beginning with either K-ras or EGFR analysis, with ALK analysis reserved for K-ras and EGFR negative specimens. Concomitant testing is not necessary unless sequential testing causes delay in treatment.
These results show the promise of therapy targeted to specific DNA alterations in advanced lung adenocarcinoma and emphasize the importance of acquiring diagnostic specimens that provide sufficient material for molecular testing. It is expected that the indications will expand in terms of the number of molecular tests needed and in terms of the tumor types from which testing will be required. Clinical trials are ongoing to determine the feasibility of performing sequencing for multiple DNA mutations that may be clinically relevant and of triaging treatment arms on the basis of DNA mutation analysis (19, 20). Currently, lung cancer specimens should and do provide sufficient tissue for histologic subtyping and for two molecular assays (EGFR and ALK) that are required for current therapy of advanced lung cancer. As additional assays are tested and validated, they will be introduced into routine clinical care and specimen acquisition and processing protocols will be adjusted accordingly. Current research of next-generation sequencing methodology is directed toward analysis of single cells and small specimens. These advances will be particularly applicable to needle aspiration specimens and will be an important future research direction.
Emergence of Endobronchial Ultrasound–Guided Transbronchial Needle Aspiration as the Procedure of Choice for Sampling Intrathoracic Lymph Nodes and Establishing Lung Cancer Diagnosis in Locally Advanced NSCLC
There are multiple approaches to sampling suspected NSCLC in the thorax (21). Suspected primary or metastatic parenchymal lesions in the periphery may be amenable to percutaneous CT-guided needle biopsy. Central primary and metastatic lesions can be accessed bronchoscopically with saline lavage or washing, cytologic brushing, forceps biopsy, or needle aspiration. Multiple surgical options are available, including mediastinoscopy, video-assisted thoracoscopic biopsy, and open thoracotomy. The overriding goal is to obtain the requisite information for diagnosis and staging with the least risk to the patient. For example, in a patient with suspected thoracic nodal metastasis, an ideal procedure is one in which diagnosis and staging is done in a single step with sampling directed toward the affected lymph node that indicates the highest clinical stage.
Until recently, surgical mediastinoscopy was the preferred initial approach for staging the mediastinum. Endobronchial ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) has gained wide acceptance as a preferred procedure for sampling intrathoracic lesions in patients with suspected or known lung cancer, now often supplanting mediastinoscopy as a first-line approach for diagnosis and staging (22).
There are several advantages of EBUS over mediastinoscopy. Multiple studies have reported superior sensitivity and specificity of EBUS-TBNA compared with those reported for surgical mediastinoscopy (23). Ernst and colleagues (24), using a prospective crossover design, showed higher sensitivity and higher negative predictive value for EBUS-TBNA compared with surgical mediastinoscopy. In a large, randomized trial comparing surgical staging alone with a strategy that used both endobronchial and transesophageal ultrasound-guided TBNA, Annema and colleagues (25) showed that the combined endosonographic approach had greater sensitivity for detecting nodal metastases and resulted in fewer unnecessary thoracotomies.
Although mediastinoscopy requires general anesthesia in all cases, EBUS can be performed outside an operating room, with topical anesthetic agents and moderate sedation. This results in reduced risk and may result in lower overall healthcare costs (26, 27). EBUS-TBNA has access to a greater range of nodal stations because hilar nodes can be accessed in many patients (28). Thus, for patients with suspected cancer and suspected nodal involvement, staging and diagnosis can preferentially be performed by EBUS in a single, minimally invasive procedure.
There are limitations to EBUS-TBNA. The negative predictive value is high, but it is not 100%. In one large study involving 494 patients, the overall negative predictive value was 81%, whereas the negative predictive value for individual nodes was 86% (29). Nondiagnostic findings, in which an aspiration contains neither malignant cells nor lymphocytes, occur in as many as 20% of individual aspirates and 10% of cases (30–32). Most studies report a positive predictive value of 100% for EBUS-TBNA, and therefore confirmatory mediastinoscopy in patients with a positive EBUS-TBNA is not needed. However, we and others believe that confirmatory surgical staging is indicated for negative and nondiagnostic needle aspiration cases (29, 33, 34), and that EBUS-TBNA is best regarded as complementary to, but not as a substitute for, surgical staging of the mediastinum (23).
The original EBUS-TBNA needle was 22-gauge. A 21-gauge needle is now available. Saji and colleagues (35) reported higher sample volumes and higher diagnostic yield with the 21-gauge EBUS-TBNA needle. Nakajima and colleagues (36) reported similar diagnostic yield with both needles but better preservation of histologic structure with the 21-gauge needle, at the expense of greater blood contamination. Despite the paucity of data, most centers have switched to the 21-gauge needle.
Regardless of needle size, the amount of material collected with EBUS-TBNA is small relative to the specimen sizes obtained by mediastinoscopy. As sample size has gotten smaller, the amount of pathologic and molecular information clinicians wish to extract from these samples has grown larger. Potentially all treatment decisions, at the time of diagnosis and later, are based on the information obtainable from that specimen. Therefore, it is essential that the specimen collection and processing procedures be optimized to ensure that the specimen's quality and quantity are adequate to provide answers to all of the questions that may be asked of it. Several studies have shown that cytologic specimens obtained by EBUS-TBNA are suitable for molecular testing for EGFR, K-ras, and ALK, although the exact yield for this testing is unknown (37–40). The bronchoscopist, in collaboration with the cytologist, must take responsibility for ensuring the adequacy and proper processing of the specimen to facilitate this testing.
Cytology of NSCLC
Currently, there is an underappreciated problem with EBUS-TBNA and for percutaneous aspiration specimens: the potential for an “inadequate” positive. This refers to a specimen in which neoplastic cells are identified but for which histologic subtyping is not possible (e.g., a diagnosis of NSCLC, not otherwise specified [NSCLC-NOS]). Subclassifying a non–small cell carcinoma on a small biopsy is occasionally difficult, because of biopsy size, tumor heterogeneity, and limited architectural detail (41, 42). Recent literature, based primarily on CT-guided aspiration biopsies, has demonstrated that adenocarcinomas and squamous cell carcinomas can be diagnosed in most instances, especially with the aid of cell blocks and immunohistochemistry (IHC) in cases of poorly differentiated carcinomas (39, 43, 44). Although there is no widely accepted standard terminology for diagnosing lung carcinomas on small biopsies or cytology specimens (42), it was recommended at the recent International Multidisciplinary Classification of Lung Adenocarcinoma (45) that the term “NSCLC-NOS” be used as little as possible, and that it be applied only when a more specific diagnosis is not possible by morphology or special stains.
Optimization of EBUS-TBNA Specimen Procurement and Processing
Rapid on-site cytologic evaluation (ROSE) of the aspirated specimens with cell block preparation has been shown to be effective in optimizing the yield and efficiency of EBUS-TBNA (44, 46). In a large metaanalysis, ROSE was associated with increased sensitivity of EBUS-TBNA from 80–88% without increasing procedure length (47). Because of staffing, time, and cost constraints (48, 49), ROSE is not available in every institution. However, recent data convincingly demonstrate that aspirates performed with ROSE optimize the use of cell aspiration procedure (50, 51). It allows the bronchoscopist to acquire repeated passes from sites known to yield diagnostic specimens. These additional specimens are triaged for processing to maximize histologic and molecular data yields. The appropriate triaging of small biopsy specimens for cytologic, pathologic, and molecular analysis is crucial, yet there are no established guidelines for triaging lung aspirates (51). The bronchoscopist and ROSE cytologist together can ensure that sufficient material is collected for cytologic diagnosis and for cell block that will be used for ancillary testing, including IHC and molecular analysis. Thus, it is recommended that bronchoscopists performing EBUS-TBNA should strongly advocate for institutional availability of ROSE.
The Procedures for EBUS-TBNA Specimen Processing
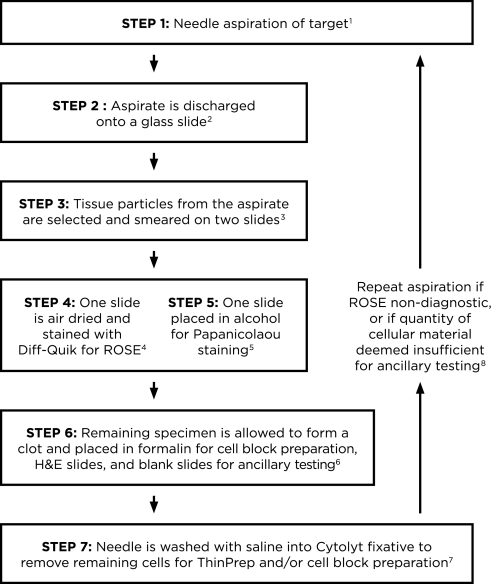
Figure 1 provides an algorithm for processing EBUS-TBNA specimens. Each aspirate is discharged onto a glass slide, either by blowing air with a syringe through the needle or by replacing the needle stylet, and then saline is passed with a syringe through the EBUS needle into CytoLyt solution (Hologic Inc., Marlborough, MA) to discharge any remaining cells. From the material dispelled onto the glass slide, a few tan–white color tissue particles are selected from the blood and mucus and smeared on two slides (52). The remaining specimen on the original slide is allowed to clot. One slide smear is air-dried for on-site assessment using Diff-Quik (Richard Allen Scientific, Kalamazoo, MI) stain, and the second is placed in alcohol for Papanicolaou staining, which enhances nuclear cytologic detail. Cell blocks are made from the material in CytoLyt solution or the clot, which is placed in formalin, centrifuged in the pathology laboratory, and submitted for histologic processing. Alternatively, the EBUS-TBNA specimen can be discharged into RPMI medium for flow cytometry if lymphoma is under consideration, or into saline if infection is suspected. Techniques using discharge of the needle contents directly into RPMI (53), saline, formalin (31, 32), CytoLyt solution (49, 54), and onto filter paper for formalin fixation have also been described (51). Likewise, different methods are used to form a cohesive pellet, including use of agar or a combination of thrombin-human plasma (54). The authors find that spontaneous pellet formation or discharge into CytoLyt provide sufficient material for cell blocks. Regardless, for a specimen to be deemed adequate by ROSE, there should be several tissue particles available for cell block; otherwise, repeat aspirates may be necessary.
Figure 1.
Algorithm for processing endoscopic ultrasound transbronchial needle aspiration (EBUS-TBNA) specimens. 1Use of the 21-gauge needle may improve diagnostic yield and may provide better preservation of histologic structure compared with the 22-gauge needle. 2The aspirate from the needle is discharged onto a glass slide by blowing air with a 20-ml syringe. If there is resistance because of clot formation, and material cannot be expelled by air, the stylet is reinserted to propel the material onto the slide. The specimen is usually placed in its entirety onto a glass slide; it may be expelled onto two slides if there is excessive blood. 3A few tissue particles, which are often tan–white, are selected from the expelled material to make smears. If the specimen has clotted, the clot is gently pressed between two slides to expose tissue particles, which are then smeared onto slides. Note that although a clot can be smeared, its thickness does not allow cells within it to be visualized on a smear. 4One smear is air-dried completely for on-site Diff-Quik staining and rapid on-site cytologic evaluation (ROSE). 5The second smear is fixed in alcohol immediately for Papanicolaou staining in the laboratory. Any air-drying creates artifacts that preclude accurate interpretation. 6The remaining specimen is allowed to clot and is placed in formalin to create a cell block for hematoxylin and eosin (H&E) slides and blank slides for immunocytochemistry, molecular, or fluorescence in situ hybridization testing, if indicated. If lymphoma is suspected on clinical grounds or after ROSE, the remainder of the specimen is placed in RPMI or saline for flow cytometry analysis. An aliquot is placed in saline for cultures in cases where granulomas are noted or infection is suspected. 7Saline (∼1 ml) is passed through the needle into Cytolyt fixative to remove any additional cells for ThinPrep or cell block preparation. 8In addition to assessing diagnostic adequacy, ROSE must include an overall quantitative assessment of the amount of cellular material triaged for cell block and ancillary testing (immunohistochemistry and molecular testing). If this is considered to be potentially insufficient, the needle aspiration should be repeated from the diagnostic location.
Hematoxylin and eosin–stained and blank slides are prepared from the cell block upfront to minimize loss of tissue by recutting for ancillary testing. These cell block preparations provide an additional source of information, such as cellular detail (i.e., intercellular bridges between squamous cells) that is complementary or supplementary to the smears or liquid-based preparations. Cell block slides can aid in identification of adenocarcinoma architectural patterns (i.e., lepidic, papillary, and micropapillary) (44) that are important for the proposed classification of small biopsies and aspirates (42). Also, cell blocks yield multiple slides in a concentrated format for subsequent IHC (55) that permits accurate histologic subtyping of NSCLC, especially for poorly differentiated carcinomas (43, 44). In the authors’ institution, a review of all diagnoses of malignancy by EBUS-TBNA over a 1-year period showed that 15 (34%) of 44 cases were classified as NSCLC-NOS by standard criteria based on cytologic review of smears alone. In 13 (87%) of those cases, the cell block specimen was adequate for IHC, and in 11 (85%) of those 13, histologic subtyping was possible (56). Equally importantly, cell blocks provide paraffin-embedded tissue for DNA mutation and FISH testing, thus permitting determination of EGFR, K-ras, and ALK translocation status (57).
It should be noted that molecular studies can use cytologic slides. EGFR and K-ras mutation testing can be performed on DNA extracted from Papanicolau smears (53), ThinPreps (Hologic Inc.), and fresh samples (58, 59). However, formalin-fixed paraffin-embedded tissue generated from cell blocks permits long-term sample preservation and serves as a source of archival DNA, available for future studies as required.
Conclusions
Recent advances in therapy for NSCLC have shown that a personalized approach to treatment has the potential to significantly reduce mortality. Concurrently, EBUS-TBNA has emerged as an accurate and sensitive tool for the diagnosis and staging of this disease. As knowledge of the molecular mechanisms that drive lung cancer progression increases, the amount of information that must be derived from a tumor specimen will also increase. Recent clinical studies have demonstrated that small specimens acquired by EBUS are sufficient for molecular testing if specimen acquisition and processing are done with these needs in mind. It is no longer the case that surgical specimens are required in many cases to permit the sophisticated molecular testing required for a personalized approach to therapy. It is increasingly appreciated that sequential biopsies may be required to evaluate somatic mutations and histologic changes that occur in resistant and recurrent disease (60). These sequential biopsies will most likely be acquired using small specimen techniques; thus the need to implement procedures to maximize specimen yield will have increasing importance.
Optimum use of EBUS-TBNA requires a coordinated effort between the bronchoscopist and the cytopathologist to collect and triage specimens for diagnostic testing. When feasible, ROSE should be performed to assess the specimen for diagnostic quality and quantity and to allocate the specimen for cell block and possible IHC and molecular studies. It is necessary for the pulmonologist to understand the rationale for histologic and molecular testing of lung cancer diagnostic specimens and to ensure that specimens are acquired and processed in a fashion that provides information from small cytologic specimens that is sufficient to guide treatment in the era of targeted therapy.
Supplementary Material
Footnotes
Supported by NIH grant RO1CA120174 (C.A.P.).
Author Contributions: manuscript conception and design, W.B., A.S., and C.A.P.
Originally Published in Press as DOI: 10.1164/rccm.201107-1199CI on October 27, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300 [DOI] [PubMed] [Google Scholar]
- 2.Halmos B, Powell CA. Update in lung cancer and oncological disorders 2010. Am J Respir Crit Care Med 2011;184:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulman WA, Powell CA. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis and staging of thoracic malignancy. Am J Respir Crit Care Med 2010;181:A5160 [Google Scholar]
- 4.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, III, Gaudreault J, Damico LA, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184–2191 [DOI] [PubMed] [Google Scholar]
- 5.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542–2550 [DOI] [PubMed] [Google Scholar]
- 6.Scagliotti G, Brodowicz T, Shepherd FA, Zielinski C, Vansteenkiste J, Manegold C, Simms L, Fossella F, Sugarman K, Belani CP. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:64–70 [DOI] [PubMed] [Google Scholar]
- 7.Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol 2010;28:5311–5320 [DOI] [PubMed] [Google Scholar]
- 8.Hirsch FR, Varella-Garcia M, Cappuzzo F. Predictive value of EGFR and her2 overexpression in advanced non-small-cell lung cancer. Oncogene 2009;28:S32–S37 [DOI] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139 [DOI] [PubMed] [Google Scholar]
- 10.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–1500 [DOI] [PubMed] [Google Scholar]
- 11.Tokumo M, Toyooka S, Kiura K, Shigematsu H, Tomii K, Aoe M, Ichimura K, Tsuda T, Yano M, Tsukuda K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res 2005;11:1167–1173 [PubMed] [Google Scholar]
- 12.John T, Liu G, Tsao MS. Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene 2009;28:S14–S23 [DOI] [PubMed] [Google Scholar]
- 13.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–2388 [DOI] [PubMed] [Google Scholar]
- 14.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (wjtog3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–128 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Stiegler AL, Boggon TJ, Kobayashi S, Halmos B. EGFR-mutated lung cancer: a paradigm of molecular oncology. Oncotarget 2010;1:497–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keedy VL, Temin S, Somerfield MR, Beasley MB, Johnson DH, McShane LM, Milton DT, Strawn JR, Wakelee HA, Giaccone G. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 2011;29:2121–2127 [DOI] [PubMed] [Google Scholar]
- 17.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, et al. Identification of the transforming eml4-alk fusion gene in non-small-cell lung cancer. Nature 2007;448:561–566 [DOI] [PubMed] [Google Scholar]
- 18.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunn PA, Jr, Hirsch FR, Doebele RC, Camidge DR, Varella-Garcia M, Franklin W. Biomarkers are here to stay for clinical research and standard care. J Thorac Oncol 2010;5:1113–1115 [DOI] [PubMed] [Google Scholar]
- 20.Gold KA, Kim ES, Lee JJ, Wistuba II, Farhangfar CJ, Hong WK. The battle to personalize lung cancer prevention through reverse migration. Cancer Prev Res (Phila Pa) 2011;4:962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera MP, Mehta AC. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131S–148S [DOI] [PubMed] [Google Scholar]
- 22.Adams K, Shah PL, Edmonds L, Lim E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757–762 [DOI] [PubMed] [Google Scholar]
- 23.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S–220S [DOI] [PubMed] [Google Scholar]
- 24.Ernst A, Anantham D, Eberhardt R, Krasnik M, Herth FJ. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577–582 [DOI] [PubMed] [Google Scholar]
- 25.Annema JT, van Meerbeeck JP, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, De Leyn P, Braun J, Carroll NR, Praet M, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245–2252 [DOI] [PubMed] [Google Scholar]
- 26.Harewood GC, Pascual J, Raimondo M, Woodward T, Johnson M, McComb B, Odell J, Jamil LH, Gill KR, Wallace MB. Economic analysis of combined endoscopic and endobronchial ultrasound in the evaluation of patients with suspected non-small cell lung cancer. Lung Cancer 2010;67:366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, Rice TW, Murthy SC, DeCamp MM, Pierce CD, Karchmer DP, Rybicki LA, Blackstone EH. Combined bronchoscopy, mediastinoscopy, and thoracotomy for lung cancer: Who benefits? J Thorac Cardiovasc Surg 2004;127:850–856 [DOI] [PubMed] [Google Scholar]
- 28.Medford AR, Bennett JA, Free CM, Agrawal S. Mediastinal staging procedures in lung cancer: EBUS, TBNA and mediastinoscopy. Curr Opin Pulm Med 2009;15:334–342 [DOI] [PubMed] [Google Scholar]
- 29.Defranchi SA, Edell ES, Daniels CE, Prakash UB, Swanson KL, Utz JP, Allen MS, Cassivi SD, Deschamps C, Nichols FC, III, et al. Mediastinoscopy in patients with lung cancer and negative endobronchial ultrasound guided needle aspiration. Ann Thorac Surg 2010;90:1753–1757 [DOI] [PubMed] [Google Scholar]
- 30.Stoll LM, Yung RC, Clark DP, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration versus conventional transbronchial needle aspiration. Cancer Cytopathol 2010;118:278–286 [DOI] [PubMed] [Google Scholar]
- 31.Nayak A, Sugrue C, Koenig S, Wasserman PG, Hoda S, Morgenstern NJ. Endobronchial ultrasound-guided transbronchial needle aspirate (EBUS-TBNA): a proposal for on-site adequacy criteria. Diagn Cytopathol (In press) [DOI] [PubMed] [Google Scholar]
- 32.Alsharif M, Andrade RS, Groth SS, Stelow EB, Pambuccian SE. Endobronchial ultrasound-guided transbronchial fine-needle aspiration: the University of Minnesota experience, with emphasis on usefulness, adequacy assessment, and diagnostic difficulties. Am J Clin Pathol 2008;130:434–443 [DOI] [PubMed] [Google Scholar]
- 33.Szlubowski A, Kuzdzal J, Kolodziej M, Soja J, Pankowski J, Obrochta A, Kopinski P, Zielinski M. Endobronchial ultrasound-guided needle aspiration in the non-small cell lung cancer staging. Eur J Cardiothorac Surg 2009;35:332–335, discussion 335–336 [DOI] [PubMed] [Google Scholar]
- 34.Gilbert S, Wilson DO, Christie NA, Pennathur A, Luketich JD, Landreneau RJ, Close JM, Schuchert MJ. Endobronchial ultrasound as a diagnostic tool in patients with mediastinal lymphadenopathy. Ann Thorac Surg 2009;88:896–900; discussion 901–892 [DOI] [PubMed] [Google Scholar]
- 35.Saji J, Kurimoto N, Morita K, Nakamura M, Inoue T, Nakamura H, Miyazawa T. Comparison of 21-gauge and 22-gauge needles for endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. J Bronchol Intervent Pulmonol 2011;18:239–246 [DOI] [PubMed] [Google Scholar]
- 36.Nakajima T, Yasufuku K, Takahashi R, Shingyoji M, Hirata T, Itami M, Matsui Y, Itakura M, Iizasa T, Kimura H. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90–94 [DOI] [PubMed] [Google Scholar]
- 37.Sakairi Y, Nakajima T, Yasufuku K, Ikebe D, Kageyama H, Soda M, Takeuchi K, Itami M, Iizasa T, Yoshino I, et al. Eml4-alk fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Clin Cancer Res 2010;16:4938–4945 [DOI] [PubMed] [Google Scholar]
- 38.Nakajima T, Yasufuku K, Nakagawara A, Kimura H, Yoshino I. Multi-gene mutation analysis of metastatic lymph nodes in non-small cell lung cancer diagnosed by EBUS-TBNA. Chest 2011;140:1319–1324 [DOI] [PubMed] [Google Scholar]
- 39.Rekhtman N, Brandt SM, Sigel CS, Friedlander MA, Riely GJ, Travis WD, Zakowski MF, Moreira AL. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and kras molecular testing. J Thorac Oncol 2011;6:451–458 [DOI] [PubMed] [Google Scholar]
- 40.Nakajima T, Kimura H, Takeuchi K, Soda M, Mano H, Yasufuku K, Iizasa T. Treatment of lung cancer with an ALK inhibitor after eml4-alk fusion gene detection using endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Oncol 2010;5:2041–2043 [DOI] [PubMed] [Google Scholar]
- 41.Roggli VL, Vollmer RT, Greenberg SD, McGavran MH, Spjut HJ, Yesner R. Lung cancer heterogeneity: a blinded and randomized study of 100 consecutive cases. Hum Pathol 1985;16:569–579 [DOI] [PubMed] [Google Scholar]
- 42.Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: strategic management of tissue for molecular testing. Semin Respir Crit Care Med 2011;32:22–31 [DOI] [PubMed] [Google Scholar]
- 43.Vazquez MF, Koizumi JH, Henschke CI, Yankelevitz DF. Reliability of cytologic diagnosis of early lung cancer. Cancer 2007;111:252–258 [DOI] [PubMed] [Google Scholar]
- 44.Loukeris K, Vazquez MF, Sica G, Wagner P, Yankelevitz DF, Henschke CI, Cham MD, Saqi A. Cytological cell blocks: predictors of squamous cell carcinoma and adenocarcinoma subtypes. Diagn Cytopathol 2010;39:92–100 [DOI] [PubMed] [Google Scholar]
- 45.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace WA, Rassl DM. Accuracy of cell typing in non-small cell lung cancer by EBUS/EUS - FNA cytology samples. Eur Respir J 2011;38:911–917 [DOI] [PubMed] [Google Scholar]
- 47.Micames CG, McCrory DC, Pavey DA, Jowell PS, Gress FG. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: a systematic review and metaanalysis. Chest 2007;131:539–548 [DOI] [PubMed] [Google Scholar]
- 48.Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: a cost and compensation analysis. Cancer 2001;93:319–322 [DOI] [PubMed] [Google Scholar]
- 49.Wallace WA, Monaghan HM, Salter DM, Gibbons MA, Skwarski KM. Endobronchial ultrasound-guided fine-needle aspiration and liquid-based thin-layer cytology. J Clin Pathol 2007;60:388–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasuti JF, Gupta PK, Baloch ZW. Diagnostic value and cost-effectiveness of on-site evaluation of fine-needle aspiration specimens: review of 5,688 cases. Diagn Cytopathol 2002;27:1–4 [DOI] [PubMed] [Google Scholar]
- 51.Nakajima T, Yasufuku K. How I do it: optimal methodology for multidirectional analysis of endobronchial ultrasound-guided transbronchial needle aspiration samples. J Thorac Oncol 2011;6:203–206 [DOI] [PubMed] [Google Scholar]
- 52.Giri D, Vazquez MF. “Pick and smear” tissue concentration technique for bloody aspirates. Acta Cytol 2001;45:889–890 [PubMed] [Google Scholar]
- 53.Billah S, Stewart J, Staerkel G, Chen S, Gong Y, Guo M. EGFR and kras mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol 2011;119:111–117 [DOI] [PubMed] [Google Scholar]
- 54.Natu S, Hoffman J, Siddiqui M, Hobday C, Shrimankar J, Harrison R. The role of endobronchial ultrasound guided transbronchial needle aspiration cytology in the investigation of mediastinal lymphadenopathy and masses. The North Tees experience. J Clin Pathol 2010;63:445–451 [DOI] [PubMed] [Google Scholar]
- 55.Kung IT, Yuen RW, Chan JK. Optimal formalin fixation and processing schedule of cell blocks from fine needle aspirates. Pathology 1989;21:143–145 [DOI] [PubMed] [Google Scholar]
- 56.Bulman WA, Saqi A, Turk A, Maxfield R, Powell CA. Endobronchial ultrasound-guided transbronchial needle aspiration for diagnosis and staging of thoracic malignancy: sensitivity and optimization of the cytologic specimen. Presented at the American Thoracic Society 2010 International Conference; May 14–19, 2010, New Orleans, LA [Google Scholar]
- 57.Travis WD, Rekhtman N, Riley GJ, Geisinger KR, Asamura H, Brambilla E, Garg K, Hirsch FR, Noguchi M, Powell CA, et al. Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology: a paradigm shift. J Thorac Oncol 2010;5:411–414 [DOI] [PubMed] [Google Scholar]
- 58.Lozano MD, Zulueta JJ, Echeveste JI, Gurpide A, Seijo LM, Martin-Algarra S, Del Barrio A, Pio R, Idoate MA, Labiano T, et al. Assessment of epidermal growth factor receptor and k-ras mutation status in cytological stained smears of non-small cell lung cancer patients: correlation with clinical outcomes. Oncologist 2011;16:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schuurbiers OC, Looijen-Salamon MG, Ligtenberg MJ, van der Heijden HF. A brief retrospective report on the feasibility of epidermal growth factor receptor and kras mutation analysis in transesophageal ultrasound- and endobronchial ultrasound-guided fine needle cytological aspirates. J Thorac Oncol 2010;5:1664–1667 [DOI] [PubMed] [Google Scholar]
- 60.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Science Translational Medicine 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.